Abstract
In this article, a novel efficient and environmental reductive roasting-acid leaching technique was proposed by a combined pretreatment of the microwave heating and reduction of alkali lignin. Factors influencing the leaching of manganese from manganese oxide ores were investigated, such as heating modes (microwave heating and conventional heating), microwave roasting parameters and acid leaching parameters. The results indicated that the leaching ratio of manganese by microwave heating was significantly higher than that by conventional heating under the same conditions. The reduction of manganese oxide ores by microwave heating was completed at a roasting temperature of 150°C and a roasting time of 5 min with the addition of 50% alkali lignin with the reduction sequence of MnO2 → Mn2O3 → Mn3O4 → MnO. A 97.43% leaching ratio of manganese was obtained from the roasted ore, which was leached by a 1.5 mol/L concentration of sulfuric acid at a 50°C leaching temperature, 50 rpm stirring speed and a 10:1 liquid-to-solid ratio for 5 min. Compared to the conventional reductive roasting-acid methods, the proposed technique was performed at a considerably lower temperature and shorter time, with higher reducing agent utilization ratio to processing the different manganese oxide ores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese ores are commonly used as a raw material in many fields, such as the steel and iron industry, food additive industry, agriculture, and preparation of cells and fine chemicals.1,2 Among them, the steel and iron industry is the biggest consumer of manganese ores, and manganese for steel production comprises 80% of total manganese production.3,4 The world demand for manganese has increased yearly because of the rapid development of the steel and iron industry. Manganese in manganese ores often occurs in MnCO3 and MnO2 in nature. Recently, much attention has been paid to the processing and utilization of manganese oxide ores due to the large consumption of manganese carbonate ores.5,6,7 Since MnO2 is stable under direct acidic and alkaline conditions, the key to manganese oxide ore processing technology is to convert insoluble Mn(IV) to soluble Mn(II) in manganese oxide ore under the action of reducing agents, followed by acid leaching extraction. The selection of an efficient, economical and environmentally friendly manganese oxide ore reduction technology is a vital issue that needs to be urgently addressed in the development of the manganese industry.
The common reducing agents used in the wet reduction leaching process of manganese oxide ore are pyrite, ferrous sulfate, sulfur dioxide, hydrogen peroxide and organic solvents, etc., which have achieved good manganese dissolution results.8,9,10 In addition, the extraction of molybdenum, copper, cobalt, nickel and zinc from ores and concentrates by the hydrometallurgical process has also been applied.11,12 However, the wet reduction leaching process is also restricted by high reaction cost, energy consumption and severe pollution, which has yet to be applied on a large scale. In contrast, the reductive roasting-acid leaching process was proved to be the most feasible technique to recover manganese from manganese oxide ores, and the reducing agent played a critical role in the manganese leaching performance because of the stability of MnO2 in manganese oxide ores during the acid leaching process.13,14
Reductive roasting using coal as reducing agent is the most commonly reductive roasting technique in treating manganese oxide ores.10 However, it often takes a long time (4–8 h) to reduce MnO2 in manganese oxide ores into MnO under a high-temperature roasting condition (1000–1350°C),15,16,17,18 and the process often involves environmental pollution and terrible working conditions. Moreover, the investment and operating costs are far from satisfactory. Therefore, developing a highly efficient, clean and energy-conservative reductive roasting-acid leaching technology for manganese oxide ores is imperative for the sustainable development of the manganese industry.
