Abstract
Background
Heart failure has the highest rates of adult hospitalisations, the highest mortality rates and significant costs associated with its care. The cost of heart failure is expected continue to grow on a global scale, with $108 billion spent on heart failure in 2012. Mortality rates are high, with incident cases of heart failure resulting in 30% 1-year mortality, and in hospital mortality of acute heart failure, 28%.
Methods and Results
This article reviews the devices currently in use for the treatment of heart failure, as well as those that are under investigation. A review of the mechanism of action of devices, the literature supporting their application as therapy, and the cost effectiveness associated with their use are discussed. Conventional techniques discussed herein include the guideline-supported therapies of mechanical circulatory support (MCS) and cardiac resynchronisation therapy (CRT). Novel devices that are discussed include invasive physiological monitoring, neuromodulation, percutaneous ventricular assist devices (VADs) and cardiac contractility modulation (CCM). There has been advancement in mechanical circulatory support devices for the treatment of both acute and chronic heart failure. In addition to MCS, only CRT has resulted in reduced mortality.
Conclusion
Due to the clinical and economic arguments, treatment of heart failure is said to be the biggest unmet need in cardiology today. The data reviewed herein support this statement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure is a complex syndrome with many aetiologies, a broad spectrum of clinical features, and various clinical subsets. It results from impairment in the ability if the heart to pump sufficient amounts of blood into the circulation during systole. An ejection fraction of ≤40% on echocardiography indicates impaired left ventricular systolic function or heart failure with reduced ejection fraction (HFrEF) [1]. Heart failure that occurs with normal left ventricular (LV) systolic function or with en ejection fraction of >50% is known as heart failure with preserved ejection fraction (HFpEF) [2]. Acute heart failure is a heterogeneous set of syndromes. Acute heart failure syndromes (AHFSs) are present in three forms [1]: (1) acute pulmonary oedema, (2) cardiogenic Shock (5–8% of STEMI and 2.5% of non-STEMI) [3], and (3) acute decompensation of CHF. This review presents the current state of the art devices currently in use for the treatment of heart failure, as well as those that are under investigation. The authors have categorized the devices based on their placement within the body and/or the mode of action of the device.
The AHA cites an annual incidence of 670,000 new cases of heart failure annually in the United States with an estimated prevalence of 5.8 million or 2.2% of the population [1, 4, 5]. Incidence for the disease approaches 10 per 1000 population after the age of 65. European data on the prevalence of heart failure are provided by the ESC statistics. According to their data on a cohort of 900 million people, prevalence is estimated at 15 Million patients [5]. Therefore, in developed countries, 1–2% of the adult population has a diagnosis of heart failure, with the prevalence rising to >10% in persons 70 and older. The annual incidence is 5–10 per 1000 persons per year [1]. The prevalence of heart failure with a preserved EF (HFpEF) in those with a diagnosis of heart failure is between 30 and 60%, with an incidence of 4.4% [6–8]. The 1-year mortality with incident cases of heart failure is 30%, with 5-year mortality about 50% and at 10 years 10% [9]. Acute heart failure Syndrome has associated with an in-hospital mortality of 28% and cardiogenic shock, the most severe form of acute heart failure, has mortality of 40–80% [10].
Heart failure has the highest rate of adult hospitalizations, highest mortality rates and as a result of this and the costs associated with its care, it has been described as an epidemic [11, 12]. This imposes a significant economic burden on governments and health care organizations.
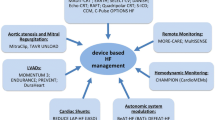
As an estimate of percentage spending of total health care globally, heart failure spending in 2012 was estimated at $108 billion [11]. The United States has the highest spending on HF per annum, $37 Billion in 2009. The AHA estimates that in the US by 2020, heart failure will cost $57 Billion and by 2030 $77 Billion [13]. The major part of this expenditure is related to hospitalizations, with an estimated cost of $20.1 Billion in 2009 [12]. In Europe, heart failure treatment takes up 1–2% of the European health spending budget, of which 75% are hospital costs [10]. As a result of variety of clinical and economic arguments, it has been said that treatment of HF is the largest unmet clinical need in cardiology today [14]. Figure 1 shows heart failure devices divided by groups (1–11) based on their locations.
