Abstract
Background/aims
Adjuvant endocrine therapy for at least 5 years improves oncological outcomes in oestrogen receptor-positive breast cancer. Adherence rates to prescribed endocrine therapy are low and the search for modifiable causes of this continues. The aim of this study was to assess adherence rates in an Irish cohort of breast cancer patients prescribed adjuvant endocrine therapy and to assess modifiable factors associated with suboptimal adherence.
Methods
A cross-sectional anonymous survey was performed on 261 patients currently prescribed endocrine therapy. Data were collected regarding demographics, treatment, social and emotional factors and medication side effects. Each patient completed a medication adherence score and provided information about discontinuation of therapy and reasons for same.
Results
Only 67.8 % of patients assessed demonstrated complete medication adherence on the medication adherence scale. Twenty-nine patients (10.9 %) permanently stopped taking their prescribed endocrine therapy. Suboptimal adherence was more likely in younger patients (p < 0.001), those in employment (p = 0.005), those who experienced side effects (p = 0.006), those who perceived themselves to have low levels of emotional support (p < 0.001) and those who use the internet to read about their illness (p = 0.003).
Conclusions
Endocrine therapy adherence is suboptimal in almost one-third of patients in our cohort. Appropriate assessment and management of side effects and negative emotions, combined with direction of patients to accurate internet sources of information, could help improve endocrine therapy adherence in women with early-stage breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic treatment of oestrogen receptor (ER) and progesterone receptor (PR) expressing breast cancers includes the use of oral anti-oestrogen therapies (endocrine therapy). There are two main classes of anti-oestrogen therapy—selective oestrogen receptor modulators (SERMs), of which tamoxifen is in mainstream clinical use, and aromatase inhibitors (AIs), of which anastrazole, exemestane and letrozole are widely commercially available.
Although tamoxifen was first trialled in breast cancer in the 1970s, it was only in 1998 a survival benefit was shown for its use in ER-positive breast cancers [1]. Five years of adjuvant tamoxifen therapy was shown to reduce breast cancer recurrence rates by 47 % and mortality by 26 % over a 10-year period [1]. More recent studies have suggested a benefit from extended duration of tamoxifen therapy [2, 3]. Tamoxifen has also been shown to be beneficial in reducing breast cancer risk in ER-positive patients with ductal carcinoma in situ (DCIS) [4].
Aromatase inhibitors were also first developed in the 1970s, but toxicity limited usefulness of early drugs of this class [5]. Newer AIs were initially introduced as second-line therapy for patients with advanced breast cancer, who had disease progression whilst taking tamoxifen [6, 7]. However, anastrazole was subsequently shown to be an effective adjuvant therapy for early breast cancer in postmenopausal women [8]. Five years of anastrazole treatment significantly prolongs disease-free survival, time-to-recurrence, time-to-distant-metastases and contralateral breast cancer incidence compared to tamoxifen therapy in postmenopausal women [8]. Studies are ongoing to assess the benefit of extended duration AI therapy for early breast cancer also [9].
Incomplete adherence to prescribed endocrine therapy can, therefore, have significant effects on patient outcomes. A recent study of patients who were non-adherent (defined as omitting endocrine therapy for ≥180 days) showed an adjusted cancer recurrence odds ratio of 2.88 compared to adherent patients [10]. Previous studies have indicated some factors that have been shown to be associated with poor adherence or non-adherence to hormonal therapy including lower financial status/medication cost [11, 12], age extremes [11], patients who perceive their need to be lower [11–14], frequency and/or efficacy of physician communication [11, 13–15], medication concerns/side effects [11, 13, 14], lack of social support [11, 16] and negative emotions [12, 17]. Patients with better understanding of their hormone receptor positivity were more likely to be adherent in one study [18]. It is clear that adherence rates are widely variable across different study populations, and reasons for non-adherence are broad in their nature.
The aim of our study was to assess endocrine therapy adherence rates in a population of Irish women with ER/PR-positive breast cancer and to look for modifiable factors associated with non-adherence.
Methods
A cross-sectional study was performed on 261 patients attending follow-up at breast and oncology clinics in a regional tertiary referral centre for breast cancer in a university teaching hospital over a 6-month period in 2013/2014. All patients were attending within 5 years of their diagnosis of early-stage breast cancer, had ER/PR-positive tumours, and the majority were prescribed therapeutic adjuvant endocrine therapy, although a smaller number were on risk-reducing hormonal treatment, with some participating in the IBIS-II DCIS trial [19]. Choice of hormonal therapy is generally left to the discretion of the prescribing physician, which could be either a surgeon or a medical oncologist. Low or intermediate risk tumours tend to be prescribed tamoxifen and some change to an AI after 2 or 3 years. Patients with higher risk tumours are often offered an AI initially for at least 5 years.
