Abstract
This study was conducted to investigate the kinetics of dissolution of pure thorium silicate (thorite mineral). This compound was synthesized by the hydrothermal method with a purity of 97.8%. The parameters affecting the dissolution kinetics of synthetic thorium silicate were optimized. The agitation speed of 450 rpm, sulfuric acid concentration of 4 M, and temperature of 100°C were determined as optimal conditions. According to scanning electron microscopy (SEM) images, as well as dynamic light scattering (DLS) and Brunauer–Emmett–Teller (BET) analyses before and after dissolution, the dissolution kinetics model of thorium silicate was determined based on the shrinking particle model. Using the Arrhenius equation, the activation energy was calculated to be 78.41 kJ/ mole. The thorium silicate dissolution mechanism was identified based on chemical control reaction. The reaction order of the concentration of sulfuric acid at different temperatures was 1.64 ± 0.44.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The abundance of thorium is 3–4 times more than uranium and is widely distributed in nature as an easily exploitable resource in many countries, but it has not thus far been commercially exploited. Concerning the decline of uranium ore mining resources in the future, the supply of nuclear energy from alternative sources will be one of the concerns of countries. In addition, the presence of only low levels of uranium in some countries has led them to seek other sources of radioactive material. Hence, the thorium fuel cycle is being considered by countries having thorium reserves in their long-term nuclear energy planning.
Unlike natural uranium, which contains ~0.7% ‘fissile’ 235U isotope, natural thorium does not have any ‘fissile’ material and is composed of the ‘fertile’ 232Th isotope. 232Th is converted to 233Pa and finally to 233U with consecutive β decays. Thorium nuclear fuels are an attractive way to produce long-term nuclear energy with low radio-toxicity waste.1 Thorium is found in nature as various structures, such as an oxide, phosphate, and silicate. Among these, thorite (ThSiO4) is the most abundant thorium mineral with an orthosilicate structure, and is isostructural with zircon (ZrSiO4) and coffinite (USiO4)2,3,4,5 crystallized in the tetragonal system. In examining the dissolution kinetics, it should be noted that dissolution processes are heterogeneous reactions involving the mass transfer of the reactants and ion products during one or more reaction steps. The most important in choosing a good model is to have the conditions closest to reality and without too much mathematical complexity. Accordingly, among dissolution kinetic models, the shrinking core/particle kinetics model has the mechanism of diffusion control from liquid film or an ash layer, and the chemical reaction control was investigated in the thorium silicate dissolution process. According to this model, the reaction first occurs in the outer layer of the particles, and then the reaction zone is transferred to the solid. Assuming spherical particles, this model has been developed in five successive reaction steps, including diffusion of the reactant from the liquid film around the particles to the solid surface, diffusion of the reactant from the ash layer to the unreacted surface, the reaction of the reactant to the solid surface, diffusion of the aqueous product from the ash layer to the outer solid surface, and diffusion of the aqueous product film to the solution. In the shrinking particle model, due to lack of an ash layer, there is no diffusion from this layer.6 In previous works, the dissolution kinetics of various thorium compounds, such as thorium oxide (thorianite)7,8,9,10,11,12,13,14 and thorium phosphate (monazite),15,16 has been studied, but no reports or articles about the dissolution kinetics of thorium silicate (thorite) have been found. Therefore, it was decided to study the kinetics and control mechanism of thorium silicate dissolution. According to the identification of thorium silicate sources, the behavior of this compound in leaching processes should be investigated and compared with a pure sample. Since a pure sample of thorium silicate was not available, the compound was synthesized in the laboratory and its dissolution kinetics was investigated.
To identify appropriate ligands for the dissolution of thorium oxide, Hubert et al.7 investigated ClO4-, Cl-, NO3-, and SO42- ligands. Accordingly, the effect of sulfuric acid on the dissolution of thorium was higher than that of other ligands. Since no oxidation and reduction reaction is expected for Th4+, they determined the dissolution mechanism of ThO2 based on complexation at the solid/solution interface. In investigating the effect of sulfate ions on the dissolution of uranium, thorium, yttrium, and rare-earth elements from Denison Eliot Lake conglomerate rock, Sapsford et al.8 found that, in the absence of sulfate at pH 1.5, the insoluble compound of ThO2 was formed, but with the addition of sulfate, the solubility of thorium was increased. Accordingly, sulfuric acid has been selected as the solvent for thorium silicate in our dissolution kinetics experiments.