Heart failure devices divided by groups (1–11) based on their locations. RA right atrium, LA left atrium, RV right ventricle, LV left ventricle, LVAD left ventricular assist device, MCD mechanical circulatory device, IABP intra-aortic balloon pump, CRT cardiac resynchronization therapy, IHDM implantable haemodynamic monitoring device, ECMO extracorporeal membrane oxygenation, CCM cardiac contractility modulation, VA veno-arterial, VV veno-venous
Group 1: Left ventricle
Left ventricular assist devices
Ventricular assist devices (VADs) function as mechanical pumps that take over the function of the ventricle and restore normal haemodynamics and end-organ flow [15]. Cardiac output is improved, thereby decreasing preload, cardiac workload and neurohormonal response with resultant increase in systemic circulation and tissue perfusion. Blood is taken from the left ventricle and exits the pump via a connection to the ascending aorta via surgical anastomosis.
In those patients who are cancer free and not in cardiogenic shock, the 2-year survival of those implanted with a continuous flow left ventricular assist device is 80% [16]. Before 2008, all VADs implanted in the US outside of clinical trials were electrically or pneumatically driven volume displacement pump, i.e. 1st generation pulsatile flow devices.
2nd generation devices are continuous axial flow pumps. In 2009, an RCT demonstrated significantly better survival for those treated with continuous flow device (or 2nd generation pumps) with one and 2-year survival 68 and 58% compared to 55 and 24% with the pulsatile device [17]. Continuous flow pumps have been the dominant technology since 2008 and account for 100% of patients receiving destination therapy since 2010 [18].
3rd generation VADS are continuous flow pumps with non-contact bearings that are currently under investigation and have so far shown non-inferiority to contemporaneously available devices [13, 19].
From 2008 to 2011, 24.8% of patients with a diagnosis of chronic heart failure received a permanent device with resultant survival benefit and improved quality of life [20]. 10% of patients with an LVAD develop significant device malfunctions. Bleeding, right heart failure, stroke, infection and device failure are among the main complications [21].
Uptake of use of VADs in the US remains much higher than Europe: the number of new VAD at >1700/year in the US vs. 430 per year in Europe. Reimbursement within the US for the device and procedures has encouraged this development. Contemporaneous analysis of the REMTACH trial estimated that the IECR was $802700/QOLY. More recently, continuous flow devices were estimated to cost $198,184/QOLY [22]. In a review of use of LVADs within the NHS, LVADS cost £ 80,569 lb by 2011 prices and a review in 2014 estimated a probabilistic incremental cost effectiveness ratio at £53527/QOLY ($84,963) over a lifetime horizon. It concluded that VADs were cost effective comparable to medical management [23].
The mortality advantage of VADs now has a 2-year equivalency to total heart replacement. Iterations of VADs and smaller size of the device continue to address the disadvantages, namely bulky, noisy devices with high complication rates. However, what still remains an issue with VADs is that specialized units are required involving heart failure specialists, infrastructure investment, and reimbursement protocols. The evolution of the LVADs encompassing size (gm) and generation is summarized in Table 1.
Centrimag
Centrimag (Thoratec) is a surgically implanted left ventricular assists device that is designed for short-term extracorporeal support in cardiogenic shock [24]. It is a 3rd generation continuous flow pump that is capable of providing up to 10 L/min of blood flow and has an FDA approval for LV support for up to 6 h [25]. A multicenter study provided evidence of its ability to provide short-term circulatory support for left, right or biventricular support, with a 30-day mortality of 47% [26].
Temporary mechanical circulatory support
Temporary mechanical circulatory support (MCS) devices are an effective means of providing support in the acute setting of cardiogenic shock or during high-risk procedures such as (percutaneous intervention) PCI. The aims of temporary MCS are to decrease preload and afterload and augment cardiac output with a goal of achieving adequate organ perfusion and oxygen delivery. To do this, they can be used to mechanically unload the left ventricle and right ventricle or provide biventricular support. Use of temporary non-percutaneous devices increased 101% from 2007 to 2011. Percutaneous devices showed the fastest growth of MCS from 2007 to 2011, with a 1511% increase in use [20]. A meta-analysis of percutaneous LVAD compared to IABP did not show any trends towards a reduced 30 mortality rate; however, superior haemodynamic support was observed with percutaneous LVAD over IABP during acute cardiogenic shock [27]. The AHA/ACC assigns a class IIb/C recommendation for LV assist devices in refractory cardiogenic shock and in the European guidelines; a class IIB recommendation is given [28].
TandemHeart™ device
The TandemHeart (CardiacAssist, Inc.) consists of venous transeptal inflow cannula, an extracorporeal continuous flow centrifugal pump that draws blood from a catheter that has been placed through the venous system across the interatrial septum and into the left atrium. The oxygenated blood from the left atrium is then pumped to the system circulation via a femoral artery catheter [29]. With its use in those with cardiogenic shock, it has been shown to improve cardiac output, increase MAP and reduce pulmonary wedge pressure [30]. TandemHeart has similar 30-day mortality rates when compared to IABP [31]. TandemHeart right VAD (RVAD) has implanted successfully both surgically and percutaneously with acute haemodynamic improvements in a broad range of clinical scenarios [32].