Patients meeting the inclusion criteria were invited to participate by clinic staff at their routine follow-up appointment. Within our unit, each patient attends the surgical clinic annually for clinical review for 5 years post-operatively and they also undergo annual mammography. Patients who undergo chemotherapy and radiotherapy also attend medical and radiation oncology follow-up clinics at a frequency which varies depending on their treatment regime. Each consenting patient completed a written anonymous questionnaire, providing self-reported information regarding diagnosis, treatment, comorbidities, demographics, household finances, follow-up and social/emotional support. Each patient also completed a validated medication adherence score (Table 1) [20] and provided information regarding discontinuation of therapy (temporary discontinuation being defined as <6 months, permanent being defined as discontinuation ≥6 months duration), reasons for discontinuation and side effects experienced. The medication adherence score assesses patients’ day-to-day use of their medication, along with their attitudes towards their medication use. Patients who agreed to participate were shown to a separate area of the clinic in order to complete their questionnaires privately and were assured of their anonymity.
Ethics approval for the study was obtained from the Ethics Committee of the Cork Teaching Hospitals and each patient provided written informed consent.
Data were entered into Excel (Microsoft, US) and analysed using SPSS Version 20 (IBM). Continuous variables were analysed via t test, whereas categorical variables were compared via Chi-squared and Fisher’s Exact tests. Variables identified as associated with suboptimal adherence with a p value ≤0.200 were analysed in a multivariate regression model using binary logistic regression.
Results
261 patients completed questionnaires and their demographics are shown in Table 2. Overall, 29 patients (10.9 %) permanently stopped taking a prescribed endocrine therapy. Reasons for this are shown in Table 3. Twenty-two patients (8.3 %) temporarily discontinued endocrine therapy; reasons and duration of discontinuation are shown in Table 3. Unbearable side effects feature highly in both groups.
Only 67.2 % (n = 178) of patients correctly understood why they were prescribed endocrine therapy (Fig. 1a). However, understanding did not correlate with adherence (p = 0.194) (Table 4). Overall, 67.8 % of patients assessed demonstrated complete medication adherence on the medication adherence scale (Fig. 1b).
Patients were then divided into those fully adherent and those with suboptimal adherence in an attempt to determine factors associated with complete adherence. Suboptimal adherence was more likely in younger patients (p < 0.001), those in employment (p = 0.005), those with higher household incomes (p = 0.023), those who experienced side effects (p = 0.006), those who perceived themselves to have low levels of emotional support (p < 0.001) and those who use the internet to read about their illness (p = 0.003) (Table 4).
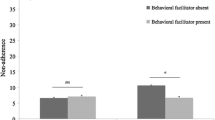
The only specific side effect associated with suboptimal adherence was mood disorder, in patients taking tamoxifen (p = 0.033), (Fig. 2). When these factors were analysed by multivariate analysis, it was found that employment, side effects and emotional support level remained significantly associated with suboptimal adherence (Table 5).
Comparison of side effects experienced by those with complete adherence versus suboptimal adherence. Values are expressed as percentages of each cohort. There were no differences seen between the two groups except for mood disorder, which was more common in the suboptimal adherence group taking tamoxifen (*p = 0.033, Fisher’s Exact Test). All other comparisons were non-significant
Patients were then analysed according to endocrine therapy sub-group (tamoxifen versus aromatase inhibitors) to assess for any differences (Table 6). As expected, patients taking tamoxifen were younger (p < 0.001) and had been diagnosed for a shorter time (p = 0.002). They also had higher incomes (p = 0.026), spent less money on their medication (p < 0.001) and were less likely to have state assistance in funding for their medication (a medical card) (p < 0.001). Patients prescribed an aromatase inhibitor were more likely to have undergone axillary lymph node dissection (p = 0.007), and received chemotherapy (p = 0.006) and trastuzumab (p = 0.048). AI prescribing trended towards being more commonly performed by medical oncologists (p = 0.060). AI users were more likely to have switched endocrine therapy (p < 0.001) and this was equally split between those who did so due to a planned tamoxifen to AI switch and those who experienced unbearable side effects. Overall, there was no difference in side effect rate reported by the two groups (p = 0.183, Fig. 3). On analysis of specific side effects, sweats/flushes and per vaginal (PV) bleeding were more common in the tamoxifen group (p = 0.001, p = 0.009, respectively); there were no specific side effects significantly more common in the AI group.
