Abstract
High-temperature oxidation resistance of Nd–Fe–B magnetic powder is of great importance for its application in bonded magnet fields. In this work, the refinement and surface modification of Nd–Fe–B magnetic powders were integrated and achieved simultaneously. The effects of phosphoric acid, isopropyl tris-(dioctyl pyrophosphate acyloxy) titanate (ITDT), and the synergistic addition of them on the oxidation resistance of the powders were investigated. Also, the formation mechanism of the protective layer on the Nd–Fe–B powder surface has been proposed. The surface morphology, phase structure, oxidation resistance, and magnetic properties were studied. The results show that the synergistic surface modification exhibited optimal oxidation resistance on the refined magnetic powders. Furthermore, the obtained powders were bonded with polyphenylene sulfide to illustrate the effect of high-temperature oxidation resistance in practical application. The ITDT showed excellent protective performance for the magnetic properties at room temperature, whereas the phosphate showed an excellent oxidation resistance at high temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because bonded magnets prepared via injection molding have many excellent properties, including high size precision, good chemical stability and corrosion resistance, stable magnetic properties, low eddy current loss, and various complex shapes,1,2,3,4 they are used for energy conversion and signal transmission in a wide range of areas, such as electric motors, household electrical appliances, and automobiles.5,6,7,8 The rare-earth iron-based intermetallic compound Nd–Fe–B is a known attractive magnetic material,9,10,11 and bonded magnets are typically produced by mixing the hard magnetic powder like Nd–Fe–B with a resin binder, followed via injection molding.12,13,14 The Nd–Fe–B powder is the key raw material for developing high-performance bonded magnets.15 Currently, commercially available Nd–Fe–B magnetic powders are commonly produced via quick quenching,16,17 and always show a scaly and flaky shape.18 The energy product of bonded magnets is proportional to the properties of the magnetic powders and the square of the magnetic filler’s volume fraction.19 The reduction in particle size could effectively improve the melt flow performance and increase the packing percentage of the magnetic powder filler into the polymer matrices.20,21 Ball-milling is an effective way to achieve the refinement of Nd–Fe–B powders.22 However, the Nd–Fe–B magnetic material exhibits a high chemical activity.23,24 After refinement, the activity of the magnetic powders increases further, due to the increase of specific surface area, and this causes the powders to be easily oxidized. Moreover, the magnetic powders must be blended with polymer at a temperature higher than 200°C when applied in preparing bonded magnets,19 and this requires excellent high-temperature oxidation resistance for the Nd–Fe–B powder. Therefore, the refined Nd–Fe–B magnetic powder must be surface-modified to increase the oxidation resistance. In recent years, several works devoted to surface modification research on rare-earth magnetic powders have been published. For example, soaking and coating using silicone organics18,25,26 or a phosphorizing agent,27 coating of parylene C by chemical vapor deposition polymerization,28 and metal layer coating by electrochemical deposition29 have been reported. Among them, phosphoric acid shows fairly good effects in the aspect of anti-oxidation. However, the research on the anti-oxidation mechanism and the effect of phosphate chemical conversion coatings focuses on sintered magnets, while have been rarely reported on the refined Nd–Fe–B powders used for bonded magnets. Isopropyl tris-(dioctyl pyrophosphate acyloxy) titanate (ITDT) is an effective surface treatment agent. The application of synergistic addition of ITDT and phosphoric acid on Nd–Fe–B powders to improve oxidation resistance has not been reported in the literature.
In this work, the Nd–Fe–B magnetic powders are simultaneously refined by ball-milling and were surface-modified using ITDT and phosphoric acid. The morphology, phase, oxidation resistance, and magnetic properties were studied. Refined Nd–Fe–B powders with excellent high-temperature oxidation resistance were obtained, and the mechanism of the formation of protective coatings on the Nd–Fe–B powder surface has been proposed.
Experimental
Materials
Nd–Fe–B magnetic powders (Grade: MQP-12-8HD) used in this study were obtained from Magnequench. Phosphoric acid (H3PO4, CAS: 7664-38-2) was supplied by Shanghai Lingfeng Chemical Reagent, China. Isopropyl tris-(dioctyl pyrophosphate acyloxy) titanate (ITDT, CAS: 67691-13-8) was supplied by Hangzhou Jessica Chemical, China. Absolute ethanol was purchased from Anhui Ante Food, China. PPS resin (Grade: Ryton XE5501BL) was obtained from Solvay.