Impella device
The Impella (Abiomed) is an axial flow, rotary blood pump that is inserted through the arterial system and placed into the left ventricle by means of a retrograde fashion across the aortic valve. It provides non-pulsatile forward blood from the left ventricular outflow tract and results in unloading of the left ventricle with expulsion into the aorta. Impella has demonstrated haemodynamic benefits with use in the acute setting. It has proven to be equivalent to the IAPB with respect to mean adverse outcomes [33]. (Mortality)There are no RCTs on the higher functioning Impella device as of yet (Impella 5.0). Impella is indicated for use in acute cardiogenic shock and high-risk PCI [33].
HeartMate percutaneous heart pump (PHP)
The PHP (St. Jude Medical) works as a left ventricular unloading device, similar the Impella, with a flow rate of 5 L/min through a 14F sheath. It has a collapsible impellar blade that crosses the aortic valve, which is connected to an extracorporeal motor via a cable. Its efficacy and safety for use during high-risk PCI were established in SHIELD I RCT. (Presented at Transcatheter Cardiovascular Therapeutics in 2015).
Whilst there have been innovations with respect to percutaneous mechanical circulatory support, they have not resulted in improved mortality rates. The large increase in their use possibly reflects their relative ease of use, minimal invasiveness, and the improved haemodynamic profile associated with their use. It is the author’s opinion that a need still exists to treat acute heart failure that will result in statistically significant and reproducible improved mortality rates, an endpoint that has not yet been achieved.
Group 2: Aorta
Intra-aortic balloon pump (IABP)
The IABP is placed into the descending aorta via arterial access. It is inflated during diastole and deflated during systole. The result is an augmented diastolic pressure and improved coronary perfusion with reduced afterload and enhanced ventricular contraction [25]. Its use in clinical practice has shown equivocal benefit, with a meta-analysis not supporting its use for MI complicated by cardiogenic shock. The IABP-SHOCK II trial revealed no improvement of 30-day mortality rates in those with acute cardiogenic shock [34]. One analysis associated the use of IABP with a 25.2% increase in the cost of hospital stay [20]. Despite these data, it continues to be used and are frequently used as a comparison in the investigation of novel devices.
Reitan catheter pump
This is an axial flow pump similar to the Impella. However, it is placed in the descending Aorta, as opposed to the left ventricle. It functions to create a pressure gradient within the aorta that results in decreased afterload and increased organ perfusion. There are no RCTs demonstrating its efficacy in heart failure but its use in high-risk PCI has been shown to be safe and efficacious [35].
Group 3: Cardiac resynchronization therapy
When the left ventricle contracts synchronously, it results in efficient ejection of blood. However, if electrical disturbances of the heart result in sites of premature stimulation, such as LBBB, regions of early and delayed contraction can occur. This results in a decline in cardiac out and efficiency with a decrease in systolic function by 20% [24, 25].
With CRT, a left ventricular lead is placed in a tributary of the coronary sinus in addition to a right ventricular and atrial lead enabling pacing and subsequent resynchronization of the impaired mechanical contraction patterns to improve myocardial efficiency [22].
With CRT therapy in heart failure, all-cause mortality is reduced by 22% [21, 22]. CRT has also been shown to reduce heart failure hospitalizations as well as improvements in NYHA class, QOL scores, exercise capacity and LV function [36, 37].
International guidelines including ESC [37] and the ACC/AHA/HRS [38] recommended CRT for patients with EF ≤35%, NYHA III or IV with symptoms despite treatment wide QRS duration (>120 ms) and Sinus Rhythm. With the prevalence of QRS prolongation at around a third of the cohort of heart failure patients and with the current guideline criteria in mind, it is estimated that between 5 and 10% of the HF population are indicated for CRT [39]. Careful selection of patients who are likely to respond to CRT, remains an issue [40]. 20–30% of patients in major trials did not have a response to CRT [41]. Significant attention has been placed on the implant procedure with adverse events related to device implantation 12.7% in one meta-analysis [42]. Cost-effectiveness analysis of the major trials with substantial follow-up periods reports the incremental cost per Quality adjusted life-year gained as: $19,600 for Companion [43] $38,202 in the analysis of CARE-HF data, $32,822 in analysis of data from the longest CRT RCTs [44]. Furthermore, analysis from the CARE HF trial confirmed that CRT alone was cost effective in all age groups [45].