Recently, microwave heating indicated potential feasibility to be an alternative heat mode in mineral processing with the advantages of reducing energy consumption, enhancing beneficiation results and improving the work condition.19,20 For example, recently, many published investigations have indicated that microwave irradiation can be successfully used as an efficient heating source for the pretreatment processes of refractory iron ores, such as hematite ores,21 laterite ores22,23 and goethite ores.24 Mehdilo et al. compared the effects of microwave irradiation and conventional heating on the physicochemical properties of ilmenite and its flotation behavior. The results indicated that a more remarkable improvement in the recovery of TiO2 was achieved after pretreatment by microwave irradiation than conventional heating.25 Besides, the investigation by Li et al. indicated that microwave heating was an effective pretreatment process for recovering iron from red mud, in which a combination of 6% Na2CO3, 6% Na2SO4 and three times ore dosage of lignite was used as the roasting additives, and the roasting conditions were fixed at 1050°C for 60 min. Under these conditions, an iron concentrate with a 90.2% iron content and 95.0% recovery ratio was obtained.26 Similar findings were also revealed under microwave treatment during the iron recovery process from red mud.27,28 Recently, Ye et al. applied the pretreatment of microwave heating to the preparation of reduced iron powder and the reduction roasting process of pyrolusite, respectively. In their study of the reduction of iron ore concentrate for the preparation of reduced iron powder, they found that microwave heating technology can be successfully applied as an effective and efficient route for processing iron ore concentrate. A reduced iron powder with an iron content of 98.56% and a metallization ratio of 99.25% could be obtained by fixing the roasting temperature at 1150°C and a roasting time for 50 min through a hierarchical and homogeneous addition of wood charcoal.29 As for the study on pyrolusite, the results indicated that the reduction of low-grade pyrolusite by microwave heating was achieved at a roasting temperature of 800°C and a roasting time of 40 min using coal as reducing agent, and under these conditions, a 97.2% reduction ratio of pyrolusite was obtained.30 Obviously, compared to the conventional reductive roasting technique of manganese oxide ores, microwave heating could reduce the roasting temperature to some extent. However, the technique still required a higher roasting temperature and a longer roasting time and used coal as reducing agent, which would inevitably cause problems to the ecological environment, and that is against the efficiency, economy and growing environmental concerns. Recent studies found that biomass, such as wheat stalk,31 moso bamboo,32 sawdust,33 cornstalk,34 bagasse35 and straw,36 could be successfully used as an alternative reducing agent to coal in the reductive roasting process of manganese oxide ores characterized by a remarkable energy conservation and environmental improvement effect. Typically, the reduction of MnO2 in manganese oxide ores using biomass as a reducing agent by conventional heating could be achieved at a roasting temperature of approximately 500°C for > 30 min.
Given that alkali lignin is a cheap and abundant biomass waste from the pulp and paper industry, in this study, a novel efficient and environmentally friendly reductive roasting-acid leaching technique of manganese oxide ores was proposed by a combined pretreatment of the microwave heating and reduction by alkali lignin. The effects of microwave roasting parameters, heating modes, and acid leaching parameters on the leaching ratio of manganese were investigated. Furthermore, the roasting behavior of manganese oxide ores was also analyzed. The X-ray diffraction (XRD) and scanning electron microscopy (SEM) studies of manganese oxide ores before and after treatment were used to verify further the feasibility of recovering manganese from manganese oxide ores by the technique proposed in this paper.
Materials and Methods
Materials
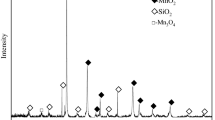
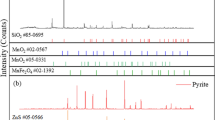
The manganese oxide ore sample used in the present work was collected from Guangxi Province of China. Two other manganese oxide ore samples from Gabon with Mn content of 45.62% and from Xiangxi, China, with Mn content of 20.59% were also used as ore samples for comparative experiments. All the ore samples were crushed and ground to a particle size < 0.25 mm (+ 60 mesh) in a ball grinder. The results of chemical analysis and X-ray diffraction (XRD) of the ore samples are presented in Table I and Fig. 1, respectively. Alkali lignin was purchased from Shandong Tranlin Group, China and used without further treatment. To facilitate the understanding and analysis of the roasting behavior by microwave heating, manganese oxide reagent with Mn content of 56.42% was used as a substitute for manganese oxide ores.
Experimental Procedures
Microwave roasting was conducted in a microwave reaction apparatus (model MAS-II). The roasting temperature was instantly controlled by varying the microwave power automatically according to a feedback control signal (the microwave power was at most 600 W), and the temperature was measured by an infrared temperature probe. The mixture of ore (or manganese oxide reagent) and alkali lignin was first put into a corundum crucible, which was covered with a lid and then placed in the reaction apparatus. In each test, 20 g of ore sample was used. After roasting for a designated time, the roasted ore was left inside the microwave reaction apparatus and cooled to room temperature. Subsequently, the cooled roasted ore was ground to a particle size < 0.25 mm (+ 60 mesh) and prepared for leaching experiments.
Before leaching experiments, a round-bottomed flask containing a specific concentration of sulfuric acid was placed in advance in a water bath with a pre-setting temperature. Then, 10 g of ground roasted ores was transferred into the flask, and the solution was stirred continuously for some time. After completion of the leaching, the leaching solution was filtered and the manganese in the filtrate was measured by ammonium iron (II) sulfate titrimetric method according to the national standards (GB/T 1506-2002). The leaching ratio of manganese was calculated from this result.
