Abstract
This study has developed an efficient low-temperature resistant quartz collector, named KDB. The effect of KDB and the conventional collector sodium oleate (NaOL) on the reverse flotation behavior of hematite was investigated by flotation tests on pure minerals and binary mixed ores. On this basis, adsorption thermodynamics, surface tension determination, surface contact angle determination, and Fourier-transform infrared spectroscopy (FTIR) analysis were used to investigate the interaction mechanism of the two collectors at different temperatures. The results showed that both can enhance hematite reverse flotation at room temperature, and that the collection performance of KDB is stronger than that of NaOL. At low temperatures, the collection performance of KDB still obtained better flotation indices compared with NaOL. The binary mixed ore flotation test showed that, at 15°C, the grade and recovery with KDB as the collector was improved by 10.77% and 14.32% compared with those of NaOL, respectively. The contact angle of the quartz surface under the KDB system at 15°C is 55.56° higher than that of NaOL. The FTIR analysis showed that KDB can significantly enhance the hydrophobicity of quartz, and that the adsorption strength on the quartz surface at low temperatures is much higher than that of NaOL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron and steel are essential materials in both national security and economic development. However, as iron ore resources are continually exploited, the quality of the raw ore decreases, and the particles of valuable minerals become finer, complicating their separation.1 As a result, the problem of sorting is becoming more and more serious, and the rational development and utilization of iron ore resources has become a major issue. Quartz is a common veinstone mineral in iron ore,2,3 so the “enhancement of iron and reduction of silicon” is crucial for the efficient utilization of iron ore. Practice has proved that reverse flotation technology is one of the effective means of obtaining high-quality iron ore concentrates.4,5 On the one hand, reverse flotation is more economical in terms of reagents and has better flotation results than direct flotation. On the other hand, reverse flotation, especially anionic reverse flotation, often has a synergistic effect of multiple reagents to obtain better flotation indices. Based on a series of advantages, such as high sludge tolerance, cheap price, high tolerance, and fast defoaming of anionic collectors,6 anionic reverse flotation is considered to be one of the most widely and economically applied technologies. Currently, the reverse flotation process in iron ore beneficiation plants widely uses fatty acid-based anionic agents.7 Currently, the collector NaOL is commonly used in flotation plants, but it has low solubility and poor dispersibility at low temperatures, which causes a sharp decline in flotation indicators. Therefore, most flotation plants in China (such as the Anshan Iron and Steel Group Qidashan concentrator) requires the slurry to be heated to over 35–40°C in order to achieve a satisfactory flotation efficiency,8 which greatly increases the energy cost and consumption of the flotation operations.
To address the challenge of low-temperature flotation, researchers have modified the polar group of fatty acid reagents by adding various functional groups, including halogenated, sulfonated, and hydroxylated groups. These modifications have been demonstrated to improve the reagents' dispersion and selectivity and to reduce their temperature sensitivity. Meanwhile, the synergistic effect between the two polar groups is also more conducive to the collector molecules in the form of chelating on the surface of the adsorbed minerals, which effectively improves the separation effect of the reagents.9 For example, the Changsha Institute of Mining and Metallurgy has developed the RA series of reagents (RA-315, RA-515, and RA-715) mainly based on chlorinated tar oil.10 They have improved the selectivity of flotation by introducing Cl-atom reactive groups on the α-carbon position of the fatty acids. RA-315 is used as the beneficiation reagent in the combined magnetic separation–flotation process of Qidashan iron ore, which has the advantages of strong adaptability to the ore sludge and stable foam. In addition, by introducing chlorine and hydroxyl active groups, RA-915 can improve the activity of the reagent while also improving the flotation effect of the circulating complexing agents. However, chlorine is a highly toxic gas with great potential danger. Some researchers11,12 have other achievements in hematite reverse flotation collectors. The α-bromo lauric acid prepared in the laboratory of mineral processing chemicals at the Northeastern University has enhanced the activity and has better low-temperature resistance. Luo et al.12 synthesized a new type of fatty acid-based collector, a-BDA, for the flotation of quartz minerals. The recovery of quartz after Ca2+ activation was as high as 99.50% under the conditions of 16°C and a pH of 11.50. However, this method has many problems, such as long reaction time, high bromine consumption, and the need to recycle and dispose of excess Br2 and HBr, which makes it difficult to be widely used.