In examining the dissolution kinetics and the mechanism, Heisbourg et al. investigated the dissolution kinetics of Th1-xUxO2 solid solutions in nitric media. They found that an increase in the mole fraction of uranium in the solid solution caused an increase in the kinetics rate and reaction order.9 They proposed a dissolution mechanism in three steps as oxidation of the uranium site on the surface, then fast protonation of different sites from the surface layer, and finally detachment of metal ions.9,10 In thorium oxide, the first stage of the dissolution mechanism was eliminated.9 They showed that nitrate ions did not influence the dissolution mechanisms.9 They found that the increasing mole fraction of thorium in the solid solution resulted in the formation of a protective layer of hydrated thorium oxide or thorium hydroxide on the surface, which eventually reduced the reaction rate and kinetics. According to the activation energy of 20 ± 3 kJ/ mole, the dissolution mechanism was identified based on diffusion control through a thin layer of thorium hydroxide.11 In a study conducted by Claparede et al. on the effect of temperature and concentration of nitric acid on the dissolution of thorium, the best conditions for dissolution were obtained at 90°C and an acid concentration of 6 molars.10 These conditions were selected as preliminary conditions for the dissolution of pure thorium silicate.
In the dissolution of ThO2 by the HNO3-HF mixture, Simonnet et al.12,13 observed that fluoride ions, along with nitric acid, led to the best dissolution rate of thorium compared to other compounds, such as oxalate and sulfate ions. They also found that the presence of an optimal concentration of HF was essential for the dissolution of ThO2. In fact, if HF is not added to the system or too much is added, complete dissolution of thorium will not be achieved. The results also indicated that high concentrations of HNO3 caused faster dissolution, but that this acid could not lead to complete dissolution of thorium oxide without the presence of HF, revealing the synergistic effect of HF and HNO3. Based on the obtained activation energy values (63–80 kJ/mole), they predicted the reaction mechanism as an adsorption reaction with a catalytic reaction. Keshtkar and Abbasizadeh14 predicted the dissolution mechanism of ThO2 in the HNO3-HF mixture according to the surface chemical reaction at the solid/solution interface. In a similar case, Takeuchi et al.15 identified the dissolution of thorium as a reaction of the surface sites of ThO2 to the formation of a fluoride surface as a precipitate, then adsorption of the nitric acid molecule on the precipitation surface.
Arinicheva et al.16 showed that the dissolution kinetics of monazite-LaPO4 was controlled by the chemical reaction in nitric acid at the temperature range of 50–90°C. However, Gausse et al.17 investigated the dissolution mechanism at different temperature ranges. At temperatures below 313 K, the dissolution process is controlled by the chemical reaction and in the temperature range of 313–363 K, controlled by diffusion.
Based on studies conducted by Hubert et al.,7 Sapsford et al.,8 and Klapardeh et al.,10 preliminary conditions for the dissolution of pure thorium silicate were selected by a sulfate ligand such as sulfuric acid in a concentration of 6 M and at a temperature of 90°C. According to this, parameters affecting the dissolution, including agitation speed, acid concentration, and temperature, were optimized. Then, the kinetics of dissolution of thorite was determined in sulfate media.
Experimental
Synthesis and Preparation of Thorium Silicate
Pure thorium silicate (thorite) was synthesized by using the hydrothermal method with a mixture of 0.14 M thorium chloride (ThCl4) solution and sodium silicate containing 0.14 M SiO2.18 According to the method proposed by Costin et al.,19 in the first stage, thorium tetrachloride (ThCl4) was prepared by multiple successive batches of dissolution of hydrated thorium nitrate (Th(NO3)4.5H2O) in 8 M HCl and evaporation, resulting in the elimination of nitrate and the substitution of chloride. Furthermore, 0.14 M ThCl4 solution was added dropwise to a 0.14 M SiO2 solution at a volumetric ratio of SiO2:ThCl4 0.9. Afterward, 8 M NaOH solution was added to the mixture dropwise to reach a pH of 8–8.5. At this point, a white gelatinous phase was formed. Finally, the pH of the gelatinous phase was buffered around 8–9 by adding 0.5 M NaHCO3. The gel containing Th(OH)4 and Si(OH)4 was poured into a PTFE Footnote 1autoclave (volume = 200 ml), and then was heated to 250°C for 24 h. After heating, the thorium silicate precipitate was filtered from the supernatant solution. To remove soluble compounds and probably organic compounds, the precipitate was washed with deionized water and ethanol. Finally, the precipitate was dried at 60°C for 24 h. The flow diagram of thorium silicate synthesis is shown in Fig. 1. To evaluate the characteristics quality of the synthesized sample, diagnostic analyzes were conducted. Figure 2 shows the XRD analysis of the synthesized thorium silicate. Thorium silicate and thorium oxide were identified in the sample, and by measuring the atomic ratio of Th:Si using energy dispersive x-ray spectroscopy (EDXS) analysis, the chemical composition of the sample was identified as Th1.03±0.18SiO4. The sample purity and the average particle size of the synthesized thorium silicate were determined as 97.80% and 731.30 nm, respectively.