Unless otherwise noted, the roasting experiments including microwave roasting and conventional roasting experiments were carried out at the weight ratio of alkali lignin to ore of 70% and the roasting temperature of 200°C for 10 min, and the leaching experiments were conducted in a 2 mol/L sulfuric acid solution at the leaching temperature of 50°C, stirring speed of 300 rpm and the liquid-to-solid ratio of 10:1 for 10 min. The flowchart of the experiments refers to online supplementary material (see online supplementary Fig. S1).
Analysis Methods
XRD results were recorded by a Theta Rotating Anode X-ray Diffractometer using Cu Kα radiation at 10°/min and a step size of 0.02 from 10° to 80°. SEM images of all samples were obtained on a scanning electron microscopy (model TESCAN MIRA3 LMU/X-Max20/H1002). TOC (total organic carbon) values of leached liquors were measured by Shimadzu TOC-VCPH analyzer.
Results and Discussions
Effects of Roasting Parameters and Heating Modes on Leaching Ratio of Manganese
Effects of Weight Ratio of Alkali Lignin to Ore and Heating Modes
Figure 2a shows the effect of the weight ratio of alkali lignin to ore on the leaching ratio of manganese by microwave heating. Obviously, the leaching ratio of manganese by microwave heating increased rapidly with an increase in the weight ratio of alkali lignin to ore from 0% to 10%, and it became slower when further increasing the dosage of alkali lignin. At 70%, approximately 98.21% leaching ratio of manganese was obtained. Then, the tendency was gradually decreased when the ratio was > 70%. Figure 2b shows the XRD results of raw ore and roasted ores obtained by microwave heating with the addition of different dosages of alkali lignin. Figure 2b shows that many peaks of MnO phase accompanied by a small amount of Mn3O4 phase appeared in roasted ore by adding 70% alkali lignin while the peaks for MnO2 phase in raw ore disappeared; however, the peaks of MnO phase in roasted ore were gradually decreased on increasing the dosage of alkali lignin from 70% to 150%, whereas the peaks for Mn3O4 were enhanced, meaning that the reductive effect of manganese oxide ore at 70% was better than that at 150%, which could be used to explain the decrease in the manganese leaching ratio at a higher dosage of alkali lignin, shown in Fig. 2a. The reduction of manganese oxide ores by biomass was mainly attributed to the thermal degradation productions of biomass, such as liquid, non-condensable gas and solid, among which liquid was the primary reducing agent during the reductive roasting process of manganese oxide ores.37,38 Generally, biomass is not a good microwave absorber, and alkali lignin is one of them. Thus, in this article, the thermal degradation of alkali lignin by microwave heating can only be induced by mixing alkali lignin with manganese oxide ore since manganese oxides in ores are good microwave absorbers.39,40,41 According to the results shown in Fig. 2b, Mn4+ in ore was reduced into low valence states of Mn (MnO and Mn3O4) during the microwave roasting with the addition of a lower dosage of alkali lignin. However, further increasing the dosage of alkali lignin resulted in an oxidization of MnO, which might be related to the different oxidation–reduction properties of liquids produced by the microwave pyrolysis of alkali lignin. Investigations on pyrolysis of alkali lignin have been conducted in many studies, and phenolics, ketones and organic acids were the common liquid products.42,43,44 Abubakar et al. observed that the raw materials ratio had a significant influence on the compositions and yield of thermally degradation productions of biomass.45 Therefore, at a higher dosage of alkali lignin, the reduced MnO was likely to be oxidized to Mn3O4 by abundant oxidizing liquids, similar to the reaction process in the production of O2 from H2O2 using MnO2 as catalyst, where the MnO2 is first reduced and then oxidized by H2O2. The microwave pyrolysis mechanism of alkali lignin in the presence of manganese oxide ores and its effects on the reductive process will be investigated in a future study. According to the plot of leaching ratio of manganese as a function of the dosage of alkali lignin by microwave heating, the appropriate dosage range of alkali lignin was 30~90%, where the leaching ratio was > 90% (Fig. 2a). In addition, for comparison, the leaching results of roasted ores treated by conventional heating are also displayed in Fig. 2a, which shows that, compared with conventional heating, the microwave roasting of manganese oxide ore brought out a higher leaching ratio of manganese even in a lower dosage of alkali lignin, and the leaching ratio by microwave heating at 70% alkali lignin was 2.67 times higher than that by conventional heating, implying that microwave heating has a significant strengthening effect as a clean and efficient way to roast manganese oxide ore, which could be related to its unique heating mechanism with the advantages of non-contact heating, rapid heating, selective heating and volumetric heating.46,47,48