The preceding paragraph highlighted numerous literature reports discussing the utilization of low-temperature collectors for iron ore, but most of the studies are devoted to practical flotation applications, physicochemical properties,13 and the interactions with the minerals.13 Accordingly, there is a lack of exploration of their adsorption mechanisms at low temperatures.14 In this study, a new low-temperature resistant collector, KDB, was developed to solve the problem of heating the flotation slurry. We conducted flotation tests using KDB in comparison with NaOL, a collector commonly used by mining plants, in terms of pH, temperatures, and dosages of reagents. Remarkably favorable results were obtained across all the parameters examined in this comprehensive study. The low-temperature resistant collector, KDB, was thoroughly investigated through Fourier-transform infrared spectroscopy (FTIR) characterization, adsorption measurement, contact angle measurement, and surface tension measurement. Consequently, these findings hold immense significance for enhancing our understanding of the silica removal process from hematite by utilizing low-temperature resistant collectors and advancing their development and application.
Materials and Methods
Sample
The quartz pure mineral used in the experiment was obtained from the Zhuanghe Quartz Sand Factory in Dalian. The lump quartz was crushed by a ball mill, followed by wet sieving and drying to obtain a product with a particle size of 0.043–0.074 μm. The dried product was used as the pure quartz mineral sample.
The pure hematite mineral was obtained from the Diaojuntai processing plant of the Anshan Iron and Steel Company. The massive hematite ore was crushed by a jaw crusher and wet ball mill. Finally, the 0.043 μm to 0.074 μm particle size was screened by a water screen and dried as the pure hematite mineral sample.
Flotation Test
Flotation Setup and Reagents
The influence of flotation parameters on the flotation performance of minerals was investigated by using an XFD III type laboratory hanging tank flotation machine produced by Shicheng Baoxin Mineral Processing Equipment Manufacturing Factory. The flotation machine had a mechanical scraper and automatic pulp temperature control, the speed of scraper was 30 rpm, and the working temperature of the flotation tank was 15–50°C. The flotation machine impeller speed adjustment range was 1400–2600 rpm.
The KDB used in the test is a new anionic collector independently developed in the laboratory. The traditional collector (NaOL) was taken from the Diaojuntai concentrator of Anshan Iron and Steel, and was mainly sodium oleate. Dilute HCl and NaOH were selected for pH adjustment. The addition of an activator, calcium oxide (CaO), is necessary for anionic reverse flotation. The activator CaO can react with water to produce calcium hydroxide (Ca(OH)+). Some researchers have found that Ca(OH)+ can reverse the negative charge of the quartz surface to a positive charge to activate the chemisorbed fatty acid collector in the alkaline solution environment.15 The activator was CaO obtained from the Diaojuntai concentrator of Anshan Iron and Steel. The selection of causticized starch as a reagent for hematite depressant was based on its exceptional adsorption capacity and potent inhibitory effect on hematite.16 Distilled water was adopted throughout the experiment.
Flotation Conditions
-
(1)
Single-mineral flotation tests
The test was carried out in an XFD III type hanging tank flotation machine. Single-mineral flotation tests were conducted to investigate the effects of pH, collector dosage, activator dosage, and temperature on the flotation of quartz and hematite in the KDB and NaOL systems. As inspired by other studies,17 all the flotation tests were performed under the following experimental conditions unless otherwise emphasized:
-
Stirring speed 1992 rpm
-
pH 11
-
Depressant 20 mg/L
-
Activator 10 mg/L
-
Collector 20 mg/L
-
Dosing interval 3 min
-
Flotation scraping bubbles 3 min
The reagents were added in the order of (1) dilute HCl and NaOH solution as pulp pH adjuster, (2) causticized modified starch as hematite depressant, (3) CaO as quartz activator, and (4) NaOL and KDB as quartz collectors, which is consistent with other studies and the plant operating procedure.
It is worth noting that the one-factor tests were conducted under the optimal conditions that have been obtained for the test. The low-temperature flotation experiment used ice water to adjust the pulp to cool, and the entire experiment was conducted in an air-conditioned room. A thermometer was used to continuously measure the pulp temperature.
-
-
(2)
Binary mixed ore flotation tests
The binary mixed ore firstly needed to be formulated according to the quality ratio of quartz: hematite = 3:1, and then used as the feed ore for the binary mixed ore flotation test after sufficient mixing. It is worth noting that the binary mixed ore tests were carried out under optimum conditions for single mineral flotation as set out above. The test temperatures were set at 15°C and 25°C, as 25°C is the conventional room temperature and 15°C is the slurry temperature in the mine during the winter season.
FTIR Characterization
The FTIR spectral analysis of quartz and the two collectors (KDB and NaOL) were examined separately at a low temperature (15°C) using a VERTEX 70v Fourier transform infrared spectrometer from Bruker, Germany. First, a pure KBr sample was collected as the background. Secondly, 1 mg of the prepared mineral sample and 100 mg of pure KBr were ground and mixed in an agate mortar. Then, the sample was pressed into a block shape and put into the spectrometer to be tested. Finally, the changes in the intensity of the adsorption peaks of the minerals with the interaction of the two collectors were characterized.