Comparison of side effects experienced between those taking tamoxifen versus aromatase inhibitors; figures are expressed as percentages of each cohort. Overall, the side effect rate was similar between the two groups (p = 0.183). However, sweats/flushes and vaginal bleeding were more common in the tamoxifen group (p = 0.001, 0.009, respectively). All other comparisons were non-significant
Discussion
This is the first study, to our knowledge, to assess adherence for both tamoxifen and AI therapy in an Irish population and to assess factors associated with suboptimal adherence. This study has shown that suboptimal adherence with prescribed endocrine therapy regimens is common, with 32.2 % of patients not completely adhering to their prescribed medication. Ten percent of our cohort permanently discontinued one medication at some point in their therapy regimen. These figures compare with internationally reported data [13, 21–25] and confirm that endocrine therapy adherence is a significant issue. Various levels of adherence to prescribed endocrine therapy have been reported, from as low as 48 % adherence [21] up to 93 % adherence [13] and various levels in between [22–25]. A previous study of an Irish cohort of patients prescribed tamoxifen showed a 22 % non-persistence rate within 1 year of commencing treatment [26]. Adherence rates have been shown to decline over time from first prescription [15, 27, 28]. Full 5-year completion of therapy is as low as 66 % in some studies [29]. A 42 % treatment interruption rate was seen within the first 2 years of therapy in a study of patients less than 40 years [16] and younger women are more likely to be non-adherent [30]. Male breast cancer patients have similarly high discontinuation rates of endocrine therapy [31] and in fact 5-year persistence rates of just 17 % have been reported [32]. Rates of discontinuation are even higher in trials where tamoxifen is used for chemoprevention for high-risk patients, at 30–50 % [11]. In fact, endocrine therapy adherence has been shown to be much poorer than adherence with other recommended adjuvant therapies for breast cancer, namely chemotherapy and radiotherapy [25], where adherence with therapies is easily monitored and documented.
As outlined previously, endocrine therapy is associated with significant reductions in breast cancer recurrence and mortality [1, 8]. Although one study of 857 low-income women with poor adherence did not demonstrate an association between adherence and breast cancer outcomes [33]; this has not been supported by other studies [23, 32]. A study of 116 male breast cancer patients showed significant differences in outcome for both overall survival and disease-free survival in adherent patients compared to those poorly adherent [32]. A further prospective study of 417 patients in Sweden also demonstrated an association between non-adherence to endocrine therapy at 1 year and increased risk of early breast cancer events [23]. Suboptimal adherence, therefore, may have significant clinical consequences and modifiable causes of suboptimal adherence need to be sought.
Our finding that approximately one-third of patients are poorly complaint with their hormonal therapy is particularly concerning in the current era of trends towards minimally effective treatments, rather than the previous trend to giving patients maximally tolerated treatments. Genomic analyses of tumours are frequently employed currently, resulting in decreased prescribing of chemotherapy [34]. Therefore, hormonal therapy is potentially far more important to our current breast cancer patients, than it may have been to previous generations of patients. A study is currently evaluating omitting chemotherapy for some node-positive patients [35]—a treatment that would generally have been mandatory for this sub-group of breast cancer patients. Many centres have also adopted or partially adopted the ACOSOG Z-11 concept which accepts that macroscopic disease is left behind in the axilla in some patients [36]. It is possible that full adherence with systemic hormonal therapy could be critical in the future in such patients. It is possible, and indeed probable, that poor adherence with hormonal therapies has been a problem for many years, but the consequences of such poor adherence may be far more significant today than previously.
Negative emotions, mood disorders and perceived poor emotional support are common themes when examining endocrine therapy adherence. Our study has confirmed an association between poor emotional support and suboptimal adherence, which held true on multivariate analysis. Quality of life (QoL) studies have demonstrated that emotional well being decreases in conjunction with other QoL scores within 3–6 months of commencing tamoxifen [37]. However, whilst other QoL parameters improve again after this stage, emotional well being does not [37]. Other studies have confirmed the association between poor emotional well being and poorer adherence to medication [12]. In fact, negative mood prior to starting therapy has also been associated with subsequent non-adherence [17]. Stanton et al. have shown onset of therapy-related negative emotions within months of diagnosis, suggesting that assessment of emotions should be performed close to the time of initiation of therapy and may predict poor adherence [12]. Depressive symptoms in cancer patients may be responsive to psychological intervention [12] and their prompt recognition and appropriate management may lead to better medication adherence.
