Abstract
Microwave heating has the characteristics of simple control and high efficiency, in which electromagnetic force plays an important role. The electromagnetic force model in the tube was established by using the UDF function and energy equation of FLUENT software. The mixing process of cerium chloride solution and the mixing effect of Lorentz force under different heating methods, as well as the microwave heating mixing and cerium oxide preparation process under different power, were numerically simulated. CFD-Post and MATLAB software were used to post-process the data of the whole fluid region. Through the above analysis, the optimal process conditions of numerical simulation and experiment were finally determined. The results show that, compared with conventional heating, microwave heating has a better effect on the uniform mixing of cerium chloride solution, in which Lorentz force promotes the mixing; under microwave heating conditions, the conversion rate is higher, the cerium oxide concentration distribution is more uniform, and the product morphology is better. The best cerium chloride mixing method and cerium oxide preparation method are microwave heating, and the best microwave power is 5 kW.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Micro-/nano-cerium oxide is an important rare earth oxide. Firstly, it has stable chemical properties, excellent thermal stability, and good safety. It has been applied in food safety testing, food wastewater treatment, and other related fields;1,2,3 Furthermore, it is widely used in mechanical polishing in industrial chemistry and other fields due to its surface, volume, and quantum size effects.3 Ce3+ and Ce4+ in CeO2 are easy to transfer out from each other and have excellent ability to store and release oxygen.4,5,6 CeO2 is an efficient and environmentally friendly catalyst because its surface acidity and alkalinity can be adjusted, and the structure of the catalytic active center can be modified. Besides, CeO2 nano-catalysts have excellent reusability7 and are widely used in various redox catalytic reactions.8,9,10
Microwave pyrolysis is a great method to prepare rare earth oxides. Tang et al.11 treated graphene oxide, pyrolytic graphene oxide, and hydrogen-reduced pyrolytic graphene oxide by microwave-assisted heating. Pure or doped graphene was prepared in a few minutes, and a thermal deoxidization reduction mechanism was proposed to understand their microwave adsorption behavior. Under microwave irradiation, N, B, and P can be introduced into the graphene lattice in just 3 min to form N/B/P-doped graphene materials. Soren et al.12 synthesized single-phase ceria (CeO2) nanoparticles in 10 min by a microwave-mediated polyol method using different cerium precursors. Lv13 studied the effect of microwave power on the temperature field during the preparation of cerium oxide. Under suitable conditions, cerium oxide particles with good morphology, high crystallinity, and purity were prepared.
The microwave pyrolysis preparation process is simple, and has low energy consumption and good continuity, and microwave heating is currently recognized as one of the representative green heating technologies. Nowadays, under the background of emphasizing low-carbon production, the preparation of rare earth oxides and microwave heating technology are a group of promising process technology combinations. Compared with the conventional heating mode, microwave heating has a unique heating mechanism, which can make the particles more fully heated and make the product morphology have better uniformity, which are difficult to match by conventional heating.
Scanning electron microscopy (SEM) and X-ray diffractometer (XRD) are important methods to observe the morphology and composition of the products. Salma14,15,16,17,18,19,20 observed and analyzed the surface morphology of xCuxMn2O4/RGO perovskite material samples, BaGdxFe2-xO4 spinel ferrite nanoparticles, Sm-doped NiO, Fe2O3(FO) and a series of Ni-doped Fe2O3(5-NFO) nanoparticles by SEM, and then detected by XRD. The diffraction pattern basically has no impurity peak, which shows that the material has high purity. All these provide correct and strong evidence for the conclusion.
Numerical simulation can more intuitively show the process of the state change of the reactants and the preparation process of the products. Lv21,22,23 carried out numerical simulation on the preparation of cerium oxide by pyrolysis of cerium chloride. The results showed that the mixing uniformity between the materials could increase the contact area between the pyrolysis reactants and refine the droplets. Therefore, the mixing uniformity of the reactants has a significant effect on the conversion rate of cerium oxide. Microwaves have a significant effect on the improvement of mixing uniformity.