Adsorption Measurement
-
(1)
Determination of linear coefficient R
The adsorption of NaOL and KDB was determined using a TU-091 dual beam UV-visible spectrophotometer. Firstly, the NaOL and KDB solutions were configured with mass concentrations of 10 mg/L, 20 mg/L, 40 mg/L, 80 mg/L, 100 mg/L, 120 mg/L, and 160 mg/L, and the full spectral scans of the two reagents (NaOL and KDB) were carried out in the wavelength range of 190–900 nm. The characteristic adsorption peaks of NaOL and KDB were measured at 205 nm and 238 nm, respectively. The absorbance of NaOL and KDB at the optimal adsorption wavelength, λ, was determined and the standard adsorption curves were plotted. Finally, the linear coefficients were obtained by fitting. It is worth noting that the goodness of fit needs to satisfy greater than 0.99, that is R2 ≥ 0.99; both reagents were measured to essentially satisfy the Beer–Lambert law.
-
(2)
Adsorption test
An amount of 2.0 g pure quartz mineral was mixed with 25 ml of deionized water and placed in a centrifuge tube for 1 min to slurry. Then, the pH adjuster (dilute HCl and NaOH), activator (CaO), and collectors (NaOL and KDB) were added, and the mixture was stirred with a magnetic stirrer for 5 min for a complete adsorption. The temperature was controlled by ice water slurry at 5°C, 10°C, 15°C, 20°C, 25°C, and 35°C. After filtration, the supernatant was obtained by centrifugation at a rotational speed of 1,2000 rpm, and the absorbance of the supernatant was measured by a UV spectrophotometer. The concentrations of the collectors in the solution before and after the adsorption of the samples were then obtained according to the standard adsorption curves. Finally, the adsorption amount of the sample under different collector concentration conditions was calculated. The adsorption capacity of the sample was calculated according to:
$$ \Gamma = (C_{0} - C) \cdot V/m $$(1)where Γ (mg/g) is the adsorption amount of reagent on the quartz surface, C0 (mg/L) is the initial mass concentration of the agent, C (mg/L) is the residual mass concentration of the collector in the supernatant, V (L) is the volume of slurry, and m (g) is the mass of the quartz ore sample.
Surface Tension Measurement
Surface tension was determined by the platinum ring method using a fully automatic Sigma 701 surface tension meter (Biolin, Sweden), the test error of the equipment was within 3%. The test used ice water to adjust the pulp to cool, and used a thermometer for continuously measuring the pulp temperature. Without special emphasis, the concentration of the collector was 80 mg/L to carry out the test. The influence of different temperature (0°C, 5°C, 10°C, 15°C, 20°C, 25°C, 30°C, and 35°C) and different concentrations (3 mg/L, 6 mg/L, 20 mg/L, 50 mg/L, 85 mg/L, 100 mg/L, 150 mg/L, 306 mg/L, 600 mg/L, 1000 mg/L, 5000 mg/L, and 8000 mg/L) of the collector on its surface tension was tested. Each setup was tested three times, and the average value was taken as the accurate value.
Contact Angle Measurement
In order to study the difference of the contact angle, a small amount of pure mineral quartz was treated with 20 mg/L NaOL or KDB at 35°C, 25°C, 15°C, and 5°C, respectively, then dried and pressed into sheets. A JC2000C1 contact angle measuring instrument was used to measure the contact angle by the drip method. After 3 tests for each sample, the average value was taken as the contact angle of the sample.
Results and Discussion
Sample Analysis Results
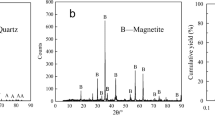
The results of the quartz single-mineral and hematite single-mineral chemical composition analyses are shown in Tables I and II. The X-ray diffraction analysis results of the two pure minerals are shown in Fig. 1.
Table I shows that SiO2 is 98.49% in the quartz pure mineral samples, and that the samples contain small amounts of Al, Mg, and other trace elements. According to Table II, it can be seen that the sample purity of hematite is 94.68% and that the content of the main impurity, SiO2, is 4.24%. Figure 1a shows that the main component of the prepared quartz monomineral samples is SiO2, which is consistent with the results of the chemical analysis of the quartz monocrystal in Table I. Figure 1b shows that the main component of the hematite monomineral is Fe2O3, and that no other obvious impurities were found. Therefore, the quartz and hematite samples can be regarded as pure minerals.