CRT has been deemed to be one of the most successful heart failure therapies to emerge in the last quarter of a century with applicability to 25–30% of patents [46]. Clinical trials demonstrating the improved mortality, reduced hospital admissions and improved quality of life, along with repeated demonstrable cost effectiveness for the use of CRT in the treatment of heart failure, should be enough to alleviate payers’ concerns with respect to use of the CRT device and justify the former statement.
Group 4: Implantable haemodynamic monitoring devices (IHMD)
The increases in intracardiac and pulmonary arterial pressures that occur as a result of decompensated heart failure are apparent weeks before the onset of symptoms [47, 48]. Studies of implantable haemodynamic monitoring systems have suggested clinical benefit [49, 50], and ongoing hypothesis being assessed in clinical trials is whether implantable haemodynamic monitoring devices could reduce heart failure hospitalizations [51].
An innovative means of assessing physiological parameters are implantable devices to collect hemodynamic data. Clinically, they are indicated for ambulatory HF patients. It is hoped by monitoring these physiological parameters, assessment of which would be by patient and or/doctor/nurse practitioners, medication changes could prevent acute decompensation and hospitalizations [52].
Right ventricular pressure monitoring
Right heart pressures can provide diagnostic, therapeutic and prognostic information with respect to heart failure. The Chronicle (Medtonic Inc) is an implantable continuous heamodynamic device that consists of an implanted memory system which is inserted subcutaneously in the pectoral area. It has a specialized transvenous lead sensor that measures intracardiac pressure at the right ventricle, which can be viewed remotely [53]. A single-blinded RCT, COMPASS HF, of 277 patients showed a non-significant reduction of all HF events [51].
Left atrial pressure monitoring device
The common pathway for acute heart failure results in an increase in left atrial pressure. This rise of LAP is gradual and precedes symptom onset [54]. Therefore, the potential exists to curb acute decompensations by accurate monitoring of LAP with resultants titration of medications [55].
The Heartpod Device (St. Jude Medical) consists of a sensor that is fixed onto the interatrial septum via cardiac catheterization and transeptal puncture; this is coupled to a coil antenna positioned in the subcutaneous tissue. It is capable of recording of left atrial pressures, an intracardiac electrogram and body temperatures. A handheld patient advisory module powers the device. The Heartpod Device was proved to be safe and feasible by means of measuring LAP [56].
The HOMEOSTASIS trial showed favourable outcomes with respect to LAP control and symptom reduction [57]. An RCT is going currently with safety and efficacy endpoints that aims to show a reduction in worsening heart failure and hospitalizations [58].
Pulmonary arterial pressure monitoring
The Cardiomems device (St. Jude Medical) is a permanently implanted wireless pulmonary artery pressure sensor. The device consists of a pressure sensitive capacitor that is inserted via catheter into the femoral vein to the deployment site in the pulmonary artery. The CHAMPION RCT randomized patients post-insertion of the device to treatment and non-treatment. The primary efficacy endpoint of reduced hospitalizations for heart failure at 6 months was achieved with a reduction of HFH of 28% (p < 0.0002) [59].
The economic cost of heart failure treatment has been discussed in “Group 2: Aorta”. With respect to this and the notoriously high hospital admission and readmission rates, interest in innovating in IHMDs is justified. However, the ideal parameter(s) to adjudge definite decompensation has not yet been identified. Once achieved and reciprocal treatment identified, IHMD could treat the clinical and economic need of treating heart failure effectively.
Group 5: Neuromodulation
Sympathetic overactivity as well as withdrawal of parasympathetic activity contribute to the development of heart failure [60]. The targeting of the sympathetic nervous system via pharmacological beta blockade in the treatment of HF reduced mortality by 35% [61]. Neurostimulatory approaches currently under investigation with respect to heart failure are also known as neuromodulation. There are currently innovative devices attempting to therapeutically increase the parasympathetic tone via afferent stimulation in the form of spinal cord stimulation and carotid sinus. Inhibiting sympathetic tone via efferent parasympathetic stimulation via cervical vagal stimulation is also under investigation [62].
Cervical vagal nerve stimulation
Stimulation of the vagus nerve in humans has been shown to be safe and tolerable [63]. A system for Cervical Vagal Nerve Stimulation (VNS) consists of a stimulator unit implanted under the skin of the chest, which is tunnelled under the skin to join an intracardiac sensing electrode that is positioned at the right ventricular apex and an electrode that is placed on the vagus nerve 3 cm below the carotid artery bifurcation [64]. Implantation of this device requires a surgeon with knowledge of head and neck, along with the cardiologist. The recently published RCT, INNOVATE-HF, compared VNS with continued medical therapy. The efficacy endpoints of death from any cause or first event for worsening heart failure were not reached. VNS did show improvements to QOL measures and NYHA class [65].