In addition to the well-known 'thermal effect', microwaves also have non-thermal effect'. When heating with microwaves, there will be a rapidly changing electromagnetic field in the reactor. The charged particles in the cerium chloride solution will be subjected to electromagnetic force to change the particle motion state, resulting in the corresponding dynamic effect. So far, the mechanism of the microwave non-thermal effect is not clear, and the results are few. Therefore, this paper takes the influence of the microwave electromagnetic effect on the distribution of cerium chloride as an example to study the microwave non-thermal effect.
In this paper, the mixing process of cerium chloride and the preparation of cerium oxide were numerically simulated. The difference between conventional heating mixing and microwave heating mixing on the mixing effect of cerium chloride solution was explored, and the influence of electromagnetic force on the mixing degree was analyzed. The preparation of cerium oxide under microwave heating and conventional heating conditions was studied, and the effect of microwave power on the mixing time and product conversion rate of cerium chloride solution is discussed.
Modeling
Circularity Tube Model
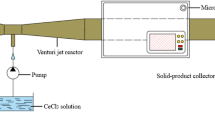
The mesh model of a circular straight tube with a diameter of 30 mm and a length of 310 mm is shown in Fig. 1. The coordinates of the left center of the model in Fig. 1a are X = 0, Y = 0.135 m, Z = 0.13 m, and the central axis of the circular tube is X = 0~0.31 m, Y = 0.135 m, Z = 0.130 m. The model is essentially the rear of a venturi jet pyrolysis reactor, and its position in the microwave oven is shown in Fig. 1.
The total number of grids is 700,000, and the minimum quality of grid is 0.74.
Governing Equations
The motion of cerium chloride in the magnetic field not only obeys the ordinary fluid mechanics equations but also obeys the Maxwell equations reflecting the electromagnetic motion. When microwave or electromagnetic mixing, the chlorinated cerium solution in the lumen will interact with the microwave. The Maxwell equations are:
where B is the magnetic flux density (Wb/m3), D the electric displacement or electric flux density (C/m2), E the electric field intensity (V/m), H the magnetic field intensity (A/m), J the current density (A/m2), and qe the electric charge density (C/m3).
The constitutive equation of the electromagnetic field is determined by Eqs. 5 and 6. The magnetic Reynolds number is low during microwave mixing and heating, so the magnetic diffusion is ignored.
where μ is the magnetic permeability, and σ the conductivity.
In the electromagnetic mixing process of cerium chloride solution, under the influence of microwaves, the charged particles will be subjected to electromagnetic forces, namely Lorentz force24 and electric field force. The formulas are derived as:
where q is the electric charge.
In the process of microwave heating, microwave energy is absorbed by droplets and converted into heat. This process can be expressed by:
where ω is the angle frequency(rad/s), ε0 the dielectric constant of free space (8.85 \(\times \) 10−12F/m), ε″ the loss factor, |E|2 the mode of the electric field, and Q the absorbing energy or invert energy (J).
The standard k–ε model and Euler–Euler multiphase flow model were selected to simulate the fluid mixing and flow in the lumen. Lorentz force and electric field force have been added to the momentum equation as source terms. The theoretical equation is:
The momentum equation:
where P is the pressure (Pa), ueff the efficient viscosity (Pa s), g the acceleration of gravity (m s-2), Fl the Lorentz force (N/m3), and Fe the electric field force (N/m3).
Energy equation:
where CP is the specific heat capacity, T the temperature, k the heat transfer coefficient of the fluid, and ST the internal heat source of the fluid and the part of the mechanical energy of the fluid converted into heat energy due to viscosity, sometimes referred to as the viscous dissipation phase. In the process of electromagnetic mixing and heating, there is a coupling of the electromagnetic field, flow field, and temperature field. The overall theoretical process is shown in Fig. 2.
Numerical Simulation
Chemical Reaction
The chemical reaction equation for preparing cerium oxide is:
Initialization Conditions
From the perspective of simulation, the distribution of space in the straight pipe is initialized. In order to make the gas phase material sufficient during the reaction, the gas phase uniform ratio was set to 0.954 according to the calculation, and the ratio of oxygen to water vapor in the gas phase was 3/7. Outside the gas phase is the liquid phase, and the composition of the liquid phase is the chlorinated cerium solution. The three different concentrations of chlorinated cerium solution are distributed at equal heights. The concentration of the middle chlorinated cerium solution was 0.1, and the green areas on both sides were 0.06. The outermost blue area was 0.02. The specific distribution is shown in Fig. 3, and the surface is the central axis section of the circular tube.