Employment status was shown to be a significant factor in our study. Our complete adherence group had a higher proportion of retired patients and patients who are full time at home compared to the suboptimal group which had higher proportions of part and full time employed patients. Reasons for this are unclear. It could be postulated that employed patients are likely to be younger than retirees or that patients experiencing side effects may discontinue medication to allow them to work. However, the effect remained significant on multivariate analysis, taking age and side effects into consideration. Other studies report mixed effects of employment—male employed patients are less likely to be adherent to medication for inflammatory bowel disease [38], whereas unemployed patients are less likely to adhere to diet and medication following renal transplant [39]. Further exploration of the effect of employment on medication adherence is required.
Side effects were commonly experienced in both groups of patients in our study but were more commonly reported in the suboptimal adherence group. Again, this is in line with the previously published data, where up to 94 % of patients prescribed endocrine therapy report some side effects [22]. In their study, Aiello-Bowles et al. found headaches to be negatively associated with adherence [22], whereas in our study we found a negative correlation with mood disorders in tamoxifen users. Management of side effects is therefore very important and it is worth noting that side effects were a common reason for therapy switch in our cohort. It has been shown previously that more than one-third of patients who fail an initial AI due to side effects may tolerate an alternative AI [40], and therefore therapy switch is an important consideration in patients reporting unbearable side effects.
Whilst most women value efficacy over side effects of their endocrine therapy, it has been shown that a proportion of women regard efficacy with less importance than they attach to side effects [41]. Patients who perceive a higher benefit/side effect ratio have better adherence to their medication [41]. Therefore, there is a cohort of patients in whom side effects will play a role in determining adherence. Knowledge of these patients beliefs may help tailor communication to suit their individual needs. Cuzick et al. have shown that onset of AI-induced symptoms is associated with better oncological outcomes [42], and therefore education of patients about the significance of their side effects may also be beneficial.
Interestingly, our study shows the novel finding that patients who use the internet to research their illness are more likely to have suboptimal adherence. This finding is particularly significant in an era where patients have increased access to and increasingly use the internet as a health information source. It has previously been shown that patients not only access the internet for medical information but that internet sourced information also helps influence their decision making [43, 44]. A large number of internet searches seeking breast cancer information are performed daily [45]. An analysis of webpages returned in common breast cancer-related searches has shown variable accuracy and applicability to patients’ queries [46]. Most webpages providing health-related information are unregulated [46]. Accurate information can be found via the right sources (education-based or government authorised websites compared to individual blogs or interest group webpages) [46], but these sources may be difficult to find for uninitiated patients. Patients should be directed to source high-quality information (rather than being exposed to potentially extreme views) if using the internet to aid their decision making, and to ensure that they are making fully informed choices.
It has been suggested that knowledge about their own hormone receptor status improves patient adherence to endocrine therapy [18]. However, our study has shown no link between understanding the reason for endocrine therapy and complete adherence. In keeping with this finding, various studies have attempted educational interventions with little success. Randomisation of patients to receive educational material in conjunction with their AI prescription had no effect on adherence rates in two studies [47, 48]. Ziller et al. did show that reminder letter and information booklets or reminder phone calls did improve adherence rates significantly in their cohort of 181 females [21]. However, the control arm of their study had only 48 % adherence at 12 months, one of the lowest reported adherence rates in the literature. A further study is being undertaken to assess cognitive behavioural side effect prevention training versus standard care or manualised supportive therapy but results from this trial are awaited [49]. Assessment of the usefulness of a text message reminder to patients to continue their endocrine therapy is currently being studied in a randomised controlled trial [50].
Studies have suggested more follow-up and follow-up with specialists rather than general practitioners (GPs) has a positive impact on adherence [13, 15, 24]. Whilst acknowledging that our patient cohort has high follow-up attendance rates in general, the percentage of reduced frequency attenders is no higher in the suboptimal adherence group than the complete adherence group. Therefore, follow-up frequency does not appear to impact adherence in our cohort but may be a factor in settings where cancer patients are discharged from specialist follow-up before completion of their endocrine therapy.