Spinal cord stimulation
Spinal cord stimulation (SCS) is aimed at reversing the sympathovagal imbalance that develops in heart failure [66]. SCS (Eon Mini Neurostimulation system, St. Jude) is delivered by inserting electrodes into the epidural space and placed to encompass various thoracic levels. The electrodes are connected to a subcutaneously implanted pulse generator. Larger trials in humans to date have had conflicting results. A pilot multicenter study, which provided SCS at T1-T3, showed improvements in NYHA class, symptoms and functional capacity that were observed in a statistically significant manner compared to non-treated patients [67]. However, a single-blinded multicenter RCT with 81 patients targeting T2–T4 showed no statistically significant changes in Left Ventricular ejection volume index, peak VO2 or ntBNP at 6 months [66].
Carotid sinus nerve stimulation
Electrical stimulation of carotid sinus nerve fibres or baroreceptor fibres causes parasympathetic efferent activation and sympathetic withdrawal [68]. To achieve carotid sinus nerve stimulation, a patch electrode is implanted on the carotid sinus, CVRx (Rheos). The electrode wire is tunneled subcutaneously to a battery powered implantable pulse generator that is implanted subcutaneously in the pectoral region. The impulse generator delivers programmable chronic activation energy to the right carotid sinus [62]. Limited clinical data exist, but subsets from hypertension studies indicate the potential for patient with heart failure [69, 70].
There remains no uncertainty that the use of pharmacological beta blockade to treat heart failure resulted in decreased mortality. With respect to neurostimulation, the early evidence has not provided any indicators so far that such substantial benefits could be obtained. The complexity of the nervous system cannot be overstated. If and once an ideal target area for neurostimulation is identified, existing and emerging devices could be tailored for treatment at these areas.
Group 6: Cardiac contractility modulation (CCM)
CCM is an electrical device-based approach that enhances failing myocardium by strengthening the contraction of left ventricular contraction. High-frequency biphasic electrical impulses are delivered to the myocardium during the absolute refractory period. This causes an increase in calcium release into the myocardial cells, thereby strengthening contraction.
CCM signals are provided by a pacemaker like impulse generator (Optimizer III, IMPULSE dynamics) that connects to the heart via two leads that are placed endocardially on the right ventricular septum. A third lead is placed in the right atrium to detect the timing of atrial activation. An algorithm is employed to detect atrial activation to ensure appropriate timing of CCM signal delivery, thereby ensuring delivery of impulse delivery without risk of inducing ventricular arrhythmias [52].
Thus far, CCM has been used in patients with a normal QRS, with an EF <35%, not indicated for CRT and an NYHA II and NYHA III [71]. To date, two RCTs [34, 35] have been performed using CCM as a treatment for heart failure. Its efficacy with respect to improving mortality and hospitalization has not been proven, but it has been shown to improve functional capacity, exercise capacity estimated by peak VO2 and QOL [72], along with NYHA classification. The cost per application of CCM was €17,278 in one Austrian study in 2008 which also suggested that the overall effectiveness and safety of use of CCM in heart failure patients are low [73]. In addressing the clinical need for treating heart failure, CCM identifies and seeks treats one of the problems of myocardial dysfunction, resulting in enhanced strength of cardiac muscle contraction. However, the clinical results and economic assessment have not justified this as a means to treat heart failure. It is the author’s opinion that it would be plausible to theorize that in conjunction with another therapy that would address other significant physiological problems associated with heart failure, CCM could potentially provide worthy clinical results.
Group 7: Extracorporeal membrane oxygenation (ECMO)
This uses a centrifugal pump to drive deoxygenated blood from the patient’s venous system, through an externalized membrane oxygenator system that results in carbon dioxide and oxygen exchange before returning to the patients arterial system. With respect to its in use in heart failure, it has similar outcomes to contemporaneous percutaneous VADs [74]. There are no RCTs demonstrating its efficacy however. ECMO-assisted PCI in the setting of cardiogenic shock as a result of AMI has been shown to improve survival [75, 76]
Group 8: Renal filtration (renal vein offloading)
Ultrafiltration is a means of removing sodium and water to improve haemodynamics in heart failure patients. The Aquadex system was inserted peripherally and provides veno-venous haeomfiltration extracorporally. The UNLOAD trial using the Aquadex system demonstrated superior efficacy over medial diuresis in reducing weight and fluid loss and a decrease in the rate and length of hospital admissions [47]. However, a meta-analysis of trials using various ultrafiltration devices reported no benefits to all-cause mortality or rehospitalizations [48]. Magenta is a percutaneous system that is placed into the renal veins to reduce venous pressure within the renal vein relative to the central venous pressure as a treatment for acute heart failure (Presented at EuroPCR Innovators day).
