Abstract
Introduction
Extrahepatic Portal Hypertension (EPH) is defined as extrahepatic hypertension of the portal venous system in the absence of liver cirrhosis. Isolated splenic vein stenosis/occlusion as one of the causes of extrahepatic portal hypertension is uncommon, comprising less than 5 % of all cases of portal hypertension. However, it is an increasingly recognised complication of both acute and chronic pancreatitis, and with the advent of more effective diagnostic methods, interventional radiological methods for its management are also becoming more effective. Often these would negate the need for invasive splenectomy surgery for the treatment of symptomatic hypersplenism and varices.
Methods
A case of a 38 year old gentleman, known to have Crohn’s disease, presented with severe acute gallstone pancreatitis with necrosis of the pancreatic neck and body. His course was very complicated, requiring two laparotomies and various interventional drainages of variceal bleeds. As a result of non resolving recurrent variceal haemorrhage, it was decided to proceed with splenic vein stenting to relieve the consequences of splenic vein stenosis. A percutaneous transhepatic splenic vein stent was deployed.
Results
Immediate decompression of the varices was noted with no further haemmorrhage.
Conclusion
There are little data to date on splenic vein stenting in the setting of EPH secondary to non-malignant pancreatic disease. We report a case managed successfully with splenic vein stenting and review the existing literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extrahepatic Portal Hypertension (EPH) is defined as extrahepatic hypertension of the portal venous system in the absence of liver cirrhosis [1]. It includes both splenic and superior mesenteric vein occlusions. Isolated splenic vein stenosis or occlusion, as a localised form of extrahepatic portal hypertension, is sometimes referred to as Regional Portal Hypertension (RPH) [2, 16].
The apparent incidence of RPH has increased recently due to improvements in imaging and diagnostic modalities [2]. However, splenic vein stenosis/occlusion as a cause of EPH only accounts for less than 5 % of all cases of portal hypertension [2]. The main clinical difference at presentation, in patients with EPH as opposed to portal hypertension, is that these uncommon cases are usually not cirrhotic and exhibit normal liver function.
Splenectomy has been considered the treatment of choice for the treatment of symptomatic RPH specifically as part of the wider spectrum of EPH [2, 17]. However, the risks of postoperative infection and portal vein thrombosis are not to be ignored.
Interventional radiological techniques of stenting the splenic vein as an effort to recanalise the stenotic or occluded vein and relieve the subsequent hypertension have not been widely studied in the literature. The limited available evidence shows promising results, negating the need for invasive surgery such as splenectomy.
In this case report, we would like to elucidate a complicated case of splenic vein stenosis, post pancreatitis, which was managed with splenic vein stenting. In addition, a literature review of this syndrome is carried out with a close look at splenic vein recanalisation as a treatment modality.
Case
This is a case of a 38 year old man, with a background history of Crohn’s disease who presented to this institution’s Emergency Department with epigastric pain of sudden onset, associated with vomiting. A diagnosis of acute pancreatitis secondary to gallstones was made, with an IMRIE [3] (Glasgow prognostic criteria) severity score of 3.
The initial admission became complicated with necrosis of the neck and proximal body of the pancreas. Two infected peripancreatic collections required radiologically guided percutaneous drainage.
However, despite best non-surgical management, the patient continued to develop peripancreatic collections and proceeded to lose both endocrine and exocrine function of the gland due to necrosis. Three months after initial presentation, laparotomy, partial pancreatic necrosectomy, cholecystectomy and bile duct exploration was performed.
Unfortunately, the patient suffered a complicated post operative course with multiple further bleeds. He required multiple radiological drainages and ultimately a second laparotomy 9 months after the initial laparotomy. However, due to continued complicated post operative course, the case was discussed at a multidisciplinary meeting with a Hepatobiliary surgeon. It was decided that due to the dangers of a third consecutive laparotomy, splenectomy was to be left as a last resort. It was thus elected that splenic vein stenting should be performed in the first instance.
In addition to surgical options, medical treatment was also initiated in the form of spirinolactone therapy and beta blockade to attempt to manage the varices (Fig. 1).