Dissolution test Procedure
Dissolution experiments on pure thorium silicate were conducted in a 1-L flat-bottomed balloon with four ports. The main port was utilized for the mechanical stirrer, and the three other ports were used for the inclusion of the condenser tube, thermometer, and feeding/sampling. The reaction vessel was heated via a Heidolph MR 3001 K magnetic stirring hotplate.
The procedure for all the dissolution experiments was as follows:
-
1.
Addition of 500 ml of sulfuric acid solution with a certain concentration to the balloon;
-
2.
Mechanical mixing of the acidic solution with an agitation speed of 450 rpm;
-
3.
Adjustment of the temperature of the solution in the desired temperature range (temp ± 3°C);
-
4.
Addition of synthetic thorium silicate to the acid solution and the start of the test;
-
5.
Sampling of 3 ml of a combination of solid and liquid at specified time intervals by pipette;
-
6.
Centrifuging of samples collected for separation of liquor from residual solid.
The solutions were analyzed for the concentration of Th using inductively coupled plasma atomic emission spectroscopy (Optima 2000; Perkin-Elmer). The analysis error of measuring thorium is ±10%.
The morphology of thorium silicate before and after dissolution was determined by scanning electron microscope (SEM). EDXS analysis was conducted on a ZEISS EVO 18 SEM operating at 25 kV with 200-nm resolution at a working distance of 8.5 mm and an EDAX SSD detector. The powdery sample was pressed onto an adhesive carbon tab, and then coated with gold.
To measure the surface area, volume, and size of the pore, Brunauer–Emmett–Teller (BET) analysis was conducted on a Quantachrome Nova Win2 instrument. The sample was degassed for 4 h at a temperature of 290°C. Nitrogen was used as the analysis gas.
Size distribution of the sample was determined by a Malvern Zetasizer Nano ZS (red badge) (ZEN 3600; Malvern, UK). To measure the size distribution, a diluted suspension of the sample was prepared. Then, 2 mL of this mixture was poured into a polymer cuvette and inserted into the DLS instrument to measure the size distribution. The temperature of the measurement was set at 25°C.
The general conditions of dissolution experiments for synthetic thorium silicate are as follows:
-
Sulfuric acid concentration (molar): 1–8
-
Solvent volume (ml): 500
-
Sample weight (mmole): 2.16
-
Dissolution temperature (°C): 70, 80, 90, 100
-
Mechanical agitation speed (rpm): 450
Results and Discussion
Effect of Agitation Speed on Dissolution of Thorium Silicate
The dissolution experiments were performed at several agitation speeds of 150 rpm, 300 rpm, 450 rpm, and 600 rpm, and without agitation to select the appropriate agitation speed. Figure 3 shows the percentage of thorium dissolved at different agitation speeds.
Figure 3 shows that the thorium dissolution was increased with increasing the agitation speed up to 450 rpm, and it was decreased as the agitation speed was increased to 600 rpm. This behavior can be attributed to an increase in the centrifugal force and a decrease in the contact of the solvent with the solute. Hence, the mechanical mixing at 450 rpm was selected for further dissolution test.
Effect of Sulfuric Acid Concentration on Dissolution of Thorium Silicate
To investigate the effect of sulfuric acid concentration on the dissolution of pure thorium silicate, the experiments were performed at a temperature of 90°C and sulfuric acid with a concentration of 1–8 M. Figure 4 shows the effect of the sulfuric acid concentration at 90°C on thorium dissolution.