Influence of KDB and NaOL on the Flotation of Pure Minerals
Effect of pH on Quartz and Hematite Flotation
The test results of the effect of slurry pH on the recovery are shown in Fig. 2. The recovery of quartz under the two types of collector systems increased with the increase of pH up to 11.0 and then decreased. The main reason why quartz has a lower recovery under acidic conditions is that the negative charge on the quartz surface is lower at a pH below 9, which makes the electrostatic adsorption between quartz and collector weaker.18 As the pH of the pulp was increased from 9.0 to 11.0, the quartz recovery was 82.68% under the NaOL system, but was enhanced to 94.54% under the KDB system. When the pH value is raised to 11, the concentration of H+ ions in the slurry is low, and, at this time, the collector ionizes to produce more anionic ions, so the flotation effect is best, which is consistent with the report by Luo et al.19 As the pH value of the slurry increases to 13.0, the recovery rate of quartz decreases sharply. At higher pH values, there is competitive adsorption between individual collector molecules (micelle formation) and the quartz particles. This will reduce the hydrophilicity of the quartz, thus inhibiting the flotation of the quartz to a certain extent.
For hematite, the recovery rate is low in the whole pH range of the test. Previous studies have shown that the surface negative charge of hematite is about − 30 mV at pH 11,20 so it is difficult to be captured by anionic collectors.21 At pH 11, the recoveries were 19.21% and 21.63% under NaOL and KDB systems, respectively. At this point, the differences between the hematite and quartz flotation recovery of the two systems were 63.47% and 72.91%, respectively. As the pH was further increased to 12 and 13, the recovery rate of hematite increased. The analysis indicates that, in strongly alkaline conditions, the RCOO− and (RCOO)22− ions in the sodium oleate solution adsorb monolayers chemically onto the maghemite surface without adsorbing starch, making the surface hydrophobic and leading to a higher recovery rate. This is consistent with the study by Xie et al.22 When the pH was 12, the differences between the hematite and quartz flotation recovery in the two systems were 54.52% and 60.07%, respectively. Therefore, the differences between the hematite and quartz flotation recovery of the two systems were most obvious at pH 11, which is most conducive to the separation of quartz and hematite. In summary, the optimal pH was determined to be 11.0, and the recovery of quartz and hematite in KDB system were 94.54% and 21.63%, respectively.
Effect of Collector Dosage on Quartz and Hematite Flotation
The results of the effect of collector dosage on the flotation of quartz and hematite are shown in Fig. 3. According to Fig. 3a, the quartz recovery with the increase of collector dosage firstly gradually increases and then tends to be stable. It is clearly seen that the overall quartz recovery under the NaOL system is lower than under the KDB system. These results indicate that KDB has a stronger flotation effect on quartz than NaOL. The maximum recovery of quartz under the KDB system could reach 94.54% when the collector dosage was 20 mg/L, while the maximum recovery of quartz under the NaOL system was only 82.68%. At this time, the recoveries of hematite were 21.63% and 19.21% for the two collector systems, respectively. The maximum difference in recovery between pure mineral quartz and hematite is favorable for sorting. In conclusion, the optimum dosage of the collector was selected as 20 mg/L.
Effect of Activator Dosage on Quartz and Hematite Flotation
The effect of activator dosage on the recovery of quartz and hematite are shown in Fig. 4. The experimental results indicate that the effect of KDB on the collection of quartz was better than that of NaOL. The trend of quartz recovery rate under the two collector systems was consistent, with the quartz recovery reaching the maximum at 10 mg/L, and then the recovery decreased slightly, which was consistent with the experimental results of Min et al.23 It is speculated that it may be the interaction between the excess Ca2+ in solution and the collector which reduces the effective concentration of the collector, thus reducing the recovery of quartz.24 In conclusion, the optimum dosage of activator was selected as 10 mg/L. In both collection systems, the trend of the hematite recovery rate is also roughly the same. When the dosage of the activator was 10 mg/L, the recoveries of hematite were 19.21% and 21.63%. At this time, the difference in recovery between pure mineral quartz and hematite is significant, which is favorable for sorting. In conclusion, the optimum dosage of activator was selected as 10 mg/L.
Effect of Temperature on Quartz and Hematite Flotation
The results for the flotation recovery rates of quartz and hematite with temperature variation are shown in Fig. 5, from which it can be seen that the recovery of quartz decreases under both kinds of collector systems with the decrease of temperature, but the recovery of hematite does not change much. It was found that there was little difference in the quartz recovery rate between the two collectors at 35°C, but the difference gradually increased with the decrease of temperature, indicating that the flotation performance of NaOL is more affected by temperature than that of KDB. The flotation performance of NaOL is poor at low temperatures, which may be related to the fact that at such temperatures NaOL tends to precipitate. As for KDB, the reagent is also stable at low temperatures. Under the NaOL system, the recovery rate of quartz rapidly decreases to 62.45% when the temperature is below 15°C, and is only 28.52% when the temperature is 0.5°C. With the temperature decreasing, the recovery of quartz in the KDB system decreases very slowly. At all the test temperatures, the recovery of quartz in the NaOL system was higher than that in the KDB system.