Effect of Roasting Temperature
Experiments were also performed at a roasting temperature from 50°C to 250°C to investigate the effect of roasting temperature on the leaching ratio of manganese, and the results obtained are shown in Fig. 3. As observed, the leaching ratio of manganese increased from 51.45% to 98.49% as the roasting temperature increased from 50°C to 150°C, after which it remained almost unchanged. To clearly observe the phase transformation of manganese at different roasting temperatures during the microwave heating process, manganese oxide reagent was used as a substitute for manganese oxide ore, and the XRD results of manganese oxide and roasted manganese oxides were shown in Fig. 3b, which shows that the peaks of MnO2 phase disappeared at 50°C, and no other obvious new peaks were observed, implying that the MnO2 phase was transformed into an amorphous phase on increasing the roasting temperature to 50°C that could not be detected by XRD analysis. As the temperature increased from 50°C to 100°C, some new peaks belonging to MnO phase occurred, and further increasing the temperature led to an enhancement of these peaks. According to the leaching curve shown in Fig. 3a, the maximum leaching ratio, 98.49%, was achieved in the roasted ore treated at a roasting temperature of 150°C. For comparison, the conventional roasting-leaching techniques for manganese oxide ores run at 1000–1350°C for almost 4–8 h.11,12,13
Effect of Roasting Time
The effect of roasting time on the leaching ratio of manganese was also studied, and the results are shown in Fig. 4. It indicated that the roasting time has little effect on the leaching ratio of manganese. Thus, 5 min was found to be sufficient for the reductive roasting of manganese oxide ore, and a 98.21% leaching ratio of manganese could be achieved under the conditions, meaning that compared to the conventional reductive roasting-acid processes of manganese oxide ores, the proposed technique could sharply shorten the roasting time needed to reduce manganese oxide ores.
Effects of Leaching Parameters on Leaching Ratio of Manganese
The effect of sulfuric acid concentration on the leaching ratio of manganese is depicted in Fig. 5a. The leaching ratio of manganese increased significantly with an increase in the sulfuric acid concentration ranging from 0 mol/L to 1.5 mol/L and then stayed almost unchanged when the concentration was > 1.5 mol/L, indicating that the acid leaching process had nearly reached equilibrium, and the optimal concentration of sulfuric acid is at 1.5 mol/L. The effect of leaching temperature on the leaching ratio of manganese is shown in Fig. 5b. The leaching temperature had a minimal effect on the leaching ratio of manganese. When the leaching temperature was > 50°C, the increase of manganese leaching ratio was slow. Therefore, considering economic and other factors, the optimum leaching temperature was chosen as 50°C. Figure 5c presents the effect of liquid-to-solid ratio on the leaching ratio of manganese. With increasing the liquid-to-solid ratio, the leaching performance was improved, especially in the lower liquid-to-solid ratio range. By increasing the liquid-to-solid ratio from 2/1 to 10/1, the leaching ratio of manganese increased from 51.06% to 98.21%. When the liquid-to-solid ratio was > 10/1, there was no significant difference in the leaching ratio of manganese. Considering the leaching cost, the optimum liquid-to-solid ratio was 10/1. The effects of leaching time and stirring speed on manganese leaching ratio are given in Fig. 5d and e, respectively. Both the leaching time and stirring speed had little influence on the leaching ratio of manganese, and 5 min and 50 rpm were found to be sufficient for the leaching of manganese during the acid leaching process. Therefore, a leaching time of 5 min and a stirring speed of 50 rpm were recommended in this study.
Full Flowsheet Test for the Reductive Roasting-Acid Leaching of Manganese Oxide Ore
According to the results mentioned above, microwave reductive roasting-acid leaching process using alkali lignin as reducing agent was carried out under the following optimum conditions: 50% dosage of alkali lignin, 150°C roasting temperature, 5 min roasting time, 1.5 mol/L sulfuric acid solution, 50°C leaching temperature, 50 rpm stirring speed, 10:1 liquid-to-solid ratio and 5 min leaching time. The analysis results of leached liquor are listed in Table II. This process shows that a leached liquor assaying 21.49 g/L Mn with a 97.43% leaching ratio of manganese was obtained. Furthermore, the leaching liquor contained lower values of metal ion impurities and TOC, thereby reducing the load of a downstream purification procedure.