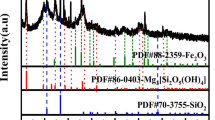
As observed, with increasing the sulfuric acid concentration up to 8 M, the rate of thorium dissolution was increased. In the sulfuric acid concentration higher than 4 M, the thorium levels were decreased after a while in the solution. Accordingly, an appropriate concentration of sulfuric acid was selected at 4 M for the dissolution of thorium silicate. A noteworthy point in the dissolution conditions was that at sulfuric acid concentrations lower than 4 M, with the completion of the dissolution process, the solution became clear. In sulfuric acid concentrations higher than 4 M, after a while, a white gelatinous compound was formed. The entry of thorium into the aqueous phase by washing the gelatinous precipitate with distilled water showed that thorium was in the gelatinous precipitate phase. This behavior indicated that the thorium was dissolved from the pure thorium silicate compound, but that it entered the precipitate phase for unknown reasons. To investigate this phenomenon, the formed precipitate was filtered and dried. Table I and Fig. 5 show the XRF and XRD analysis of the gelatinous precipitate at \(\text{C}_{\text{H}_{2}\text{S{O}}_{4}}\)= 8 M, respectively. As can be seen, thorium and sulfur were identified as the main elements in the precipitate. The XRD analysis also identified Th(SO4)2.4H2O in white color as the predominant compound. In the study of various species and components formed in the thorium dissolution process with sulfuric acid, the mononuclear complexes of thorium sulfate as Th(SO4)n4-2n with n = 1–4 and three different thorium compounds in various sulfuric acid concentrations are formed. Th(SO4)2.9H2O (cr) forms from low concentrations to 3.9 M H2SO4, and Th(SO4)2.8H2O (cr) forms from 6.1 to 7.1 M as well as Th(SO4)2.4H2O (cr) at 11.2 M H2SO4 forms at 25°C.20
The XRD analysis of precipitate formed during the dissolution of thorium silicate with H2SO4 = 8M shows Th(SO4)2.4H2O as the major compound in the gelatinous precipitate. Since the color of this compound is white, the precipitate formed is also white. The reason for the gelatinization of the precipitate can be due to the increase of the H+ concentration and the ionic strength of the medium, leading to formation of colloidal particles and then three-dimensional networks of silica gel and amorphous silica in the solution.21,22 In general, with increasing the concentration of sulfuric acid (increasing H+ concentration), two phenomena were observed in the dissolution process:
-
1.
Formation of thorium sulfate hydrated crystals such as Th(SO4)2.4H2O
-
2.
Faster formation of three-dimensional networks of silica gel
As shown in Fig. 4, behavior change in dissolution process can be due to the faster formation of silica gel with increasing H+ concentration, which leads to trapped thorium sulfate hydrated crystals in the silica gel structure and ultimately reduces the concentration of thorium in the resulting solution.
The general reaction of dissolution of thorium silicate in different concentrations of sulfuric acid could be as below:
Effect of Temperature on Dissolution of Thorium Silicate
Thorium dissolution from pure thorium silicate was investigated at temperatures of 70°C, 80°C, 90°C, and 100°C in the sulfuric acid concentration of 4 M. Figure 6 shows the evolution of thorium dissolution for pure thorium silicate at different temperatures. As observed, with increasing the temperature from 70°C to 100°C, the dissolution kinetics of thorium becomes faster. Accordingly, the highest dissolution rate was observed at 100°C and selected as the optimal temperature for kinetics studies of thorium silicate leaching.
Identification of Dissolution Kinetics and Mechanism of Thorium Silicate
To identify the dissolution kinetics model of thorium silicate, the dissolution test was conducted under the conditions of 4 M sulfuric acid concentration, temperature of 100°C, and mixing time of 60 min at a mechanical agitation speed of 450 rpm. SEM images in Fig. 7 show a significant reduction in particle size after dissolution.
With DLS and BET analyses, the surface area and the particle size of the sample, before and after dissolution were determined 20.52 m2/g, 40.15 m2/g, 731.34 nm, and 195.6 nm, respectively. These results confirmed the reduction of the particle size in the dissolution process.