Influence of KDB and NaOL on the Flotation of Binary Mixed Minerals
The results of the binary mixed ore flotation tests of NaOL and KDB at 15°C and 25°C are shown in Fig. 6. The results show that the flotation indices of the binary mixed ores under KDB system are better than those under the NaOL system at 15°C and 25°C. When KDB is used as a collector, good indicators of a concentrate grade of 65.02% and a recovery of 90.34% can be obtained at 25°C, with the grade and recovery improved by 3.56% and 7.70%, respectively, compared with using NaOL as a collector. When KDB is the collector at 15°C, the concentrate grade is 64.13% and the recovery is 89.50%, which is 5.77% and 14.32% higher than those when NaOL is the collector. In conclusion, KDB can obtain good flotation indices at both room and low temperatures. This is because the new low-temperature-tolerant collector, KDB, is less affected by temperature, and its components and effects are also relatively stable at low temperature.
Study on the Mechanism of KDB to Enhance Hematite Reverse Flotation
FTIR Analysis
The results of the FTIR spectra of quartz before and after interaction with KDB and NaOL are shown in Fig. 7. According to Fig. 7a, it can be seen that: the vibrational adsorption peak of the O-H bond between adsorbed water is at 3436.92 cm−1; 1085.85 cm−1 is the asymmetric telescopic vibrational adsorption peak of Si-O with a broader and stronger adsorption band, which is the first characteristic adsorption optical band of quartz pure minerals;25 785.94 cm−1 and 684.59 cm−1 are the symmetric telescopic vibrational adsorption peaks of quartz Si-O-Si;26 and the bending vibrational adsorption peaks of O-Si-O is at 464.81 cm−1, which is the second characteristic adsorption band of quartz.27
Comparing Fig. 7a and b, it can be seen that the first and second characteristic adsorption peaks of quartz are shifted to the direction of a low wavelength by 3.86 cm−1 and 1.93 cm−1, respectively; the smaller shifts are presumed to be probably due to the electrostatic adsorption. The Si-O-Si symmetric telescopic vibrational adsorption peaks are shifted by 4.82 cm−1 in the low-wavenumber direction and 5.88 cm−1 in the high-wavenumber direction, respectively, which is speculated to be a possible hydrogen bonding effect. Comparing Fig. 7a and c, it can be seen that the characteristic adsorption peak of the O-H bond at 3436.92 cm−1 of quartz under the KDB system is shifted to 3431.14 cm−1, which indicates that there exists a hydrogen-bonding adsorption between the KDB and the quartz. The first and second characteristic adsorption peaks of quartz after the action of KDB were shifted to the direction of a low wavenumber by 5.78 cm−1 and 3.86 cm−1, respectively, which also indicated that there was a hydrogen-bonding adsorption between the KDB and the quartz surface. The Si-O-Si symmetric telescopic vibrational adsorption peaks are shifted by 6.75 cm−1 and 9.74 cm−1, respectively, which are much larger than those of quartz in the NaOL system. In general, the adsorption strength of KDB on the quartz surface is much higher than the physical adsorption between NaOL and quartz, and more inclined to chemical adsorption.
The presence of oxygen-containing groups on the mineral surface affects its hydrophobicity.28 Some researchers have used the strength of the A-OH peak (water peak) in the FTIR spectra to reveal the hydrophobicity of mineral surfaces. For example, Tang et al.30 showed that the characteristic peaks of the A-OH peaks of graphite samples treated with nanobubbles were significantly reduced, indicating that the hydrophobicity of the graphite surface was significantly enhanced. Zheng et al.31 also characterized the hydrophobic strength of non-coking coal surfaces by characterizing the intensity change of the A-OH peaks. By comparison of Fig. 7b and c, it can be seen that the intensity of the A-OH peak near 3431.14 cm−1 of quartz under the KDB system is significantly lower than that under the NaOL system, which indicates that KDB can effectively cover the surface of the quartz and thus enhance the hydrophobicity of the quartz surface. It is worth mentioning that the limited increase in FTIR peak intensity is related to the nature of physical adsorption of the agent on the quartz surface.31 It can be seen from Fig. 7 that the characteristic peak intensities of quartz with KDB are significantly higher than those of quartz with NaOL near 1080 cm−1, 780 cm−1, 690 cm−1, and 460 cm−1, which suggests that KDB has a higher adsorption intensity on the surface of quartz at low temperatures to further improve the quartz surface hydrophobicity.