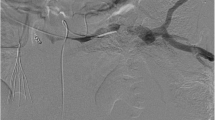
The patient underwent a transhepatic portogram, portal pressure measurement, splenic venography and stenting of the splenic vein (Fig. 2). Ultrasound guidance was employed to gain access transhepatically into a right sided portal vein branch. A guidewire was inserted into the main portal vein and subsequently into the splenic vein. A 6 French sheath was placed. Venography identified the superior, inferior mesenteric veins and splenic veins. There was evidence of a very tight stenosis within the portal vein confluence and splenic vein with a pressure gradient of 20 mmHg. Multiple large varices throughout were noted. A 10 mm × 10 cm Bard Luminexx (Bard PV, Tempe, AZ, USA) stent was placed across the tight stenosis at the splenic vein (Fig. 3). Completion venography showed no filling of the large varices and good patency of the stent. The stent was ballooned to 8 mm. The transhepatic tract was subsequently coil embolised to achieve haemostasis. The patient was subsequently discharged to the Primary team and did not require post operative anti coagulation.
Ultrasound examination one day post procedure showed normal hepatopedal flow within the splenic and portal veins. The loculated fluid collections had significantly reduced in size compared with previous imaging. The last remaining drain (LUQ) was removed 5 days post stenting as there was minimal output.
Two months post stenting a CT was performed demonstrating a widely patent splenic vein stent and little ascites (Figs. 4, 5, 6). In addition, the spleen had reduced in size, measuring 13 cm as opposed to 15 cm previously.
The patient was subsequently lost to follow up as he relocated to another country. However, 2.5 months post stenting the patient was significantly less symptomatic without further variceal bleeding.
Discussion
Pathophysiology
Extrahepatic Portal Hypertension (EPH) due to occlusion of the extrahepatic venous systems surrounding the pancreas may be confined to either the superior mesenteric or splenic venous branch or may involve the whole splenomesentericoportal axis [5].
Conditions associated with EPH include extrinsic venous compression by pancreatic pseudocysts [6–8] or pseudotumours as a complication of chronic pancreatitis [8], pseudoaneurysms of the splenic artery [9], arteriovenous fistulas [8], or retroperitoneal fibrosis [6, 9]. EPH is characterized by either a complete occlusion of branches of the venous splanchnic system (occlusive form) or a subtotal obstruction of one or more of these branches (nonocclusive form) [10, 11].
In this case, isolated splenic vein occlusion/stenosis was the cause of the EPH. This is uncommon as it is now widely believed that in the setting of pancreatitis, that every aspect of the splenomesentericoportal venous axis may be involved [12].
This generally would result in either compromise (nonocclusive variant) or obstruction (occlusive variant) of the lumen and hence of portal venous flow [11].
Isolated splenic vein occlusion in the setting of pancreatitis, has been described in the form of splenic vein thrombosis more frequently than stenosis [13, 14]. In the setting of both acute and chronic pancreatitis, the anatomic location of the splenic vein along the entire posterior aspect of the pancreatic tail, where it lies in direct contact with the peripancreatic inflammatory tissue, makes it subject to perivenous inflammation and hence splenic vein thrombosis [13]. The exact mechanism of the thrombosis is likely multifactorial. Intrinsic endothelial damage and/or extrinsic damage secondary to venous compression from fibrosis, adjacent pseudocysts or oedema have all been documented in the literature [13, 14].
In addition to thrombosis, stenotic obstruction of the splenic vein may also be caused by benignly enlarged retroperitoneal lymph nodes or by pancreatic or perisplenic nodes that are located near the splenic artery, superior to the splenic vein. These nodes lie adjacent to the pancreas and splenic vein and compress the splenic vein when involved in an inflammatory process [5].