Indices of Mixing Uniformity
The mixing degree is an important indicator of the mixing ability of microwave mixing and conventional heating mixing on the cerium chloride solution. Therefore, the mixing uniformity formula is introduced to represent the mixing degree of cerium chloride solution. The closer M is to 100%, the higher the mixing degree:
The mixing uniformity reaches about 95%, which is a micro-mixing state. When it reaches 98%, it is considered to be uniformly mixed.
Condition Hypothesis
For the convenience of calculation, this simulation makes the following assumptions:
-
a)
Fluid is incompressible;
-
b)
The magnetic diffusion phenomenon is not considered;
-
c)
The microwave frequency is constant at 2.45 GHz;
-
d)
The initial temperature in the reactor is uniform at 300 K; and
-
e)
Permeability is constant at 1.
Results and Discussion
Comparative Analysis of the Mixing Effect
Microwave Heating Mixing and Conventional Heating Mixing
The cerium chloride solution distributed in Fig. 3 is mixed in two ways: microwave heating mixing and conventional heating mixing. The mixing effects of the two mixing methods have been compared.
Firstly, the microwave heating mixing was carried out, the microwave power was 4 kW, the frequency was 2.45 GHz, and the numerical simulation was carried out in 0~2.4 s. Then, the model was placed in a tube furnace for conventional heating. In the numerical simulation, the bulk heating power at the wall surface when the microwave mixing is completed is used as the heating power of the wall surface, and the thermal effect of the wall surface heating is used to mix the cerium chloride solution in the initial distribution state. The mixing effect in 2.4 s is shown in Fig. 4. The color of the digit describing the time corresponds to the color of the curves in this figure.
CFD-post was used to extract the concentration data for cerium chloride of all the grid units in the fluid domain, and formulae. (13) was compiled by MATLAB for processing and calculation. Finally, the mixing uniformity at different times under the two mixing modes was obtained. The mixing uniformity of the two mixing methods has been compared, as shown in Fig. 4.
It can be seen from Fig. 4 that, under the mixing mode of microwave heating, a uniform mixing state has reached in 0.4 s, which is much quicker than the 2.2 s of conventional heating, while the mixing uniformity of cerium chloride under conventional heating mode at each time within 0~2.4 s is lower than that of electromagnetic–thermal mixing. It can be seen that the microwave heating mixing has a shorter mixing time and better mixing effect on cerium chloride.
Microwave Mixing
From the previous section, it is concluded that the microwave heating mixing effect is better, which is the result of the electromagnetic force and thermal diffusion. In this process, in addition to the thermal effect of the microwaves, electromagnetic force also plays an important role. Under the combined action of Lorentz force and electric field force, the charged particles in the cerium chloride solution will move along the direction of the resultant force. At the microwave frequency of 2.45 GHz, the direction and size of the electromagnetic force change rapidly, so the motion state of the particles will also change continuously, thus realizing the mixing of the cerium chloride solution. In order to explore the role of electromagnetic force in microwave mixing, the mixing effect of electromagnetic force on the cerium chloride solution was numerically simulated separately. The simulation results show that, under the condition of a microwave power of 4 kW, the mixing uniformity of cerium chloride is close to 95% at 2.4 s, reaching the micro-mixing state, and the concentration distribution is relatively uniform. The details are shown in Fig. 5. The color of the digit describing the time corresponds to the color of the curve in this figure.
The DEFINE _ ADJUST macro of UDF was used to derive the electromagnetic force data of the center coordinates of all the grid units at 2.4 s, and the electromagnetic forces of the central axis planes of the three circular tubes, x = 0.15 m, y = 0.135 m, and z = 0.13 m, inside the model have been plotted and analyzed. From the derived data, the electric field force on the particle is very small and negligible. This analysis mainly discusses the Lorentz force, and the vector distribution of the Lorentz force on the three planes is shown in Fig. 6.
It can be seen from Fig. 6a that the mode of Lorentz force has good continuity between different positions, and that there are many peak areas, which ensures the continuous effect of Lorentz force on the cerium chloride. On the whole, the mode and direction of Lorentz force change with different positions, which reflects its good volatility.