Adsorption Thermodynamic Differences
Figure 8 depicts the adsorption capacity of the adsorption of NaOL and KDB on the quartz surface. The adsorption capacity of NaOL on the quartz surface decreased with the decrease of temperature. When the temperature is lower than 15°C, the adsorption capacity rapidly decreases to 0.364 mg/g, and even decreases to 0.032 mg/g when the temperature reaches 0.5°C. This indicates that low temperature will hinder the adsorption of NaOL by quartz, thus reducing the surface hydrophobicity of the quartz.19,32 Of course, the lower the hydrophobicity, the worse the flotation efficiency of quartz, which is consistent with the poor flotation efficiency of NaOL at low temperatures. NaOL components cannot be adsorbed on the quartz surface because NaOL precipitates more easily and does so especially at low temperatures. In addition, the effective ion concentration in the pulp is low, so the adsorption capacity of NaOL on the quartz surface decreases sharply, which affects the activation of calcium oxide and the adsorption of fatty acid root ions.33 In contrast, the adsorption capacity of KDB on the quartz surface decreases more slowly with the decrease of temperature than NaOL. The adsorption capacity was 1.312 mg/g at 25°C and the adsorption capacity could still reach 1.145 mg/g at 15°C, which confirmed that the KDB collector had better adaptability to temperature, good collecting ability, and strong dispersibility.
Differences in Surface Tension of Slurries
The results of the surface tension determination of the quartz pulp by NaOL and KDB at different concentrations are shown in Fig. 9, from which it can be seen that the addition of KDB or NaOL can reduce the surface tension with the increase of collector concentration, but the difference is that the surface tension with KDB is always lower than that with NaOL. For example, the surface tension with KDB is 36.21 mN/m when the KDB concentration is 20 mg/L, while the tension with NaOL is 38.34 mN/m; that is, the tension with KDB is 2.13 mN/m lower than that with NaOL. It should be noted that the tension with KDB a to minimum value of 22.24 mN/m (γCMC) when the KDB concentration was 85 mg/L (critical micelle concentration; CMC). Similarly, the tension with NaOL was 23.68 mN/m under the condition of the CMC 306 mg/L. Therefore, it can be clearly seen that the CMC with KDB is 221 mg/L lower than with NaOL. The CMC is one of the important indices for measuring the efficiency of reducing the surface tension. In general, the lower the CMC value of the collector, the better the collection performance.34 In other words, the KDB collector has a stronger ability to form micelles and a better hydrophobicity.35 In addition, the reduction of surface tension is beneficial for improving the solubility and foaming properties of the collector.36,37
The effects of NaOL and KDB on the surface tension of quartz pulp at different temperatures are shown in Fig. 10, from which it can be seen that the lowest surface tension of KDB is always lower than that of NaOL under different temperature conditions. In addition, the lower the pulp temperature, the larger the γCMC. According to previous studies, the surface tension decreases with the increase of temperature,38 which will intensify the intermolecular thermal motion and increase the distance between the liquid molecules. Therefore, the mutual attraction between the molecules decreases, and the surface energy decreases. The decrease of the surface tension of the pulp is conducive to the formation of a stable foam layer, thus improving the flotation performance.39
It also can be seen from Fig. 10 that, in the range of 35–20°C, the surface tension of the two reagent systems gently changed with the decrease of temperature. When the temperature was lower than 20°C, the surface tension of NaOL system rapidly increased while that of KDB system slowly increased. For example, the γCMC of the pulp in the NaOL system at 35°C is 21.65 mN/m, and the γCMC of the pulp in the NaOL system at 5°C is 41.69 mN/m, and the surface tension at 5°C is 19.74 mN/m higher than that at 35°C, which is not conducive to mineral flotation. NaOL forms precipitates readily at low temperatures (below 20°C), which reduces the effective concentration of oleate ions in solution and thus decreases the surface activity and greatly weakens the ability to reduce the surface tension.40,41
It should be added that, under the condition of 5°C, the surface tensions of KDB and NaOL are 27.36 mN/m and 41.69 mN/m, respectively; that is, the surface tension of KDB is 14.33 mN/m lower than that of NaOL. Therefore, the influence of temperature change on surface tension in the KDB system is much smaller than that in the NaOL system, which is one of the main reasons why KDB has a more satisfactory flotation efficiency than NaOL. Further, it is speculated that the synergistic effect between the oxygen-containing groups and oleate in the KDB collector reduces the surface tension of the pulp, the total free energy of the system, and the pressure in the bubbles, so that stable small bubbles can be formed at low temperatures to improve the flotation efficiency. CHO et al. showed42,45 that lowering the surface tension is favorable for obtaining small-diameter blisters with a larger gas–liquid interfacial volume, which is conducive to the entrainment of uplifted minerals. In other words, KDB can stabilize the bubbles by reducing the surface tension while the stabilized bubbles can improve the flotation performance.