To further observe the phase transformation and microstructure variation of manganese oxide ore during the proposed roasting-leaching process, the raw ore, roasted ore and leached residue were characterized by XRD and SEM analysis, respectively, and the results are shown in Figs. 6 and 7, respectively. From the XRD results shown in Fig. 6, the major phases in raw ore were MnO2, SiO2 and Al2SiO5, and after roasting process, MnO2 phases were no longer observed. The main Mn mineral phase was MnO, meaning that the reduction of MnO2 in manganese oxide ores was completed at a microwave roasting temperature of 150°C and a roasting time of 5 min using 50% alkali lignin as reducing agent. Then, the MnO phases disappeared in leached residue, indicating that MnO2 in raw ore was reduced and then entirely leached by sulfuric acid solution. Figure 7 shows the results of the SEM analysis. It was evident that the original ore had a smooth and dense structure, which is not conducive to the reaction, and the surface of the ore sample was rough and had many cracked holes after roasting. Compared with the SEM images of roasted ore by conventional heating using biomass as reductant reported by Zhang et al.,30 significant damage was observed for the microwave roasted ore, which may be the reason why the manganese in roasted ore obtained by microwave heating could be extracted by a lower concentration of sulfuric acid under a milder leaching condition (Fig. 5). Interestingly, after the leaching process, the surface of ore became smooth with porous embedded (see Fig. 7c), further confirming the successful leaching of manganese in roasted ore by acid leaching.
Roasting Behavior of Manganese Oxide
To clearly investigate the roasting behavior of manganese oxide in manganese oxide ore under the optimum conditions, the experiments were performed on manganese oxide reagent, which was used as a substitute for manganese oxide ore. First, based on the leaching ratio of manganese, the dosage of alkali lignin was optimized under the optimum conditions (150°C roasting temperature, 5 min roasting time, 1.5 mol/L sulfuric acid solution, 50°C leaching temperature, 50 rpm stirring speed, 10:1 liquid-to-solid ratio and 5 min leaching time), and the results are shown in Fig. 8a. Furthermore, the values of TOC of leached liquors were also measured, providing an important reference index for the optimization of alkali lignin dosage (Fig. 8a). It was apparent that increasing the weight ratio of alkali lignin to Mn in manganese oxide from 0.18 to 0.42 dramatically increased the leaching ratio of manganese from 6.66% to 68.53% with a slight increase of the value of TOC. Further increase of the dosage of alkali lignin to 1.06 caused a continued and slower increase of the leaching ratio of manganese, while for TOC, it was pretty much constant, implying that the majority of alkali lignin added participated in the reductive reaction with manganese oxide, achieving an improvement in the utilization ratio of alkali lignin. When further increasing the dosage of alkali lignin from 1.06 to 3.54, no significant increase in the leaching ratio was achieved, however, accompanied by a significant increase in TOC in leached liquor from 186.38 mg/L to 1218.02 mg/L. Therefore, considering the cost and utilization ratio of alkali lignin, an appropriate weight ratio of alkali lignin to Mn in manganese oxide was recommended as 1.06. Figure 8b shows the roasting behavior of manganese oxide during the roasting process under the optimum roasting conditions. It was apparent that by adding a 0.18 weight ratio of alkali lignin to Mn in manganese oxide, peaks attributed to MnO2 phase disappeared, while the phases of Mn2O3 and Mn3O4 were observed, meaning that MnO2 could be reduced by alkali lignin. Further increasing the dosage of alkali lignin resulted in the disappearance of phases of Mn2O3 and Mn3O4 in sequence and the appearance and enhancement of peaks of MnO phase. In conclusion, Mn in manganese oxide was reduced by alkali lignin in the microwave field in the following sequence: MnO2 → Mn2O3 → Mn3O4 → MnO.