From Fig. 6b and c, the Lorentz force has a large proportion of symmetry on the plane of x = 0.15 m, which makes the motion state of cerium chloride in the left and right parts of the tube similar, so as to realize the symmetrical distribution of cerium chloride concentration in the mixing process, as shown in Fig. 5.
According to the microwave characteristics, the current density and magnetic field intensity inside the model are periodic, and the frequency is constant, so the Lorentz force is also periodic. In Fig. 6, the Lorentz force vector data at a certain position may be the state of the next position.
Comparative Analysis of Reaction Effect
Conventional Heating Reaction
The initial cerium chloride solution was conventionally heated, and the wall heating power was the same as in Section 3.1.1. The concentration distribution of cerium oxide after heating was analyzed, and its concentration distribution at the end of the reaction is shown in Fig. 9a:
It can be seen that, under the condition of conventional heating, the concentration of cerium oxide generated by the reaction is unevenly distributed and concentrated in the center of the tube. The mixing uniformity was calculated to be 85.94%. The conversion rate of the target product was calculated according to the initial liquid phase mass flow rate, the percentage of solute, the solute molecular weight, the solid mass flow rate at the end of the reaction, the mass fraction of the target product, and the molecular weight of the target product. The reaction conversion rate was 91.33%.
There are some areas with large concentration differences on both sides of the tube, which may be due to the generation of gas during the reaction process. There is a certain pressure in the area, which reduces the diffusion rate of cerium oxide to its interior.
Reaction of Microwave Heating
During microwave heating, each part of the medium is heated at the same time without forming a temperature difference between the inside and the outside. Not only can this heating method improve the heating efficiency and reduce energy consumption but it can also make the temperature distribution of the system more balanced, thus contributing to the successful completion of the reaction.25 The synthesis process of cerium chloride by microwave pyrolysis is shown in Fig. 4.
It can be seen from the figure that the hydrogen chloride produced in the process of preparing cerium oxide by microwave pyrolysis can be effectively recovered and no waste will be produced (Fig. 7).
Under the condition of a heating power of 4 kW and a frequency of 2.45 GHz, the initial distribution of cerium chloride solution was heated by microwaves to reach the temperature condition of preparing cerium oxide, and then cerium oxide was generated. The concentration distribution of cerium oxide during microwave heating is shown in Fig. 8.
During the preparation of cerium oxide, the diffusion of water vapor generated by microwave heating, the generation of hydrogen chloride gas, and microwave mixing all have a certain influence on the concentration distribution of cerium oxide.
It can be seen from Fig. 8 that, during the whole reaction process, the concentration of cerium oxide is basically symmetrically distributed, and that, before a good uniform distribution is achieved, there is a relatively concentrated high-concentration symmetrical area near the central axis of the tube. From these areas outward, the cerium oxide concentration gradually decreases. As the reaction progresses, this phenomenon gradually disappears, and finally the uniform distribution of cerium oxide is achieved, which shows the good heating characteristics of microwaves and their auxiliary effect on the diffusion of cerium oxide.
For the process of preparing cerium oxide by microwave heating, the product reached uniform distribution at 2.0 s, and the uniformity reached 99.5779% at 2.4 s. The final conversion of the reaction was 91.96%, which was higher than that of the conventional heating method (Fig. 9).
Analysis of the Structure of the Product
SEM images of nano-CeO2 particles obtained in this study are shown in Fig. 10b. The particles were sphere-like and smaller size, and there was less agglomeration. The above results show that pure CeO2 can be obtained by microwave heating, and that the morphology is regular.
The XRD pattern of the product is shown in Fig. 10a. The results showed that single phase CeO2 was obtained under microwave heating. The intensity of the diffraction peak is high, and there is no other impurity peak, indicating that CeO2 has high crystallinity and high purity.
Power Optimization
The above calculations were carried out under the condition of a microwave power of 4 kW, and the calculation results under different powers may be slightly different. In order to obtain the mixing effect of cerium chloride solution and the conversion rate of cerium oxide under different powers, and then optimize the microwave power to achieve the ideal results, the electromagnetic mixing and cerium oxide preparation reaction under the power conditions of 3 kW, 3.5 kW, 4.5 kW, and 5 kW were also numerically simulated, and the simulation results are shown in Fig. 11.