Refinement and Surface Modification
Nd–Fe–B magnetic powders were refined using a planetary ball mill. Typically, 40 g of Nd–Fe–B powders and 400 g of zirconia balls (300g φ2 mm and 100g φ10 mm) were added into a 0.5-L ball-milling tank and spun at 250 rpm for the set time. Phosphoric acid (0.612 g, 85% in H2O), ITDT (0.4 g), and the mixture of them were each used as a protective agent for surface modification. The remaining space of the ball-milling tank was filled with ethanol and Ar gas to eliminate air during the ball-milling process. Subsequently, the milled powders were separated from the zirconia balls and then rinsed with ethanol several times. Finally, the powders were dried at 50°C in a vacuum oven for 3 h.
Characterizations
The surface morphology and particle size were observed using scanning electron microscopy (SEM; SU-1510; Hitachi, Japan). The particle size distribution (PSD) of the Nd–Fe–B powders were measured via a Sympatec Helos/Rodos laser diffraction particle size analyzer (Sympatec, Germany), and the measuring ranges of R1, R3, and R5 in the laser diffraction system were used. Thermogravimetric–differential scanning calorimetry (TG-DSC; Q5000IR; TA Instruments, USA) was conducted to obtain the curves from room temperature to 300°C with a heating rate of 10 K min−1 under air atmosphere. The oxygen content was measured using the Oxygen/Nitrogen/Hydrogen Analyzer (ONH 836; Leco, USA). The elemental analysis was carried out using X-ray fluorescence spectrometry (XRF; ZSX Primus-II; Rigaku, Japan). The X-ray diffraction (XRD) patterns were measured on an X'pert PRO Model powder diffractometer (PANalytical, Netherlands) operating at 40 kV and 30 mA using Cu Kα radiation (λ = 1.5404 Å). The diffractograms were recorded in the 2θ range from 20° to 80°. The magnetic behavior of the Nd–Fe–B powders was investigated using a vibrating sample magnetometer (VSM; Lakeshore 7307, USA) with a maximum magnetic field of 1350 kA m-1. A B-H analyzer (FE-2100H; Hunan Forever Elegance Technology, China) was used to test the magnetic properties of the bonded magnets. To fabricate the bonded magnets for testing, the magnetic compound was first prepared. The Nd–Fe–B powders (89.7 wt.%) and PPS resin (10.3 wt.%) were pre-mixed and then fed into a twin-screw extruder using a single-screw conveyor. In the extrusion process, the temperature was set at 300°C for the feed zone and the die zone, and at 315°C for the melting and melt-pumping zones. Then, the obtained magnetic compound was injected, forming cubic magnets of 10 × 10 × 10 mm, with the barrel temperature of 320°C and the mold temperature of 120°C.
Results and Discussion
Mechanism of Modification and Refinement
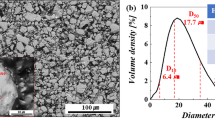
The morphology of the initial quick-quenched magnetic powders and the obtained Nd–Fe–B powders with different milling times and different additions are shown in Fig. 1a–f, and the corresponding PSD curves are illustrated in Fig. 1g. Dimensionless logarithmic differential (density) distribution, q3lg, was exhibited in PSD curves to observe the discrete degree and central tendency of the data more intuitively and quickly, and a logarithmic x-axis was used to show better resolution over the entire distribution width. The magnetic powder particles were first refined to D50 = 18 μm after 30 min of ball milling, as shown in Fig. 1b and g. Because the quick-quenched Nd–Fe–B powders were scaly and possessed high diameter-to-thickness ratios, the fracture of the powders occurred preferentially along the length direction during the early ball-milling stage. Then, the particle size was decreased to a size finer than 10 μm after 3 h of ball milling, as shown in Fig. 1c–g. It can be concluded from the PSD curves in Fig. 1g that ball milling with the addition of ITDT caused the magnetic particle size distribution to be more concentrated and uniform by acting as a surfactant.30 The refined Nd–Fe–B powder with the synergistic addition of phosphoric acid and ITDT showed the most uniform and concentrated size distribution, and the PSD curve combined the distribution characteristics of the samples with single phosphoric acid or ITDT addition.