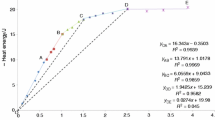
Owing to the reduction of the particle size in the dissolution process and the complete dissolution of particles at the end of the process, the dissolution process should follow the shrinking particle kinetics model. By assuming spherical particles, the shrinking particle kinetics models were fitted for the dissolution kinetics curves at temperatures of 70°C, 80°C, 90°C, and 100°C and the sulfuric acid concentration of 4 M. Figure 8a and b show the fitted model based on the two mechanisms of the diffusion control from the liquid film layer or the chemical control reaction of the dissolution process.
Kinetics parameters of thorium silicate dissolution. (a, b) Plot of shrinking particle kinetics model based on diffusion control from the liquid film layer and chemical reaction control, respectively. (c) Arrhenius plot for thorium silicate dissolution based on shrinking particle kinetics model with two mechanism control. (d–f) Plot of shrinking particle kinetics model based on chemical control reaction at temperatures of 70°C, 90°C, and 100°C. (g) Log [H2SO4] versus log k based on shrinking particle kinetics model with chemical reaction control at different temperatures (Vsolution = 500 ml, \(C_{{{\text{H}}_{2} {\text{SO}}_{4} }} \)= 4 M, \(M_{{{\text{ThSiO}}_{4} }} \)= 2.16 mmole, agitation speed = 450 rpm).
According to Fig. 8a and b, both models properly fitted the diffusion control from the liquid film layer or the chemical control reaction (R2 > 0.95). Therefore, to identify the appropriate kinetics model, it is necessary to determine the activation energy for both kinetics models. Table II shows the rate constants obtained from the fitted models at temperatures of 70°C, 80°C, 90°C, and 100°C.
The Arrhenius equation expresses the relationship between temperature and rate constant as follows:
A plot of Ln(k) versus 1/T based on the Arrhenius equation is a straight line where the slope is (− Ea/R) (Fig. 8c).
According to the Arrhenius plot, the activation energies based on the shrinking particle kinetics model with diffusion and chemical control reaction mechanisms were determined 81.93 kJ/mole and 78.41 kJ/mole, respectively. Since an activation energy higher than 40 kJ/mole indicates the chemical control reaction mechanism, and values less than 20 kJ/mole are related to the diffusion mechanism, the activation energy of 81.93 kJ/mole cannot be correct for the shrinking particle kinetics model with the diffusion control mechanism from the liquid film layer. Hence, the shrinking particle kinetics model based on the chemical control mechanism was selected for the thorium dissolution from thorium silicate. The mathematical equation of the kinetics model of thorium dissolution from pure thorium silicate with the activation energy of 78.41 kJ/mole is as follows:
To determine the reaction order of the sulfuric acid concentration, the dissolution results of pure thorium silicate at sulfuric acid concentrations of 1–4 M and temperatures of 70°C, 90°C, and 100°C fitted the selected model (Fig. 8d–f).
The slope of the regression lines is the rate constant (k). By plotting the log [H2SO4] versus log k in Fig. 8g, the reaction orders at different temperatures were determined.
The reaction orders of the sulfuric acid concentration in thorium dissolution from pure thorium silicate at temperatures of 70°C, 90°C, and 100°C were determined as 1.64, 1.86, and 1.42, respectively. Moreover, with a confidence limit of 95%, the average reaction order was 1.64 ± 0.44 at all the temperatures.
Conclusion
This study aimed to investigate the kinetics of thorium silicate (thorite mineral) dissolution. For this purpose, dissolution kinetics studies were carried out on pure thorium silicate synthesized in the laboratory by the hydrothermal method.18 Parameters affecting thorium dissolution from pure thorium silicate, including agitation speed, sulfuric acid concentration, and temperature, were optimized in a glass reactor equipped with a mechanical stirrer. Optimal thorium dissolution conditions were obtained at an agitation speed of 450 rpm, a sulfuric acid concentration of 4 mole/L, and a temperature of 100°C. Despite the increase in thorium silicate dissolution kinetics with increasing the sulfuric acid concentration (to 8 mole/L), the thorium concentration in the leaching solution began to decrease with increasing the sulfuric acid concentration from 4 to 8 mole/L over time. The reason of this behavior could be due to the formation of silica gel with increasing H+ concentration, which leads to trapped thorium sulfate hydrated crystals in the silica gel structure, and ultimately reduces the concentration of thorium in the resulting solution with a sulfuric acid concentration higher than 4 mole/L.