It can be seen from Fig. 11 that, as the microwave power increases, the micro-mixing time decreases and the reaction conversion rate increases. With the decrease of micro-mixing time, it is possible that the greater the power, the greater the electromagnetic force. In the case of constant frequency, the particle force becomes larger, so the mixing ability is stronger. The conversion of cerium oxide increases with the increase of power. The possible reason is that the higher the power, the higher the temperature generated in the tube, the more obvious the thermal effect, and the higher the diffusion rate, so the reaction is more complete. Therefore, a microwave power of 5 kW is more appropriate.
Conclusion
-
(1)
Compared with conventional heating mixing, microwave heating mixing has a better mixing effect on the initial distribution of cerium chloride solution, a shorter uniform mixing time, and a wider mixing range. The main reason is that Lorentz force accelerates the movement and diffusion of cerium chloride.
-
(2)
Cerium oxide was prepared by pyrolysis. Under the conventional heating method, the concentration of the chlorinated cerium solution was uneven, concentrated in the middle of the circular tube, and it decreased from the middle to both sides. The concentration of cerium oxide gradually tends to be uniform with time under microwave heating, and the cerium oxide reached a uniform distribution in 2.0 s.
-
(3)
Under microwave heating conditions, the conversion rate of the chlorinated cerium solution and its mixing time have a good linear relationship with the microwave power. The higher the microwave power, the higher the conversion rate, and the shorter the uniform mixing time. The microwave power of 5 kW is better.
-
(4)
Pure CeO2 can be obtained by microwave heating, and the morphology is regular. Also, single-phase CeO2 was obtained under microwave heating. The intensity of the diffraction peak is high, and there is no other impurity peak, indicating that CeO2 has high crystallinity and high purity.
References
C.Y. Chen, J.M. Lv, and Q.Z. Yao, Med. Sci. Monit. (2016). https://medscimonit.com/abstract/index/idArt/899852
Y. XU, S.H. Yang, G.X. You, and J. Hou, Asian J. Ecotoxicol. (2021). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iy_Rpms2pqwbFRRUtoUImHd693HyUksC-kkAGEi01vhNL8t0cGD_sKGNRApf7C28D&uniplatform=NZKPT&src=copy
P.G. Adriana, A.G.M. Víctor, V.G.I. Luz, M.P. Jimena, V.A.L. Pedro, B.S. Marcelo, S. Peter, B.G. Luis, and P.R. Mario, PLoS ONE. https://doi.org/10.1371/journal.pone.0211653 (2019).
T. Wu, J. Li, M.X. Zhu, X.Z. Zhou, and Y.X. Li, (2020). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=XTZZ202005001&uniplatform=NZKPT&v=q4iM3nJjKctdPUjdlplluxx2RqBmLb8z0354g8N0jHNEoBx6Ol7e5zmgMdJ0XgaL
L. Zhang, Y.Q. Ma, S.Y. Yu, X.Y. Sun, and L. Fang, Packag. Eng. (2021). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iy_Rpms2pqwbFRRUtoUImHdp-2aHqKFCeSqlyoXbi47Pirr1Pa2M1CVcUfbJlUcLq&uniplatform=NZKPT&src=copy
Y.H. Quan, C. Miao, T. Li, N. Wang, M.M. Wu, N. Zhang, J.X. Zhao, and J. Ren, J. Fuel Chem. Technol. (2021). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iy_Rpms2pqwbFRRUtoUImHfaud0DFz9XpDc1rvrslDOjAOoAFJl4rbnsIn-PWhFl2&uniplatform=NZKPT&src=copy
B.A. Rzigalinski and C.S. Carfagna Jr, Nanotechnology Characterization Tools for Environment, Health, and Safety, (Heidelberg, Berlin, 2019), pp. 217-243.
M. Dadu, A. Esra, B. Aamna, J. Muhammad Saqaf, and K. Sagar, Environ. Technol. https://doi.org/10.1080/09593330.2021.1929506?journalCode=tent20 (2021).