Group 9: Interatrial shunts
Elevation of left atrial pressures, causing pulmonary congestion, is reported in 90% of patients presenting with acute heart failure. Interatrial shunts are designed to relieve left atrial excess volume [49]. Blood flow is observed being shunted from the left to right atrium. Corvia Medical received CE approval for its InterAtrial Shunt Device (IASD) (Fig. 7) for the treatment of HFpEF. A non-randomized open label trial reported improvements left atrial pressures, functional capacity and QOL after 6 months [50]. V-Wave device (V-Wave Ltd) described a first-in-man case study of a 70-year-old man with HFrEF. The results demonstrated functional improvement, QOL improvement, 3-month post interatrial shunt placement [77].
Group 10: Tissue engineering
The increased volume of the left ventricle along with the stiffening of the myocardium that occurs with ischaemic cardiomyopathy results decreased cardiac output. Alginate-Hydrogels (Algysil-LoneStar, Inc.) are an inert implant that is being assessed as a means of treating this problem. Surgically inserted via thoracotomy, Algisyl implant has resulted in improved exercise capacity and symptoms in addition to standard medical therapy [78].
Group 11: Left ventricular partitioning
Anterior myocardial infarction results in an acute loss of myocardium in the left ventricle. Remodelling of the left ventricle occurs causing an increase in left ventricular volume, which can result in heart failure [79]. The parachute is a device that is inserted percutaneously. It was developed as a means to isolate damaged myocardium, while creating a new left ventricular apex (Fig. 1). The self-expanding nitinol frame permits contraction of the underlying healthy myocardium, an ePTFE occlusive membrane and an atraumatic foot [80]. The PARACHUTE trial showed significant improvement in left ventricular volumes, with sustained improvements in NYHA class and QOL scores. The device is CE marked since 2011. A pivotal trial is currently underway in the US [81]. Table 2 summarizes heart failure devices based on the mode of action.
Conclusions
Contemporaneous clinical, economic and epidemiological evidence highlights the epidemic that is heart failure. Morbidity and mortality remain high with cost constraining the uptake of effective treatment with device therapies such as VADs, certainly in Europe. The need to treat heart failure is a validated need. Within heart failure, there exist a number of more filtered needs with both broad and narrow scopes. The need to reduce mortality in particular with respect to acute cardiogenic shock, the need to improve patients quality of life, the need to develop smaller and more durable VADs are amongst a few. New devices continue to be produced; however, efficacy and mortality improvements have not been effectively proven in clinical trials. Cost effective analysis of current and future therapies should be incorporated in clinical reviews to improve the possibility of implantation for heart failure.
References
Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93:1137–1146
Yturralde RF, Gaasch WH (2005) Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis 47:314–319
Reynolds HR, Hochman JS (2008) Cardiogenic shock: current concepts and improving outcomes. Circulation 117:686–697
Hunt SA, Abraham WT, Chin MH et al (2009) Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed. J Am Coll Cardiol 53:e1–e90
Dickstein K, Cohen-Solal A, Filippatos G et al (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur J Heart Fail 10:933–989
Yancy CW, Lopatin M, Stevenson LW et al (2006) Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A Report From the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 47:76–84
Bhatia RS, Tu JV, Lee DS et al (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355:260–269
Bleumink GS, Knetsch AM, Sturkenboom MCJM et al (2004) Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 25:1614–1619
MacIntyre K, Capewell S, Stewart S et al (2000) Evidence of improving prognosis in heart failure: trends in case fatality in 66,547 patients hospitalized between 1986 and 1995. Circulation 102:1126–1131
McMurray JJV, Adamopoulos S, Anker SD et al (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J 33:1787–1847
Cook C, Cole G, Asaria P et al (2014) The annual global economic burden of heart failure. Int J Cardiol 171:368–376
Lloyd-Jones D, Adams R, Carnethon M et al (2009) Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119:e21–e181
Slaughter MS, Pagani FD, McGee EC et al (2013) HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 32:675–683
World’s First Endovascular Neuromodulation Device Implanted in Man for the Treatment of Congestive Heart Failure|Journal of Invasive Cardiology. http://www.invasivecardiology.com/news/worlds-first-endovascular-neuromodulation-device-implanted-man-treatment-congestive-heart. Accessed 18 Feb 2016
Rose EA, Gelijns AC, Moskowitz AJ et al (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345:1435–1443