An acknowledged limitation of our study is its cross-sectional design which does not allow for the assessment of changes in adherence over time. However, it provides what we believe to be a true representation of adherence and discontinuation rates in a large cohort of patients, as well as providing information regarding some potential factors which affect adherence. The study population is potentially limited by selection bias as patients with the poorest adherence to therapy may not attend their hospital follow-up, and therefore they may not have been captured by this hospital-based study. Patient self-reported adherence rates have been shown to over state adherence when compared to actual prescription fill rates, in some studies [51, 52]. Verification of clinical information provided by the patients is precluded by the anonymous nature of the questionnaire. However, we believe that it is this anonymity which makes it likely that the responses provided are a true reflection of adherence rates, discontinuation rates, and social and emotional factors.
In conclusion, we have shown that endocrine therapy adherence is suboptimal in almost one-third of patients in our cohort. Factors associated with this include poor emotional support, employment status, medication side effects and patient internet use. Appropriate assessment and management of negative emotions and side effects, combined with direction of patients to accurate internet sources of information, could help improve endocrine therapy adherence in women with early-stage breast cancer.
References
Early Breast Cancer Trialists’ Collabroative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351(9114):1451
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805
Gray R, Rea D, Handley K et al (2013) aTTom: Long term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6593 women with early breast cancer. J Clin Oncol 31(suppl):abstr5
Allred DC, Anderson SJ, Paik S et al (2012) Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 30(12):1268–1273
Santen RJ, Santner S, Davis B et al (1978) Aminoglutethimide inhibits extraglandular estrogen production in postmenopausal women with breast carcinoma. J Clin Endocrinol Metab 47:1257–1265
Jonat W, Howell A, Blomqvist C et al (1996) A randomised trial comparing two doses of the new selective aromatase inhibitor (“Arimidex”) with megestrol acetate in postmenopausal women with advanced breast cancer. Eur J Cancer 32A:404–412
Buzdar AU, Jones SE, Vogel CL et al; The Arimidex Study Group (1997) A Phase III trial comparing anastrozole (1 and 10mg), a potent and selective aromatase inhibitor, with megestrol acetate in postmenopausal women with advanced breast cancer. Cancer (Phila.) 79:730–739
Howell A, Cuzick J, Baum M et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60
Mamounas E, Lembersky B, Jeong JH et al (2006) NSABP B-42: a clinical trial to determine the efficacy of five years of letrozole compared with placebo in patients completing five years of hormonal therapy consisting of an aromatase inhibitor (AI) or tamoxifen followed by an AI in prolonging disease-free survival in postmenopausal women with hormone receptor-positive breast cancer. Clin Breast Cancer 7(5):416
Barron T, Cahir C, Sharp L, Bennett K (2013) A nested case-control study of adjuvant hormonal therapy persistence and compliance and early breast cancer recurrence in women with stage I–III breast cancer. Br J Cancer 109:1513
Chlebowski RT, Geller ML (2006) Adherence to endocrine therapy for breast cancer. Oncology 71(1–2):1
Stanton AL, Petrie KJ, Partridge AH (2014) Contributors to non-adherence and non-persistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat 145(2):525
Simon R, Latreille J, Matte C, Desjardins P, Bergeron E (2013) Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg 57(1):26
Jacob Arriola KR, Mason TA, Bannon KA et al (2014) Modifiable risk factors for adherence to aduvant endcorine therapy among breast cancer patients. Patient Educ Couns 95(1):98
Danilak M, Chambers CR (2013) Adherence to adjuvant endocrine therapy in women with breast cancer. J Oncol Pharm Pract 19(2):105
Cluze C, Rey D, Huiart L et al (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer, determinants of interruptions vary over time. Ann Oncol 23(4):82
Bender CM, Gentry AL, Brufsky AM et al (2014) Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum 41(3):274
Albert US, Zemlin C, Hadji P et al (2011) The impact of breast care nurses on patients’ satisfaction, understanding of the disease and adherence to adjuvant endocrine therapy. Breast Care (Basel) 6(3):221
Cuzick J, Sestak I, Forbes JF et al (2014) Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383(9922):1041–1048
Morisky DE, Green LW, Levine DM (1986) Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 24(1):67
Ziller V, Kyvernitakis I, Knoll D, Storch A, Hards O, Hadji P et al (2013) Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment—the COMPAS study. BMC Cancer 13:407
Aiello Bowles EJ, Boudreau DM, Chubak J et al (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early stage breast cancer. J Oncol Pract 8(6):e149