Y. Zhang, W.J. Zhu, T. Fu, Y.H. Li, Y.M. Luo, and J.C. Lu, J. Mol. Catal. (2022). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iJTKGjg9uTdeTsOI_ra5_XcXnAt8S89sjQFklsvGdV7cCaBa1HMmh83UBpGUlNMnV&uniplatform=NZKPT
Y. Zou, S. Zhang, and Y.Q. Qu, J. Shaanxi Norm. Univ., Nat. Sci. Ed. (2022). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iJTKGjg9uTdeTsOI_ra5_XVzGSpUoNURXoZt4eTQJlXjDYQkucXYBYbB3TwA4Urtl&uniplatform=NZKPT
P. Tang, G. Hu, Y.J. Gao, W.J. Li, S.Y. Yao, Z.Y. Liu, and D. Ma, Sci. Rep. https://doi.org/10.1038/srep05901 (2014).
S. Soren, M. Bessoi, and P. Parhi, Ceram. Int. (2015) https://linkinghub.elsevier.com/retrieve/pii/S0272884215004113
C. Lv, H.X. Yin, Y.L. Liu, X.X. Chen, and M.H. Sun, Crystals. (2022). https://www.mdpi.com/2073-4352/12/6/843
S. Aman, N. Ahmad, S. Manzoor, et al., Catal. Surv. Asia. https://doi.org/10.1007/s10563-023-09389-9 (2023).
S. Aman, M.S. Waheed, K. Jabbour, et al., Ceram. Int. https://doi.org/10.1016/j.ceramint.2022.11.328 (2023).
S. Aman, S.A. Khan, S. Gouadria, et al., J. Electron. Mater. https://doi.org/10.1007/s11664-023-10324-x (2023).
S. Aman, S. Gouadria, F.F. Alharbi, et al., Appl. Phys. A. https://doi.org/10.1007/s00339-023-06591-4 (2023).
S. Aman, N. Ahmad, B.S. Almutairi, et al., J. Electron. Mater. https://doi.org/10.1007/s11664-023-10396-9 (2023).
S. Aman, N. Ahmad, M.B. Tahir, et al., Surf. Interfaces. https://doi.org/10.1016/j.surfin.2023.102857 (2023).
S. Aman, A.M. Alenad, M.S. Waheed, et al., Mater. Res. Bull. https://doi.org/10.1016/j.materresbull.2023.112306 (2023).
C. Lv, X. Lv, and Q.Y. Zhao, Chin. J. Rare Met. (2019). https://chn.oversea.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&filename=ZXJS202203011&dbname=CJFDLAST2022&uid=WEEvREcwSlJHSldSdmVqMDh6cEFFdDZCbFlkdzdNa2VNbEpPNDlIRUtPbz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw
C. Lv, T.Y. Xia, L.P. Niu, and Q.Y. Zhao, Russ. J. Non-ferrous Metals. https://doi.org/10.3103/S1067821220010101 (2020).
C. Lv, H. Yin, Y.L. Liu, X.X. Chen, and H.L. Zhao, JOM. https://doi.org/10.1007/s11837-022-05654-w (2023).
Y.J. Zhang, W.N. Qin, and X.F. Huang. China Three Gorges University. (2015) https://kns.cnki.net/kcms2/article/abstract?v=kxaUMs6x7-4I2jr5WTdXti3zQ9F92xu0x7oG-EmpY101Rjq1o9LZE9ez69XAIozwzJkV3YpVY7wZE2QA_qq-qWZV2O4zzaHR&uniplatform=NZKPT
C.P. Kang, Harbin Inst. Technol. (2020). https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkEcTGK3Qt5VuzQzk0e7M1z9yl1EKMhil_DFOIMjEC8IgJIUATmqFH1PEYGlqGue5I&uniplatform=NZKPT
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 51904069), the Fundamental Research Funds for the Central Universities (No. N2223026), the Development Fund of State Key Laboratory for Clean Utilization of Complex Nonferrous Metal Resources (No. CNMRCUKF2302).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, C., Liu, Y., Yang, T. et al. Effect of Microwave Heating on the Mixing Effect of Chlorinated Cerium Solution and the Preparation of Cerium Oxide. JOM 76, 1737–1745 (2024). https://doi.org/10.1007/s11837-024-06370-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-024-06370-3