Reduction Efficiency of Other Manganese Oxide Ores
To prove the technology proposed in this paper can be applied to other types of manganese oxide ores rather than Guangxi manganese oxide ore particularly, two other manganese content samples from different sources were tested to validate its universality under optimum conditions, and the results are shown in Fig. 9. From Fig. 9, the optimum dosage of alkali lignin for Gabon ore (60%) was 1.5 times higher than that for Xiangxi ore (40%). The leaching ratios of manganese were both > 96%, while for Guangxi ore (27.78%), the optimum dosage was 50%, demonstrating a positive correlation between the amount of alkali lignin and the content of manganese in the ore sample. The plots of the leaching ratio of manganese displayed a parabola similar to those in Fig. 2. In conclusion, the technology proposed in this paper was suitable for processing and utilization of different grades of manganese oxide ores and not just for the Guangxi manganese oxide ore sample.
Conclusion
A novel efficient and environmentally friendly reductive roasting-acid leaching technology using alkali lignin as reducing agent by microwave heating for recovering manganese from manganese oxide ores was proposed in this paper. The results indicated that manganese oxide ore can be reduced by alkali lignin by microwave roasting at a weight ratio of alkali lignin to ore 50% and a roasting temperature 150°C for 5 min, and under these roasting conditions, a leaching ratio of manganese of 97.43% was obtained from manganese oxide ore with Mn content of 27.78% using a sulfuric acid concentration 1.5 mol/L, leaching temperature 50°C, stirring speed 50 rpm and liquid-to-solid ratio of 10:1 for 5 min. Manganese oxide in manganese oxide ore can be reduced to MnO in the following sequence during microwave roasting process under optimum conditions: MnO2 → Mn2O3 → Mn3O4 → MnO. Moreover, the technology proposed in this paper was also proven suitable for utilizing different grades of manganese oxide ores and not just for Guangxi manganese oxide ore sample. This proposed method would potentially be a feasible route for processing and utilization of manganese oxide ores, which is also of great significance for offering a feasible way to utilize alkali lignin, easing the shortages of manganese and promoting the sustainable development of the steel and iron industry.
References
B. Das, S. Prakash, P.S.R. Reddy, and V.N. Misra, Resour. Conserv. Recycl. 50, 40–57 https://doi.org/10.1016/j.resconrec.2006.05.008 (2007).
P. Das, S. Upadhyay, S. Dubey, and K.K. Singh, J. Environ. Chem. Engin. 9, 105640 https://doi.org/10.1016/j.jece.2021.105640 (2021).
R. Zhang, X. Ma, X. Shen, Y. Zhai, T. Zhang, C. Ji, and J. Hong, J. Clean. Prod. 253, 119951 https://doi.org/10.1016/j.jclepro.2019.119951 (2020).
Od.S.H. Santos, Cd.F. Carvalho, GAd. Silva, and CGd. Santos, J. Environ. Manag. 147, 314–320 https://doi.org/10.1016/j.jenvman.2014.09.020 (2015).
Q.-Q. Lin, G.-H. Gu, H. Wang, R.-F. Zhu, Y.-C. Liu, and J.-G. Fu, Int. J. Miner. Metall. Mater. 23, 491–500 https://doi.org/10.1007/s12613-016-1260-x (2016).
J.D. Steenkamp, D. Chetty, A. Singh, S.A.C. Hockaday, and G.M. Denton, JOM 72, 3422–3435 https://doi.org/10.1007/s11837-020-04318-x (2020).
R. Elliott, K. Coley, S. Mostaghel, and M. Barati, JOM 70, 680–690 https://doi.org/10.1007/s11837-018-2769-4 (2018).
G. Chen, C. Jiang, and R. Liu, Sep. Purif. Technol. 277, 119472 https://doi.org/10.1016/j.seppur.2021.119472 (2021).
N. Toro, F. Rodríguez, A. Rojas, P. Robles, and Y. Ghorbani, Miner. Eng. 163, 106748 https://doi.org/10.1016/j.mineng.2020.106748 (2021).
V. Singh, T. Chakraborty, and S.K. Tripathy, Min. Proc. Ext. Met. Rev. 41(6), 417–438 https://doi.org/10.1080/08827508.2019.1634567 (2020).
A. Ganbari Arbat, E. Asghari Fesaghandis, A. Taghizadeh Tabrizi, and H. Aghajani, Trans. Indian Inst. Metals 73, 2355–2360 https://doi.org/10.1007/s12666-020-02036-1 (2020).
A. Shoghian-Alanaghi, A.J. Zamharir, H. Aghajani, and A.T. Tabrizi, Min. Metall. Explor. 39(4), 1753–1761 https://doi.org/10.1007/s42461-022-00642-9 (2022).