The dissolution kinetics of pure thorium silicate was investigated under optimized conditions. According to images of SEM, DLS, and BET analyses before and after dissolution, and assuming spherical particles, the shrinking particle kinetics model was selected for the dissolution of pure thorium silicate. To determinate the activation energy of 78.41 kJ/mole according to the Arrhenius equation, the shrinking particle kinetics model based on the chemical control reaction mechanism was selected for the dissolution of pure thorium silicate. The reaction order of the sulfuric acid concentration at different temperatures was determined as 1.64 ± 0.44 for the thorium dissolution from pure thorium silicate.
Notes
Polytetrafluoroethylene.
References
Thorium fuel cycle- Potential benefits and challenges, IAEA-TECDOC-1450, (2005).
C. Frondel, Am. Mineral. 38, 1007 (1953).
L.H. Fuchs and E. Gebert, Am. Miner. 43, 243 (1958).
I.R. Shein, K.I. Shein, and A.L. Ivanovskii, Phys. Chem. Miner. 33, 545 (2006).
M. Taylor and R.C. Ewing, Acta Cryst. B34, 1074 (1978).
O. Levenspiel, Chemical reaction engineering, 3rd edn. (Wiley, New York, 1999).
S.S. Hubert, G. Heisbourg, N. Dacheux, and P. Moisy, Inorg. Chem. 47(6), 2064 (2008).
D.J. Sapsford, R.J. Bowell, J.N. Geroni, K.M. Penman, and M. Dey, Miner. Eng. 39, 165 (2012).
G. Heisbourg, S. Hubert, N. Dacheux, and J. Ritt, J. Nucl. Mater. 321, 141. (2003).
L. Claparede, F. Tocino, S. Szenknect, A. Mesbah, N. Clavier, P. Moisy, and N. Dacheux, J. Nucl. Mater. 457, 304 (2015).
G. Heisbourg, S. Hubert, N. Dacheux, and J. Purans, J. Nucl. Mater. 335, 5 (2004).
M. Simonnet, N. Barré, R. Drot, C.L. Naour, V. Sladkov, and S. Delpech, Radiochim. Acta 104(10), 691 (2016).
M. Simonnet, N. Barré, R. Drot, C.L. Naour, V. Sladkov, and S. Delpech, Radiochim. Acta 107(4), 289 (2018).
A.R. Keshtkar and S. Abbasizadeh, Prog. Nucl. Energy 93, 362 (2016).
T. Takeuchi, C.K. Hanson, and M.E. Wadsworth, J. Inorg. Nucl. Chem. 33, 1089 (1971).
Y. Arinicheva, S. Neumeier, F. Brandt, D. Bosbach, and G. Deissmann, MRS Adv. 3(21), 1133 (2018).
C. Gausse, S. Szenknect, A. Mesbah, N. Clavier, S. Neumeier, and N. Dacheux, J. Appl. Geochem. 93, 81 (2018).
A. Ghadiri, M. Abdollahy, A. Khanchi, M.R. Khalesi, and M. Akbari, Russ. J. Inorg. Chem. 64(14), 1829 (2019).
D.T. Costin, A. Mesbah, N. Clavier, S. Szenknect, N. Dacheux, C. Poinssot, J. Ravaux, and H.P. Brau, Prog. Nucl. Energy 57, 155 (2012).
OECD/NEA, Chemical Thermodynamics of Thorium, Volume 11, Chemical Thermodynamics, (Paris OECD, 2008), pp. 274- 298.
B. Terry, Hydrometallurgy 10, 151 (1983).
R.K. Iler, The chemistry of silica: solubility, polymerization, colloid and surface properties and biochemistry (Wiley, New York, 1978), pp. 172–311.
Acknowledgements
The authors would like to thank the funders of this project by Tarbiat Modares University and the Nuclear Science Technology and Research Institute (NSTRI) of the Atomic Energy Organization of Iran (AEOI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghadiri, A., Abdollahy, M., Khanchi, A. et al. Kinetics Study of Thorium Silicate Dissolution in Sulfuric Acid Media. JOM 74, 689–696 (2022). https://doi.org/10.1007/s11837-021-05046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05046-6