Kirklin JK, Naftel DC, Pagani FD et al (2012) Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg 144:584–603
Slaughter MS, Rogers JG, Milano CA et al (2009) Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361:2241–2251
Kirklin JK, Naftel DC, Kormos RL et al (2011) Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 30:115–123
Aaronson KD, Slaughter MS, Miller LW et al (2012) Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125:3191–3200
Stretch R, Sauer CM, Yuh DD et al (2014) National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 64:1407–1415
Kirklin JK, Naftel DC, Pagani FD et al (2014) Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 33:555–564
Rogers JG, Bostic RR, Tong KB et al (2012) Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail 5:10–16
Birks EJ (2010) The comparative use of ventricular assist devices: differences between Europe and the United States. Tex Heart Inst J 37:565–567
Shuhaiber JH, Jenkins D, Berman M et al (2008) The Papworth experience with the Levitronix CentriMag ventricular assist device. J Heart Lung Transplant 27:158–164
Gilotra NA, Stevens GR (2014) Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol 8:75–85
John R, Long JW, Massey HT et al (2011) Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg 141:932–939
Cheng JM, Den Uil CA, Hoeks SE et al (2009) Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 30:2102–2108
Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) AF, Steg PG, James SK et al (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33: 2569–2619
Kirkpatrick JN, Wieselthaler G, Strueber M et al (2015) Ventricular assist devices for treatment of acute heart failure and chronic heart failure. Heart 101:1091–1096
Burkhoff D, Cohen H, Brunckhorst C et al (2006) A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 152:469(e1–e8)
Thiele H, Lauer B, Hambrecht R et al (2001) Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation 104:2917–2922
Kapur NK, Paruchuri V, Jagannathan A, et al. 307 Mechanical circulatory support for right ventricular failure: The TandemHeart in RIght VEntricular Support (THRIVE) Registry. J Hear Lung Transplant 31. doi:10.1016/j.healun.2012.01.315. (Epub ahead of print 2012)
O’Neill WW, Kleiman NS, Moses J et al (2012) A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 126:1717–1727
Thiele H, Zeymer U, Neumann F-J et al (2013) Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet (London, England) 382:1638–1645
Smith EJ, Reitan O, Keeble T et al (2009) A first-in-man study of the reitan catheter pump for circulatory support in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv 73:859–865
McAlister FA, Ezekowitz J, Hooton N et al (2007) Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 297:2502–2514
Brignole M, Auricchio A, Baron-Esquivias G et al (2013) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Eur Heart J 34:2281–2329
Tracy CM, Epstein AE, Darbar D et al (2012) 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm S. Circulation 126:1784–1800
Brignole M, Auricchio A, Baron-Esquivias G et al (2013) 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Europace 15:1070–1118
Bax JJ, Abraham T, Barold SS et al (2005) Cardiac resynchronization therapy: part 2—issues during and after device implantation and unresolved questions. J Am Coll Cardiol 46:2168–2182
Kashani A, Barold SS (2005) Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol 46:2183–2192
Adabag S, Roukoz H, Anand IS et al (2011) Cardiac resynchronization therapy in patients with minimal heart failure: a systematic review and meta-analysis. J Am Coll Cardiol 58:935–941
Feldman AM, de Lissovoy G, Bristow MR et al (2005) Cost effectiveness of cardiac resynchronization therapy in the comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) trial. J Am Coll Cardiol 46:2311–2321
Fox M, Mealing S, Anderson R et al (2007) The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess 11: iii–iv, ix-248
Yao G, Freemantle N, Calvert MJ et al (2007) The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J 28:42–51
Daubert J-C, Saxon L, Adamson PB et al (2012) 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace 14:1236–1286
Costanzo MR, Guglin ME, Saltzberg MT et al (2007) Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 49:675–683
Kwong JSW, Yu C-M (2014) Ultrafiltration for acute decompensated heart failure: a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 172:395–402
Del Trigo M, Bergeron S, Bernier M et al (2016) Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet 387:1290–1297
Hasenfuß G, Hayward C, Burkhoff D et al (2016) A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 387:1298–1304
Bourge RC, Abraham WT, Adamson PB et al (2008) Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 51:1073–1079
Kuck K-H, Bordachar P, Borggrefe M et al (2014) New devices in heart failure: an European Heart Rhythm Association report: developed by the European Heart Rhythm Association; endorsed by the Heart Failure Association. Europace 16:109–128
