Abstract
Welding is an essential fabrication step during the construction of pipelines using high-strength low-alloy steels. The base metal experiences a rapid thermal excursion in the heat-affected zone, resulting in modification of the microstructure and mechanical properties. In particular, the coarse-grain heat-affected zone closest to the weld pool is of significance for the integrity of pipelines. In the present work, the microstructure and hardness were quantified from laboratory-simulated samples, obtained at different cooling rates for 12 steels, where their chemistry was systematically varied. Electron backscattered diffraction was used to quantify the bainitic microstructures based on the density of high-angle grain boundaries (HAGBs). It was observed that an increase of the cooling rate results in (1) a reduction of the transformation start temperature, (2) a larger density of HAGBs, and (3) an increase in hardness. A Hall–Petch-type relationship is proposed to link the hardness with the HAGB density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transmission of liquids and gases by pipelines is considered the safest and most cost-effective method to deliver these products to market.1 While the transport of oil and gas continues to play a major role in today’s energy system, there is also increasing interest to consider alternative fuels, such as hydrogen, natural gas/hydrogen mixtures, biofuels, and carbon dioxide (for sequestration technologies). As a result, new pipeline projects remain an important part of the energy ecosystem. In addition, the transport of carbon-based fuels increasingly occurs from remote locations where many of these resources are located. The combination of difficult terrain and hostile environments (seismic activity and/or low temperatures) constitutes a major challenge for steel makers and pipeline constructors to produce safe and economically sound projects.
Line pipe steels are low-carbon steels micro-alloyed with Nb, Ti, and/or V. The carbon content is generally kept below 0.1 wt.% to ensure good weldability, and the Ti and N levels are carefully controlled to prevent the formation of coarse TiN particles, which are detrimental to fracture properties. In general, the requirements for the steel are based on mechanical performance, i.e., yield stress, ultimate tensile stress, crack tip opening displacement, and ductile–brittle transition temperatures. This leaves a wide latitude for steel producers to tailor chemistry and thermomechanical controlled processing (TMCP), depending on their facilities to meet strength and toughness requirements for a particular pipeline project.2,3
Currently, the industry is focused on increasing the transmission capacity of new pipelines. This can be achieved by specifying larger pipe radii and/or higher gas pressure. The latter necessitates the use of higher strength steels, and increasing strength would be an attractive solution, except that this approach is limited by concerns regarding reduced toughness and hydrogen embrittlement4,5 of higher strength steels. Over the past decades, the majority of pipeline projects have employed X70 steel, with some limited applications of X80 and X90 steels (note: X70, X80, or X90 represents the yield stress in ksi, which is equivalent to 480 MPa, 550 MPa, and 620 MPa, respectively). Based on this experience and concerns about toughness, larger wall thickness has now become the preferred solution for achieving higher flow rates.
Submerged arc welding (SAW) and gas metal arc welding (GMAW) are the two most commonly used welding processes for fabricating pipes and the subsequent construction of pipelines. During welding, the region adjacent to the weld pool, known as the heat-affected zone (HAZ), experiences rapid thermal excursion, resulting in local changes to its microstructure and properties. In particular, the subregion closest to the weld pool, where temperatures approach the melting point of steel, is known as the coarse-grain heat-affected zone (CGHAZ). In the CGHAZ, most of the micro-alloying precipitates dissolve during the thermal excursion, with the exception of TiN-based particles, and, as a result, substantial austenite grain growth occurs. The CGHAZ has been identified as a potential region of low toughness and high ductile to brittle transition temperature.6,7,8,9,10 This has motivated systematic studies on the microstructural evolution in the CGHAZ and the resulting properties.
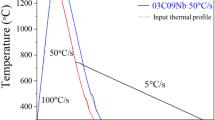
Different welding processes have different heat inputs. As a result, the thermal path experienced by the HAZ changes depending on the welding process. Welding trials11 using typical industrial conditions have shown that cooling rates from 800°C to 500°C for SAW and GMAW are approximately 5°C/s and 50°C/s, respectively. Due to the significantly different cooling rates associated with different welding methods, studying the microstructure evolution over a range of thermal paths relevant to different welding processes is of interest to both steel producers and pipeline manufacturers.