M. Petranikova, A.H. Tkaczyk, A. Bartl, A. Amato, V. Lapkovskis, and C. Tunsu, Waste Manag. 113, 521–544 https://doi.org/10.1016/j.wasman.2020.04.007 (2020).
A.P. Das, L.B. Sukla, N. Pradhan, and S. Nayak, Bioresour. Technol. 102, 7381–7387 https://doi.org/10.1016/j.biortech.2011.05.018 (2011).
Q. Zhao, L. Sun, G. Wang, C. Luo, Y. Shun, and K. Yan, Hydrometallurgy 189, 105113 https://doi.org/10.1016/j.hydromet.2019.105113 (2019).
O. Ostrovski, S.E. Olsen, M. Tangstad, and M. Yastreboff, Can. Metall. Q. 41, 309–318 (2002).
G. Akdogan, and R.H. Eric, Metall. Mater. Trans. B 26, 13–24 https://doi.org/10.1007/BF02648973 (1995).
R.K. Jana, B.D. Pandey, and Premchand, Hydrometallurgy 53, 45–56 https://doi.org/10.1016/S0304-386X(99)00031-6 (1999).
V. Nunna, S. Hapugoda, M.I. Pownceby, and G.J. Sparrow, Miner. Eng. 166, 106826 https://doi.org/10.1016/j.mineng.2021.106826 (2021).
W. Wei, Z. Shao, Y. Zhang, R. Qiao, and J. Gao, Appl. Therm. Eng. 157, 113751 https://doi.org/10.1016/j.applthermaleng.2019.113751 (2019).
Y. Sun, P. Gao, Y. Han, and D. Ren, Ind. Eng. Chem. Res. 52, 2323–2329 https://doi.org/10.1021/ie303233k (2013).
D.Q. Zhu, Y. Cui, K. Vining, S. Hapugoda, J. Douglas, J. Pan, and G.L. Zheng, Int. J. Miner. Process. 106–109, 1–7 https://doi.org/10.1016/j.minpro.2012.01.003 (2012).
G. Li, T. Shi, M. Rao, T. Jiang, and Y. Zhang, Miner. Eng. 32, 19–26 https://doi.org/10.1016/j.mineng.2012.03.012 (2012).
K.-O. Jang, V.R.M. Nunna, S. Hapugoda, A.V. Nguyen, and W.J. Bruckard, Miner. Eng. 60, 14–22 https://doi.org/10.1016/j.mineng.2014.01.021 (2014).
A. Mehdilo, and M. Irannajad, J. Ind. Eng. Chem. 33, 59–72 https://doi.org/10.1016/j.jiec.2015.09.018 (2016).
G. Li, M. Liu, M. Rao, T. Jiang, J. Zhuang, and Y. Zhang, J. Hazard. Mater. 280, 774–780 https://doi.org/10.1016/j.jhazmat.2014.09.005 (2014).
S. Agrawal, V. Rayapudi, and N. Dhawan, Miner. Eng. 132, 202–210 https://doi.org/10.1016/j.mineng.2018.12.012 (2019).
S. Agrawal, and N. Dhawan, Sustain. Mater. Technol. 27, e00246 https://doi.org/10.1016/j.susmat.2021.e00246 (2021).
Q. Ye, H. Zhu, L. Zhang, J. Ma, L. Zhou, P. Liu, J. Chen, G. Chen, and J. Peng, J. Alloys Compd. 613, 102–106 https://doi.org/10.1016/j.jallcom.2014.06.016 (2014).
Q. Ye, H. Zhu, L. Zhang, P. Liu, G. Chen, and J. Peng, RSC Adv. 4, 58164–58170 https://doi.org/10.1039/C4RA08010F).10.1039/C4RA08010F (2014).
Y. Sun, G. Fu, L. Jiang, and X. Cai, Min. Metall. Explor. 35, 215–220 https://doi.org/10.19150/mmp.8598 (2018).
S. Lin, R. Liu, and S. Guo, Renew. Energy 181, 714–724 https://doi.org/10.1016/j.renene.2021.09.055 (2022).
J. Ju, Y. Feng, H. Li, H. Yu, H. Wu, and S. Liu, Sustain. Chem. Pharm. 19, 100346 https://doi.org/10.1016/j.scp.2020.100346 (2021).