The pathophysiology of the varices in isolated splenic vein occlusion/stenosis results primarily from collateral vessels shunting blood around the occluded splenic vein. The two most common collateral pathways use the short gastric vessels. In the distal oesophagus, portosystemic collaterals connect the short gastric veins into the azygos system. Splenoportal collaterals decompress the short gastric veins through both the coronary vein into the portal vein and via the gastroepiploic arcade into the superior mesenteric vein. In either case, the hypertensive short gastric veins cause increased pressure within the submucosal veins of the gastric fundus, resulting in varices. In contrast to generalised portal hypertension, in splenic vein thrombosis flow in the coronary vein is hepatopedal rather than hepatofugal [13].
In this particular case, it was noted at laparotomy that there were engorged gastroepiploic vessels. This is a common finding at laparotomy, and maybe the only finding in some cases of occult splenic vein thrombosis [13].
Other, less common collateral variants may present in cases of splenic vein occlusion. In cases in which the coronary vein joins the splenic vein distal to the obstruction, isolated oesophageal varices may develop. This anatomic variant has been described in 17 % of cases [9]. The left gastroepiploic vein can collateralise to the left colic and inferior mesenteric veins. This has been reported to result in colonic variceal haemorrhage [9]. Other collateral venous channels may develop via the diaphragmatic and intercostal veins to the inferior vena cava [4]. The splenic vein may also collateralise to the renal vein via the adrenal vein [13].
Presentation
In many cases, isolated splenic vein stenosis/occlusion is silent and found incidentally on investigation. Regardless of the symptoms caused by underlying diseases, GI bleeding, splenomegaly, and hypersplenism are the most common clinical presentations of this syndrome. GI bleeding from gastric varices can be life threatening or may require repeated transfusions and have higher rebleeding and mortality rates. Although splenomegaly was found to be present in up to 71 % of cases, hypersplenism (leukopenia and/or thrombocytopenia) was relatively infrequent, with incidence rates ranging from 2 to 52.5 % in different patient populations [14–16].
Often, as in our case, the stenosis is diagnosed incidentally on imaging and/or laparotomy, with the presence of engorged varices.
Splenic vein stenting
There are wide spread reports of portal vein stenting in the setting of portal hypertension induced by pancreatitis. However, there is very limited literature in the setting of splenic vein recanalisation in the setting of extrahepatic portal hypertension induced by pancreatitis. And more specifically, the utilisation of the transhepatic approach to deliver the stent.
In 2013, Xuefeng Luo et al. carried out a retrospective study on 11 patients, who underwent endovascular splenic vein recanalisation due to regional portal hypertension (EPH resulting from splenic vein stenosis or occlusion) over a 2.5 year period. However, these were performed via the transjugular approach as opposed to the percutaneous transhepatic approach [17].
Reasons cited in that study for transjugular approach were twofold. It was felt firstly that the risk of intra-abdominal haemorrhage or intrahepatic haematoma could be reduced as transhepatic access through the liver capsule was to be avoided. They did however note that their patients suffered from thrombocytopaenia which relatively increased the risk of haemorrhage. Secondly, it was felt that as their cases were on oral anticoagulation post operatively, the chances of haemorrhage would be less with the transjugular approach.
Technical success was achieved in 8 of the 11 cases studied by Luo et al. Six of the eight had splenic vein stenosis and two had splenic vein occlusion. They were followed up over a median time of 17.5 months.
Three cases failed and CT showed completely occluded splenic veins with tortuous collaterals near the splenic hilum. It was noted that it would have been difficult to advance the catheter into the true lumen of the splenic vein through the perihilar splenic vein. These three patients required splenectomy.
In addition to Luo et al’s study, Stein et al. partly analysed splenic vein recanalisation as part of their efforts to evaluate the safety and efficacy of portal—splenic vein reconstruction in patients with symptomatic spleno-mesenteric-portal venous thrombosis [19].
Of the 21 cases studied, three suffered from splenic vein thrombosis alone and were treated with transhepatic recanalisation of the splenic vein. Over a mean follow up period of 15.2 months, this study showed success in splenic vein stenting of those three cases.
Our current case certainly did show immediate success with almost immediate reduction in variceal size and reduction of splenomegaly by 2 cm at 2 months post procedure. It is clear that more data is needed and longer follow up required to truly evaluate the effectiveness of splenic vein stenting.