Magalski A, Adamson P, Gadler F et al (2002) Continuous ambulatory right heart pressure measurements with an implantable hemodynamic monitor: a multicenter, 12-month follow-up study of patients with chronic heart failure. J Card Fail 8:63–70
Yu C-M, Wang L, Chau E et al (2005) Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 112:841–848
Ritzema J, Melton IC, Richards AM et al (2007) Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 116:2952–2959
Troughton RW, Ritzema J, Eigler NL et al (2011) Direct left atrial pressure monitoring in severe heart failure: long-term sensor performance. J Cardiovasc Transl Res 4:3–13
Ritzema J, Troughton R, Melton I et al (2010) Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 121:1086–1095
Maurer MS, Adamson PB, Costanzo MR et al (2015) Rationale and design of the left atrial pressure monitoring to optimize heart failure therapy study (LAPTOP-HF). J Card Fail 21:479–488
Abraham WT, Adamson PB, Bourge RC et al (2011) Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377:658–666
Olshansky B, Sabbah HN, Hauptman PJ et al (2008) Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118:863–871
Domanski M (2003) A comparative analysis of the results from 4 trials of β-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail 9:354–363
Kuck K-H, Cappato R, Siebels J et al (2000) Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 102:748–754
De Ferrari GM, Crijns HJGM, Borggrefe M et al (2011) Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32:847–855
Schwartz PJ, De Ferrari GM, Sanzo A et al (2008) Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 10:884–891
Gold MR, Van Veldhuisen DJ, Hauptman PJ et al (2016) Vagus Nerve Stimulation for the Treatment of Heart Failure: The INOVATE-HF Trial. J Am Coll Cardiol. doi:10.1016/j.jacc.2016.03.525 (Epub ahead of print 29 March 2016)
Zipes DP, Neuzil P, Theres H et al (2016) Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT-HF Study. JACC Heart Fail 4:129–136
Tse H-F, Turner S, Sanders P et al (2015) Thoracic Spinal Cord Stimulation for Heart Failure as a Restorative Treatment (SCS HEART study): first-in-man experience. Heart Rhythm 12:588–595
Lovett EG, Schafer J, Kaufman CL (2009) Chronic baroreflex activation by the Rheos system: an overview of results from European and North American feasibility studies. In: Conf Proceedings Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2009, pp 4626–4630
Georgakopoulos D, Little WC, Abraham WT et al (2011) Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail 17:167–178
Brandt MC, Madershahian N, Velden R et al (2011) Baroreflex activation as a novel therapeutic strategy for diastolic heart failure. Clin Res Cardiol 100:249–251
Abraham WT, Lindenfeld J, Reddy VY et al (2015) A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation in patients with moderately reduced left ventricular ejection fraction and a narrow QRS Duration: study rationale and design. J Card Fail 21:16–23
Giallauria F, Vigorito C, Piepoli MF et al (2014) Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: an individual patient’s data meta-analysis of randomized controlled trials. Int J Cardiol 175:352–357
Radlberger P, Adlbrecht C, Mittermayr T (2011) Cardiac contractility modulation in patients with heart failure refractory to drug treatment. Exp Clin Cardiol 16:43–46
Chamogeorgakis T, Rafael A, Shafii AE et al (2013) Which is better. ASAIO J 59:607–611
Tsao N-W, Shih C-M, Yeh J-S et al (2012) 2012 Extracorporeal membrane oxygenation–assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J Crit Care 27:530(e1–e11)
Sheu J-J, Tsai T-H, Lee F-Y et al (2010) Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med 38:1810–1817
Amat-Santos IJ, Bergeron S, Bernier M et al (2015) Left atrial decompression through unidirectional left-to-right interatrial shunt for the treatment of left heart failure: first-in-man experience with the V-Wave device. Eurointervention 10:1127–1131
Anker SD, Coats AJS, Cristian G et al (2015) A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J 36:2297–2309
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988
Cilingiroglu M, Rollefson WA, Mego D (2013) Percutaneous implantation of a parachute device for treatment of ischemic heart failure. Cardiovasc Revasc Med 14:236–240
Costa MA, Pencina M, Nikolic S et al (2013) The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles. Am Heart J 165:531–536
Acknowledgements
We thank the Health Service Executive (Ireland) that provides all of Ireland’s public health services in hospitals and communities across the country.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest for any authors.
Rights and permissions
About this article
Cite this article
Murphy, C., Zafar, H. & Sharif, F. An updated review of cardiac devices in heart failure. Ir J Med Sci 186, 909–919 (2017). https://doi.org/10.1007/s11845-017-1597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1597-9