Markkula A, Hietala M, Henningson M et al (2012) Clinical profiles predict early non-adherence to adjuvant endocrine treatment in a prospective breast cancer cohort. Cancer Prev Res (Phula) 5(5):735
Guth U, Myrick ME, Kilic N, Eppenberger-Castori S, Schmid SM et al (2012) Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res Treat 131(2):491
Dittmer C, Roeder K, Hoellen F et al (2011) Compliance to adjuvant therapy in breast cancer patients. Eur J Gynaecol Oncol 32(3):280
Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ (2007) Early discontinuation of tamoxifen. A lesson for oncologists. Cancer 109(5):832
Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C (2013) Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108(7):1515
Font R, Espinas JA, Gil-Gil M et al (2012) Prescription refill, patient self report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. Br J Cancer 107(8):1249
Guth U, Huang DJ, Schotzau A et al (2008) Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer 99(3):428
Hershman DL, Kushi LH, Shao T et al (2010) Early discontinuation and non-adherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120
Visram H, Kanji F, Dent SF (2010) Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol 17(5):17
Xu S, Yang Y, Tao W et al (2012) Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res treat 136:495
Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G et al (2013) Adherence to adjuvant hormonal therapy and it relationship to breast cancer recurrence and survival among low income women. Am J Clin Oncol 36(2):181
Carlson JJ, Roth JA (2013) The impact of the oncotype DX breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
SWOG S1007 Trial. www.clinicaltrialsregister.eu/ctr-search/search?query=2012-000576-42. Accessed 4 July 2014
Guiliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569
Sert F, Ozsaran Z, Eser E et al (2013) Quality of life assessment in women with breast cancer: a prospective study including hormonal therapy. J Breast Cancer 16(2):220
Ediger JP, Walker JR, Graff L et al (2007) Predictors of Medication adherence in inflammatory bowel disease. Am J Gastroenterol 102:1417
Kiley DJ, Lam CS, Pollak R (1993) A study of treatment compliance following kidney transplantation. Transplantation 55(1):51–56
Henry NL, Azzouz F, Desta Z et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early breast cancer. J Clin Oncol 30(9):936
Wouters H, Maatman GA, Van Dijk L et al (2013) Trade off preferences regarding adjuvant endocrine therapy among women with estrogen receptor positive breast cancer. Ann Oncol 24(9):2324
Cuzick J, Sestak I, Cella D et al (2008) Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol 9(12):1143
Van de Poll-Franse LV, van Eenbergen MC (2008) Internet use by cancer survivors: current use and future wishes. Support Care Cancer 16(10):1189e95
Castleton K, Fong T, Wang-Gillam A et al (2011) A survey of internet utilization among patients with cancer. Support Care Cancer 19(8):1183–1190
McHugh SM, Corrigan M, Morney N et al (2011) A quantitative assessment of changing trends in internet usage for cancer information. World J Surg 35(2):253
Quinn EM, Corrigan M, McHugh SM et al (2012) Breast cancer information on the internet: analysis of accessibility and accuracy. Breast 21(4):514
Neven P, Markopoulos C, Tanner M et al (2014) The impact of educational materials on compliance and persistence rates with adjuvant aromatase inhibitor treatment: first-year results from the compliance of aromatase inhibitors assessment in daily practice through educational approach (CARIATIDE) study. Breast [Epub ahead of print]
Hadji P, Blettenr M, Harbeck N et al (2013) The patient’s anastrazole compliance to therapy (PACT) program: a randomized, in-practice study on the impact of a standardized information programme on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol 24(6):1505
von Blanckenburg P, Schuricht F, Albert US, Rief W, Nestoriuc Y et al (2013) Optimizing expectations to prevent side effects and enhance quality of life in breast cancer patients undergoing endocrine therapy: study protocol of a randomized controlled trial. BMC Cancer 18(13):426
Southwest Oncology Group. S1105: text-messaging intervention to reduce early discontinuation of AI therapy in women with early-stage breast cancer. NCT01515800. http://clinicaltrials.gov/show/NCT01515800. Accessed 13 Nov 2014
Wang P, Benner J, Glynn R et al (2004) How well do patients report noncompliance with antihypertensive medications?: a comparison of self-report versus filled prescriptions. Pharmacoepidemiol Drug Saf 13(1):11–19
Garber M, Nau D, Erickson S, Aikens J, Lawrence J (2004) The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 42(7):649–652
Acknowledgments
The authors received no funding for this study.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Ethics approval for the study was obtained from the Ethics Committee of the Cork Teaching Hospitals and each patient provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quinn, E.M., Fleming, C. & O’Sullivan, M.J. Endocrine therapy adherence: a cross-sectional study of factors affecting adherence and discontinuation of therapy. Ir J Med Sci 185, 383–392 (2016). https://doi.org/10.1007/s11845-015-1307-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-015-1307-4