Differences in Contact Angle of Quartz Surfaces
The results of the contact angle test of quartz after interaction with NaOL or KDB are shown in Fig. 11. The contact angle of the pure mineral quartz raw ore was 20.98°, indicating that quartz is hydrophilic on the surface before adsorption of the collector, which is consistent with a previous study by Hu et al.44 Fig. 11 shows that the contact angles of the quartz surface after interaction with NaOL and KDB at 35°C were 85.64° and 96.36°, respectively, indicating that both collectors can better adsorb on the quartz surface. This better adsorption can enhance the hydrophobicity of the quartz surface. With the decrease of temperature, the contact angle of the surface shows a decreasing trend, but the difference of the contact angle with KDB and with NaOL are increasing; that is, the contact angle of KDB system is always higher than that of NaOL system. For example, the contact angle with KDB is 78.28° when the temperature is 5°C, while the contact angle with NaOL is 28.6°, thus the contact angle is 49.68° higher than that of the NaOL system. The long molecular hydrocarbon chain of NaOL has low solubility in water, leading to the formation of micelles and precipitation at low temperatures. As a result, the flotation recovery decreases significantly at lower temperatures. In contrast, there are oxygen-containing polar components, such as C-O alcohol in the KDB collector,45 which improve the dispersion and foaming performance of the collector.
Conclusion
-
(1)
The flotation performance of a novel quartz collector, KDB, has been studied and compared to a traditional collector by conducting flotation tests on pure minerals and binary mixed ores. The KDB has a better flotation effect than the traditional anionic collector, NaOL, at both room and low temperatures. The binary mixed ore flotation test was carried out at a slurry temperature of 15°C, which indicated that the iron grade of KDB was increased by 10.77% and the iron recovery of concentrate was increased by 14.32% compared with the NaOL.
-
(2)
The research into adsorption mechanisms at low temperatures is of great significance for the utilization low-temperature flotation agents. FTIR measurements revealed that the adsorption strength of KDB on the quartz surface at low temperatures was significantly higher than that of NaOL, which was more inclined to chemisorption with hydrogen bond formation. The reduced intensity of the A-OH characteristic peaks suggests that KDB can further enhance the hydrophobicity of quartz, which in turn improves the effectiveness of hematite reverse flotation.
-
(3)
The contact angle and adsorption thermodynamic analyses both indicated that KDB has a greater adsorption capacity on the quartz surface than NaOL. The presence of oxygen-containing polar components such as C-O alcohols in the KDB can increase the number of active sites for collecting minerals, which also have strong adsorption effects at low temperatures.
References
W.B. Liu, W.G. Liu, D.Z. Wei, M.Y. Li, Q. Zhao, and S.C. Xu, Chem. Eng. J. 309, 63 (2017).
Z.Q. Huang, H. Zhong, S. Wang, L.Y. Xia, W.B. Zou, and G.Y. Liu, Chem. Eng. J. 257, 218 (2014).
H. Sahoo, N. Sinha, S.S. Rath, and B. Das, Chem. Eng. J. 273, 46 (2015).
C.H. Veloso, L.O. Filippov, I.V. Filippova, S. Ouvrard, and A.C. Araujo, Miner. Eng. 125, 133 (2018).
L.O. Filippov, V.V. Severov, and I.V. Filippova, Int. J. Miner. Process. 127, 62 (2014).
F. Nakhaei and M. Irannajad, Miner. Process. Extr. Metall. Rev. 39, 89 (2017).
H. Sis and S. Chander, Miner. Eng. 16, 577 (2003).
K. Quast, Miner. Eng. 160, 106647 (2021).
F. Nakhaei and M. Irannajad, Miner. Process. Extr. Metall. Rev. 39, 89 (2018).
J.H. Xu, G.D. Meng, J.H. Chen, and Y.T. Li, Min. Eng. 04, 21 (2012). (Chinese)
H.L. Zhang, S.Y. Lin, Z.H. Guo, W. Sun, and C.Y. Zhang, Appl. Surf. Sci. 614, 156056 (2022).
B. Luo, Y. Zhu, C. Sun, Y. Li, and Y. Han, Miner. Eng. 77, 86 (2015).
A.Z.M. Abouzeid, Int. J. Miner. Process. 85, 59 (2008).
X. Zhang, X. Gu, Y. Han, N. Parra-Álvarez, V. Claremboux, and S.K. Kawatra, Miner. Process. Extr. Metall. Rev. 42, 184 (2021).
D. Lu, Y. Hu, Y. Li, T. Jiang, W. Sun, and Y. Wang, Physicochem. Probl. MI. 53, 724 (2017).
S.S. Rath and H. Sahoo, Miner. Process. Extr. Metall. Rev. 43, 122 (2022).