Further, as mentioned earlier, a particular line pipe grade can be manufactured with a combination of different steel chemistries and TMCP conditions. For example, the alloy content of current X70 line pipe steels vary as follows: 0.03–0.1 wt.% for carbon, 1.4–2.0 wt.% for manganese, 0.03–0.11 wt.% for niobium, 0.01–0.25 wt.% for chromium, 0.0–0.3 wt.% for molybdenum, 0.01–0.02 wt.% for titanium, up to 0.1 wt.% for vanadium, and 30–90 ppm for nitrogen. In addition, other alloying elements, such as silicon, nickel, and copper, can also exist. Therefore, a range of chemistries needs to be examined to quantify the role of these different alloying elements, in particular for the development and certification of acceptable welding procedures.
The present study aims to clarify the relationship between CGHAZ microstructures and their mechanical properties (in the current case, hardness as a measure of strength), using 12 specially cast steels that cover a chemistry range typical of X70/X80 line pipe steels and cooling rates relevant to welding of pipelines. In particular, the concentration of C, Nb, Cr, and Mo have been varied, as these elements are known to have a significant effect on the strength and toughness of line pipe steels.
Materials and Experimental Methodology
Materials
The materials used in this study were cast and hot-rolled in a laboratory-scale facility at CanmetMATERIALS, Hamilton, ON, Canada. Twelve different steels were examined, in which the C, Nb, Mo, and Cr contents were varied, as shown in Table I. Two different carbon contents (0.04 wt.% and 0.06 wt.%), three niobium levels (0.03 wt.%, 0.06 wt.%, and 0.09 wt.%), two different molybdenum contents (0.1 wt.% and 0.25 wt.%), and two chromium levels (0 wt.% and 0.25 wt.%) were examined. The Mn and Si levels were kept constant at ≈1.65 wt.% and 0.27 wt.%, respectively. The range of chemistries was selected based on a survey of steel chemistries found in X70/X80 line pipe steels currently being employed by the industry. The deviation from the nominal chemistries is a result of inherent variation caused due to the laboratory steel-making process. As shown in Table I, the Ti/N ratio for all the steels was maintained below the stoichiometric weight ratio of 3.42 (atomic ratio 1:1) to avoid the formation of coarse TiN particles, which can act as crack nucleation sites.12,13
Austenite Decomposition
A Gleeble 3500 thermomechanical simulator was used to conduct the thermal treatments under vacuum conditions (0.002 Pa) to avoid decarburization. Tubular specimens of 20 mm length with 8 mm outer diameter and 6 mm inner diameter were employed, and a Type K thermocouple was spot-welded to the midpoint of the sample length. The specimens were heated at 50°C/s to 1300°C, followed by an isothermal hold ranging between 10 s and 20 s to completely dissolve the Nb-rich precipitates and to obtain a prior austenite grain size of 60–80 μm,14 which is consistent with the grain size typically observed in the CGHAZ. Thereafter, the samples were cooled to 1000°C at 20°C/s, followed by cooling at constant rates of 3°C/s, 5°C/s, 10°C/s, 30°C/s, and 50°C/s. The cooling rate of 3°C/s was obtained under vacuum, while the faster cooling rates required helium gas quench assistance. A contact dilatometer was used to measure the change in dimensions of the samples during the heat treatment. The lever rule was used to calculate the fraction transformed from the dilation data during continuous cooling.
Characterization of Microstructures and Properties
The microstructures of the samples from the cooling experiments were characterized by electron backscatter diffraction (EBSD) mapping. For this purpose, the tubular samples were sectioned at the center at the location where the thermocouple was spot-welded. The sectioned specimens were prepared by mechanical polishing (to 1 µm diamond) followed by electropolishing, which was conducted using a solution of 95% acetic acid and 5% perchloric acid, and applying a 15-V potential difference across the sample for 15 s. EBSD mapping was then conducted using a Zeiss-Sigma field-emission scanning electron microscope at a 20-kV acceleration voltage. EBSD data were collected with an EDAX DigiView Camera and TSL orientation imaging microscopy (OIM) data collection software at a step size of 0.15 µm. Body-centered cubic (BCC) ferrite and face-centered cubic austenite were selected as the possible phases for automatic indexing by the TSL OIM Analysis software, v.6.2. The post-processing of the EBSD data included a single-step cleaning using a grain dilation algorithm, with a grain tolerance angle of 5° and a minimum grain size of two pixels. A BCC grain boundary was identified as a high-angle grain boundary (HAGB) for a misorientation angle greater than 15°. The Micro-Vickers hardness of all the continuous cooling transformation samples was measured at 1-kgf load, applied for a dwell time of 10 s. At least five indents were made for each condition. A minimum of three diagonal lengths were maintained between the indents as well as to the edge of the sample.