Conclusion
Splenic vein occlusion as a result of pancreatitis is an increasing cause of extra hepatic portal hypertension. It is a syndrome which can be indolent for some time. Hence, at presentation, it may be life threatening. In this case report we illustrate the difficulty in management of such a condition, and outline a method of splenic vein recanlisation. This is much less invasive than the historic, radical treatment of choice of splenectomy. However, there is limited date to illustrate the long term effectiveness of this treatment choice. More trials with longer follow up periods are needed.
References
Longstreth GF, Newcomer AD, Green PA (1971) Extrahepatic portal hypertension caused by chronic pancreatitis. Ann Intern Med 75:903–908
Koklu S, Coban S, Yuksel O, Arhan M (2007) Left-sided portal hypertension. Dig Dis Sci 52(5):1141–1149
Ranson JHC (1982) Etiologic and prognostic factors in human acute pancreatitis: a review. Am J Gastroenterol 77:633–638
Büchler M, Friess H, Mueller MW et al (1995) Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 169:65–70
Stringer MD, Heaton ND, Karani J et al (1994) Patterns of portal vein occlusion and their aetiological significance. Br J Surg 81:1328–1331
Little AG, Moossa AR (1981) Gastrointestinal hemorrhage from left-sided portal hypertension. An unappreciated complication of pancreatitis. Am J Surg 141:153–158
Bernades P, Baetz A, Lévy P et al (1992) Splenic and portal venous obstruction in chronic pancreatitis. A prospective longitudinal study of a medical-surgical series of 266 patients. Dig Dis Sci 37:340–360
Harnar T, Johansen K, Haskey R et al (1982) Left-sided portal hypertension from pancreatic pseudotumor. Am J Gastroenterol 77:639–641
Ghosn PB, Fontaine A, Roy P et al (1981) Arteriography and pancreatic disease. Can J Surg 24:612–614
Bloechle C, Busch C, Tesch C et al (1997) Prospective randomized study of drainage and resection on non-occlusive segmental portal hypertension in chronic pancreatitis. Br J Surg 84:477–482
Warshaw AL, Jin GL, Ottinger LW (1987) Recognition and clinical implications of mesenteric and portal vein obstruction in chronic pancreatitis. Arch Surg 122:410–415
Ribet M, Quandalle P, L’Hermine C et al (1971) Superior mesenteric venous hypertension in chronic pancreatitis. Chirurgie 97:273–282
Sharon M, Weber MD, Layton F, Rikkers MD (2003) Splenic vein thrombosis and gastrointestinal bleeding in chronic pancreatitis. World J Surgery 27:1271–1274
Ryan Heider, Samreen Azeem, Joseph A. Galanko, Kevin E. Behrns (2004) The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004; 239(6):876–882
Liu QD, Zhou NX, Zhang WZ, Wang MQ (2005) Diagnosis and management of regional portal hypertension. Chin J Dig Dis 6(2):87–92
Madsen MS, Petersen TH, Sommer H (1986) Segmental portal hypertension. Ann Surg 204(1):72–77
Sakorafas GH, Sarr MG, Farley DR, Farnell MB (2000) The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg 179(2):129–133
Luo X, Nie L, Wang Z, Tsauo J, Tang C, Li X (2014) Transjugular endovascular recanlization of splenic vein in patients with regional portal hypertension complicated by gastrointestinal bleeding. Cardiovasc Intervent Radiol 37:108–113
Stein M, Link DP (1999) Symptomatic spleno-mesenteric-portal venous thrombosis: recanalization and reconstruction with endovascular stents. J Vasc Interv Radiol. 10(3):363–371
Acknowledgments
There was no funding from any source in the selection preparation of this case report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
El Kininy, W., Kearney, L., Hosam, N. et al. Recurrent variceal haemorrhage managed with splenic vein stenting. Ir J Med Sci 186, 323–327 (2017). https://doi.org/10.1007/s11845-016-1420-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-016-1420-z