Y.-B. Zhang, Y. Zhao, Z.-X. You, D.-X. Duan, G.-H. Li, and T. Jiang, J Cent. South Univ. 22, 2515–2520 https://doi.org/10.1007/s11771-015-2780-7 (2015).
S. Yuan, W. Zhou, Y. Han, and Y. Li, Powder Technol. 361, 529–539 https://doi.org/10.1016/j.powtec.2019.11.082 (2020).
Y. Cao, Y. Sun, P. Gao, Y. Han, and Y. Li, Int. J. Min. Sci. Technol. 31, 1075–1083 https://doi.org/10.1016/j.ijmst.2021.09.008 (2021).
K. Li, J. Chen, G. Chen, J. Peng, R. Ruan, and C. Srinivasakannan, Bioresour. Technol. 286, 121381 https://doi.org/10.1016/j.biortech.2019.121381 (2019).
K. Li, G. Chen, J. Chen, J. Peng, R. Ruan, and C. Srinivasakannan, Bioresour. Technol. 291, 121838 https://doi.org/10.1016/j.biortech.2019.121838 (2019).
X. Wang, L. Mei, X. Xing, L. Liao, G. Lv, Z. Li, and L. Wu, Appl. Catal. B 160–161, 211–216 https://doi.org/10.1016/j.apcatb.2014.05.009 (2014).
F. Wu, Z. Cao, S. Wang, and H. Zhong, J. Alloys Compd. 722, 651–661 https://doi.org/10.1016/j.jallcom.2017.06.142 (2017).
F. Wu, J. Deng, B. Mi, Z. Xiao, J. Kuang, H. Liu, M. Liang, B. Liu, and P. Yu, Powder Technol. 356, 170–176 https://doi.org/10.1016/j.powtec.2019.08.020 (2019).
J. Geng, W.-L. Wang, Y.-X. Yu, J.-M. Chang, L.-P. Cai, and S.Q. Shi, Bioresour. Technol. 227, 1–6 https://doi.org/10.1016/j.biortech.2016.11.036 (2017).
D. Chen, K. Cen, X. Cao, F. Chen, J. Zhang, and J. Zhou, Renew. Sustain. Energy Rev. 136, 110444 https://doi.org/10.1016/j.rser.2020.110444 (2021).
D. Chen, Y. Wang, Y. Liu, K. Cen, X. Cao, Z. Ma, and Y. Li, Fuel 252, 1–9 https://doi.org/10.1016/j.fuel.2019.04.086 (2019).
Z. Abubakar, A.A. Salema, and F.N. Ani, Bioresour. Technol. 128, 578–585 https://doi.org/10.1016/j.biortech.2012.10.084 (2013).
B.T. Pérez-Martínez, M.A. Aboudzadeh, U.S. Schubert, J.R. Leiza, and R. Tomovska, Chem. Eng. J. 399, 125761 https://doi.org/10.1016/j.cej.2020.125761 (2020).
J. Huang, G. Xu, Y. Liang, G. Hu, and P. Chang, Fuel 266, 117022 https://doi.org/10.1016/j.fuel.2020.117022 (2020).
J. Li, J. Dai, G. Liu, H. Zhang, Z. Gao, J. Fu, Y. He, and Y. Huang, Biomass Bioenergy 94, 228–244 https://doi.org/10.1016/j.biombioe.2016.09.010 (2016).
Acknowledgements
This work was supported by Hunan Province Science and Technology Talent Support Project (grant number 2022TJ-N15); Key Scientific research project of Education Department of Hunan Province (grant number 20A245); General Project of Natural Science Foundation of Hunan Province (grant number 2021JJ30410, 2022JJ30348).
Author information
Authors and Affiliations
Contributions
JH: Conceptualization, Methodology, Formal analysis, Writing—review & editing, Funding acquisition; LC: Investigation, Data curation, Validation, Writing—original draft; JZ: Investigation, Data curation, Formal analysis; YZ: Investigation, Data curation; JZ: Conceptualization, Data curation; LC: Conceptualization, Data curation; WZ: Investigation, Data curation; HT: Investigation, Formal analysis; JY: Investigation, Data curation; FW*: Resources, Funding acquisition, Methodology, Supervision, Validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, J., Chen, L., Zhang, J. et al. A Process for Extraction of Manganese from Manganese Oxide Ores by a Novel and Efficient Roasting-Acid Leaching Technique. JOM 75, 3511–3520 (2023). https://doi.org/10.1007/s11837-023-05896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-05896-2