Results and Discussion
The transformation start temperature was defined as the temperature at which 5% of the austenite decomposition has occurred. Figure 1 shows an example for Steel I of the dependence of the transformation start temperature and hardness on the cooling rate. Here, it can be observed that the transformation start temperature decreases from ≈595°C to ≈525°C when the cooling rate increased from 3°C/s to 50°C/s. In parallel, the hardness increased from ≈215 Hv to ≈300 Hv for the same increase in cooling rate. These observations are consistent with well-established trends of the role of cooling rate on transformation temperatures and hardness for a wide range of steels9,15,16,17,18
Figure 2 shows EBSD inverse pole figure (IPF) maps obtained for Steel I at three different cooling rates. The microstructure changes from a blocky structure at a lower cooling rate to a finer elongated lath-like structure at higher cooling rates. A similar trend was observed for the other steels. As for Steel I, all the microstructures were observed to be completely bainitic. For the leaner chemistries, i.e., Steels A, B, C, and F, at a low cooling rate, the transformation start temperature is higher than 630°C, and, in these cases, ferrite films form at the prior austenite grain boundaries, i.e., these microstructures are not completely bainitic.
These microstructure observations are consistent with the bainite nucleation temperatures that were calculated to fall between 640°C and 650°C for all the 12 steels, as obtained with Thermo-Calc using database TCFE7. These calculations are based on the T0 concept, i.e., the temperature at which, for the given steel chemistry, the Gibbs free energies of austenite and ferrite are equal, assuming that a driving pressure of 400 J/mol is required for bainite formation to start at the temperature \(T_{0}^{^{\prime}}\), as had been proposed by Bhadeshia.19
To quantify the microstructure, HAGB maps were constructed from the EBSD data. Figure 3 shows the HAGB maps for Steel I at cooling rates of 3°C/s, 10°C/s, and 50°C/s. As the cooling rate increases, the microstructure becomes refined, i.e., there is an increase in the length of HAGBs. To normalize the data, the HAGB density is defined as:
and a characteristic length scale of the microstructure can be defined as the reciprocal of the HAGB density.
As can be observed in Fig. 3, the HAGB density for Steel I increases from 0.28 μm−1 to 0.99 μm−1 as the cooling rate increases from 3°C/s to 50°C/s, and similar observations were made for all the steels examined. A larger value of the HAGB density corresponds to a finer characteristic bainitic lath scale. Turning to the mechanical properties, i.e., here hardness, a first approximation suggests that the hardness increases following a Hall–Petch-type relationship, where the grain size is replaced by the characteristic length of the bainitic microstructure. As such, the hardness, Hv can be written in terms of the HAGB density as:
where \({\text{Hv}}_{o}\) is the hardness for a material with no HAGBs and \(k\) is a constant. Figure 4 shows a plot of the hardness data as a function of the square root of \(N_{A}^{{{\text{HAGB}}}}\) for all the 12 steels considered. A linear regression fit to the data gives values of \({\text{Hv}}_{o}\) = \(129\) kgf/mm2 and k = 181 kgf/mm2 μm0.5 with a correlation coefficient, R2, of 0.94. Note that the data for low cooling rates in the leaner chemistries where the microstructures were not completely bainitic were not included in this analysis.
Relationship between high-angle grain boundary density and hardness for the completely bainitic microstructures. Low-carbon (~0.04 wt.% C) and high-carbon (~0.06 wt.% C) steels are shown using hollow and solid markers, respectively. Group a includes data for low- and high-carbon steels cooled at 3°C/s, 5°C/s, and 10°C/s, group b consists primarily of low-carbon steels cooled at 30°C/s and 50°C/s, while group c contains high-carbon steels cooled at 30°C/s and 50°C/s.
It is apparent from Fig. 4 and its analysis that Eq. (2) represents a reasonable approach to describe the dependence of the hardness on the dominant microstructural feature, i.e., HAGB density. All the hardness values calculated using Eq. (2) are within a 5% deviation of the measured values, except for Steel B at 50°C/s, which has a deviation of 6%. It is worth noting that Eq. (2) is independent of chemistry, i.e., within the range of chemistry examined, the relationship can be directly used to compute hardness from the microstructure.