D. Tao, Z. Wu, and A. Sobhy, Powder Technol. 379, 12 (2021).
B. Siwek, M. Zembala, and A. Pomianowski, Int. J. Miner. Process. 8, 85 (1981).
X. Luo, Y. Wang, S. Wen, M. Ma, C. Sun, W. Yin, and Y. Ma, Int. J. Miner. Process. 152, 1 (2016).
H.M. Yu, H.J. Wang, and C.Y. Sun, Metal Mine. 11, 64 (2017). (Chinese)
D. Li, W.Z. Yin, Y.Q. Ma, and J. Yao, J. Northeast. Univ. (Nat. Sci.) 6, 865 (2016). (Chinese)
D.D. Xie, Y. Hou, G.H. Huang, D.P. Tao, C. Han, X.L. Wang, D. Jin, and J. Cent, South Univ. 50, 7 (2019). (Chinese)
C. Min, X.M. Hu, and H.Q. Zhang, Metall. Eng. 7, 37 (2017).
A.L. Shao, J. Cent. South Univ. Nat. Sci. Ed. 2, 456 (2013). (Chinese)
Y.S. Zhou, C.R. He, and X.S. Yang, Sci. China Earth Sci. 51, 1411 (2008).
H. Sahoo, S.S. Rath, and B. Das, Sep. Purif. Technol. 136, 66 (2014).
H. Sun and W. Yin, Sep. Purif. Technol. 295, 121201 (2022).
K. Zheng, W. Xia, and W. Zhang, Colloids Surf. 611, 125794 (2021).
L. Tang, F.M. Ma, T.Y. Wu, D. Zhang, Y. Wang, T.L. Zhao, Z.L. Fan, and X.Y. Liu, J. Ind. Eng. Chem. 122, 389 (2023).
K. Zheng, W. Xia, R. Wang, Y. Li, and W. Zhang, Colloids Surf. 622, 126668 (2021).
K. Zheng, W. Xia, and W. Zhang, Colloids Surf. A 611, 125794 (2021).
J. Wang, Y. Ji, S. Cheng, S. Liu, J. Cao, and P. Chen, Appl. Surf. Sci. 568, 150956 (2021).
J.Y. Chong, W.Q. Wang, Y.M. Lin, Y.T. Cui, and Y. Zheng, Nonmet. Min. 6, 77 (2018). (Chinese)
N.A. Abdel-Khalek and A.M. Omar, J. Chem. Eng. Data 44, 138 (1999).
L. Xia, H. Zhong, G. Liu, Z. Huang, and Q. Chang, Int. J. Miner. Process. 92, 74 (2009).
J.Z. Wang, Y.Z. Cheng, Y.C. Xiao, Y. Mao, J. Xue, and Y. Zhang, Flotat. Mech. Nonferrous (Met. Miner. Process. Part) 01, 100 (2019). (Chinese)
A. Liu, M.Q. Fan, and P.P. Fan, Miner. Eng. 65, 41 (2014).
X. Liu, J.B. Li, Z. Li, Y.D. Wang, Z.B. Deng, and X. Xie, Conservation and utilization of mineral. Resources 04, 115 (2019). (Chinese)
X. Huang, K. Huang, Y. Jia, S. Wang, Z. Cao, and H. Zhong, Chem. Eng. Sci. 205, 220 (2019).
H. Kursun, Part. Sci. Technol. 32, 632 (2014).
Y. Chen, H. Li, D. Feng, X. Tong, S. Hu, F. Yang, and G. Wang, Miner. Eng. 160, 106658 (2021).
Y.S. Cho and J.C. Laskowski, Int. J. Miner. Process. 64, 69 (2002).
Y.S. Cho and J.C. Laskowski, Can. J. Chem. Eng. 80, 299 (2002).
B. Hu, J. Dispers. Sci. Technol. 37, 1555 (2016).
H. Zhang, W. Liu, C. Han, and H. Hao, J. Mol. Liq. 249, 1060 (2018).
Acknowledgements
This work was supported by the Basic Scientific Research Project of Higher Education Department of Liaoning Province (LJKQZ20222340).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following financial interests/personal relationships which maybe considered as potential competing interests: KDB was developed by our team (the researchers who contributed to this achievement). Currently, this research is being applied for a patent for invention. Other than that, there are no other potential conflicts of interest related to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, S., Zheng, Y., Zhao, T. et al. Study on the Enhanced Desalinization of Hematite Reverse Flotation by a New Low-temperature Resistant Collector. JOM (2024). https://doi.org/10.1007/s11837-024-06824-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11837-024-06824-8