Figure 4 also shows that the data can be grouped into three different brackets, based on the cooling rate and the carbon content. Data in group a correspond to lower cooling rates (3°C/s, 5°C/s, and 10°C/s), typical for welding processes with high heat input like SAW, irrespective of the carbon content. Group b contains all the data examined for the low-carbon steels at higher cooling rates (30°C/s and 50°C/s). Note that a single data point in group b belongs to the high-carbon Steel I cooled at 10°C/s. Finally, group c includes data for high-carbon steels cooled at faster cooling rates (30°C/s and 50°C/s), typical for low-heat input welding processes, such as GMAW.
These observations suggest that an increase in HAGB density and hardness with an increase in cooling rate is significantly more pronounced for the high-carbon steels (~0.06 wt.% C) compared to the low-carbon steels (~0.04 wt.% C). Furthermore, the effect of increased carbon content on the microstructure and hardness is relatively small at the lower cooling rates, but becomes significant at higher cooling rates. A comparison between the low-carbon steel D (0.035 wt.% C) and the high-carbon Steel I (0.061 wt.% C) shows that the hardness increases by approximately 5 Hv at 3°C/s, compared to an increase of 45 Hv for a cooling rate of 50°C/s.
The effect of the other three alloying elements examined, i.e., Nb, Mo, and Cr, on the hardness and microstructure is relatively small, irrespective of the cooling rate. For example, an increase in the niobium content from 0.032 wt.% in Steel A to 0.1 wt.% in Steel F results in a maximum hardness increase of 12 Hv. Similarly, on increasing the molybdenum content from 0.11 wt.% in Steel C to 0.25 wt.% in Steel D, the hardness increases by about 12 Hv for all the cooling rates examined. Finally, with an increase in the chromium content from 0.023 wt.% in Steel D to 0.24 wt.% in Steel E, the hardness increases approximately 5 Hv for all the cooling rates. Based on these observations, increasing the carbon content, but not of Nb, Mo, and Cr, is an effective way to obtain a refined microstructure with high hardness in the CGHAZ for welding processes with higher cooling rates, like GMAW. For low cooling rate processes like SAW, the effect of change in the alloying content of C, Nb, Mo, and Cr on the microstructure and hardness of the CHGAZ is relatively small.
The proposed relationship between HAGB density and hardness is a simple but effective structure–property relationship for the bainitic microstructures expected in the CGHAZ of line pipe steels. Thus, microstructure refinement is a primary strengthening mechanism in the CGHAZ of line pipe steels. For the cases examined in the present study, the HAGB density varies between 0.18 μm−1 and 0.99 μm−1. The contributions of other strengthening mechanisms (solid solution, precipitation, dislocations) remain, on the other hand, relatively constant independent of steel chemistry and simulated welding procedures, such that they are all included in the constant Hvo. Based on Eq. (2), the contribution of grain refinement to the total hardness is 37% for the microstructure with the lowest HAGB density, i.e., 0.18 μm−1, and 58% for the one with the highest HAGB density, i.e., 0.99 μm−1. This is consistent with the analysis of Lu et al.,20 who found that the contribution of grain-size strengthening increases from 45% for X70 steel to 50–60% for X80 steel, and 70–75% for X100 steel i.e., the contribution of microstructure refinement towards strength increases as the microstructures get progressively finer from X70 to X100 steel. Further, Maetz et al.21 investigated four different Nb-Mo low-carbon steels for simulated hot-rolling conditions, and found about 65% of the total strength can be correlated with microstructure refinement for the as-quenched conditions, which were representative for low cooling-temperature (≈500°C) simulations with limited precipitation strengthening, reaffirming microstructure refinement as an important strengthening mechanism for micro-alloyed low-carbon steels.
Further, it had been shown that the toughness of low-carbon steels with bainitic microstructures increases with HAGB density.10,22 As toughness is a critical design criterion for pipeline operation, future work will include the measurement of Charpy impact energies and transition temperatures for the 12 steels of this study.
Conclusion
The microstructures from experimental simulations of conditions relevant to the CGHAZ in X70/X80 line pipe steels are predominantly bainitic, and the properties, at least in terms of hardness, are primarily affected by the cooling rate, which is directly linked to the heat input of the welding process. This study examined 12 steels in which the C, Nb, Mo, and Cr contents were varied, and it has been shown that the HAGB density increases with the cooling rate. For the investigated chemistry range, the hardness of the observed bainitic microstructures can be correlated with the HAGB density using a Hall–Petch-type relationship. Increasing the nominal carbon content from 0.04 wt.% to 0.06 wt.% was identified as the primary factor to obtain a markedly higher hardness (above 270 Hv) in the CGHAZ for GMAW conditions. In contrast, the studied variation of steel chemistry appears to be of the second order for the CGHAZ hardness under SAW conditions.
Future work includes expanding the quantitative relationship of the microstructure with mechanical properties like tensile strength and toughness. In addition, work is being carried out to link the transformation start temperature to the resultant microstructure. Then, the development of a chemistry-sensitive model to predict the transformation kinetics will provide an important tool to build a complete model linking the chemistry–structure-property for the CGHAZ of line pipe steels.
References
D. Furchtgott-Roth, Pipelines are safest for transportation of oil and gas. https://www.manhattan-institute.org/html/pipelines-are-safest-transportation-oil-and-gas-5716.html. Accessed 22 Dec 2021
S. Vervynckt, K. Verbeken, B. Lopez, and J.J. Jonas, Int. Mater. Rev. 57, 187. (2012).
T.N. Baker, Ironmak. Steelmak. 43, 264. (2016).
N.E. Nanninga, Y.S. Levy, E.S. Drexler, R.T. Condon, A.E. Stevenson, and A.J. Slifka, Corros. Sci. 59, 1. (2012).
T.Y. Jin, Z.Y. Lui, and Y.F. Cheng, Int. J. Hydrogen Energy 35, 8014. (2010).
C. Li, Y. Wang, T. Han, B. Han, and L. Li, J. Mater. Sci. 46, 727. (2011).
X.L. Wang, Z.Q. Wang, L.L. Dong, C.J. Shang, X.P. Ma, and S.V. Subramanian, J. Mater. Sci. Eng. A 704, 448. (2017).
X.L. Wang, Z.Q. Wang, X.P. Ma, S.V. Subramanian, Z.J. Xie, C.J. Shang, and X.C. Li, Mater. Charact. 140, 312. (2018).
Y.Q. Zhang, H.Q. Zhang, J.F. Li, and W.M. Liu, J. Iron Steel Res. Int. 16, 73. (2009).
M. Mandal, Ph.D. Thesis, The University of British Columbia, Vancouver (2020). https://doi.org/10.14288/1.0389717
M. Gaudet, Ph.D. Thesis, The University of British Columbia, Vancouver (2020). https://doi.org/10.14288/1.0166539
S.C. Wang, J. Mater. Sci. 24, 105. (1989).
W. Yan, Y.Y. Shan, and K. Yang, Metall. Mater. Trans. A 37, 2147. (2006).
N. Romualdi, M. Militzer, W. Poole, L. Collins, and R. Lazor, Proc. ASME Int. Pipeline Conf. https://doi.org/10.1115/IPC2020-9687 (2020).
S. Zhao, D. Wei, R. Li, and L. Zhang, Mater. Trans. 55, 1274. (2014).
S.W. Thompson, D.J. Colvin, and G. Krauss, Metall. Mater. Trans. A 27, 1557. (1996).
I.D.G. Robinson, T. Garcin, W.J. Poole, and M. Militzer, Proc. ASME Int. Pipeline Conf. https://doi.org/10.1115/IPC2016-64509 (2016).
J.M. Reichert and M. Militzer, HSLA Steels 2015, Microalloying 2015 Offshore Eng. Steels 2015, Conf. Proc. (2015). https://doi.org/10.1007/978-3-319-48767-0_12
H.K.D.H. Bhadeshia, Acta Metall. 29, 1117. (1981).
J. Lu, O. Omotoso, J.B. Wiskel, D.G. Ivey, and H. Henein, Metall. Mater. Trans. A 43, 3043. (2012).
J.Y. Maetz, M. Militzer, Y.W. Chen, J.R. Yang, N.H. Goo, S.J. Kim, B. Jian, and H. Mohrbacher, Metals 8, 758. (2018).
C. Wang, M. Wang, J. Shi, W. Hui, and H. Dong, Scr. Mater. 58, 492. (2008).
Acknowledgements
The authors acknowledge the Natural Sciences and Engineering Research Council (NSERC) of Canada, Evraz Inc. NA, and TC Energy for funding the project. We would like to thank CanmetMATERIALS, ON, for providing the steels for this project, and we would like to also acknowledge that this work was undertaken, in part, thanks to funding from the Canada Research Chair program (Poole).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roy, S., Romualdi, N., Yamada, K. et al. The Relationship Between Microstructure and Hardness in the Heat-Affected Zone of Line Pipe Steels. JOM 74, 2395–2401 (2022). https://doi.org/10.1007/s11837-022-05280-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05280-6