SEM images of (a) initial coarse Nd–Fe–B powders; (b) powders ball-milled in pure ethanol for 30 min; powders ball-milled for 3 h: (c) in pure ethanol, (d) in phosphoric ethanol solution, (e) in ITDT ethanol solution, and (f) in ethanol solution with both phosphoric acid and ITDT; (g) size distribution of Nd–Fe–B powders.
The schematic of the refinement and surface treatment effect is shown in Fig. 2. In the process of ball milling, the flaky powder became granular. At the same time, ITDT, phosphoric acid, or both of them reacted to the newly produced surface of fine powder, and a protective film formed. When only ITDT existed, as shown in Fig. 2b, the alkoxide group (—OR) of the ITDT could potentially react with the hydroxyl groups (—OH) on the magnetic powder surface to form covalent bonds after the release of isopropanol.31 This reaction resulted in the formation of an organic titanium monolayer on the powder surface to increase the oxidation resistance. However, the amount of hydroxyl groups on the surface of the magnetic powders was limited, because the Nd–Fe–B powder was in situ-crushed, and the surface of the refined powders was newly created. Therefore, only a very small part of the powder surface was covered by ITDT through chemical bonding. To characterize the formation of the passive film after ball milling and coating, the phosphorus content was analyzed using the XRF instrument, and the results are shown in supplementary Table S1. The measured P content in the sample with only ITDT added was 0.0180 wt.%, being slightly higher than the value of 0.0056 wt.% (which might have come from impurities in the raw materials or the manufacturing equipment) for the powders without additive agent. This is consistent with the analysis of Fig. 2b. As shown in Fig. 2c, when only phosphoric acid was added, the conversion products of ferric phosphate and neodymium phosphate formed.32,33,34 The elements of Fe and Nd in the Nd–Fe–B alloy could react with phosphoric acid as in Eqs. (1) and (2):
The insoluble conversion layer covered the surface of the magnetic powders, thereby preventing the erosion of oxygen. According to supplementary Table S1, the P element (0.2592 wt.%) was clearly present in the powders treated with phosphoric acid, indicating that the phosphate protective film formed on the surface of the powders. As shown in Fig. 2d, the addition of a mixture of ITDT and phosphoric acid led to the formation of a synergistic protective film. The effects of ITDT and phosphoric acid were intertwined and complemented each other. The formation mechanism of the synergistic film on the Nd–Fe–B powder surface with the addition of ITDT and phosphoric acid is proposed, as shown in Fig. 2e. Because there were very few hydroxyl groups (—OH) existing on the newly generated surface during the ball-milling process, ITDT was rarely directly and chemically combined with the surface of the powder. However, when phosphoric acid (H3PO4) existed simultaneously, dihydrogen phosphate (H2PO4−) and hydrogen phosphate (HPO42−) were formed and bonded on the surface of the Nd–Fe–B powders. Potentially, the H atoms of the H2PO4− and HPO42− enabled the reaction with ITDT after the release of isopropanol ((CH3)2CHOH). This reaction resulted in the formation of a dense synergistic protective layer that was connected by chemical bonds on the surface of powders to increase the anti-oxidant capacity. The XRF results in supplementary Table S1 show the highest phosphorus content (0.3839 wt.%) in this sample, which was caused by the phosphorus in the combined ITDT and phosphate, and this is consistent with the scheme proposed above.
XRD Analysis
The phase compositions of the magnetic powders that were treated using different passivation reagents were analyzed via XRD, and the results are shown in Fig. 3. As seen from the diffraction peaks, the curve (Fig. 3e) of the sample synergistically treated with the synergistic addition of phosphoric acid and ITDT contained the least impurity peaks and matched best with the diffraction peaks (Fig. 3a) of the raw Nd–Fe–B powders. Meanwhile, it can be seen in the enlarged view (20–46°) in Fig. 3 that the diffraction peaks (Fig. 3b) of the sample milled without protective addition shifted to higher angles compared to the peaks in Fig. 3a, for instance, the patterns of the crystalline planes (214), (105), (313), (224), and (314); in contrast, there were no diffraction peak shifts observed in the other samples. These observations indicate that the interplanar spacing increased for the sample without an added protective agent, which was a result of the doping of the H and O atoms into the lattice space. In summary, both ITDT and phosphoric acid showed effective protective effects on the crystalline structure of the Nd–Fe–B sample during the ball-milling process, and the synergistic addition of the two agents showed the best protective effect.
Oxidation Resistance Properties
The refined Nd–Fe–B powders, which were ball-milled for 3 h with different passivation agents, were placed at 250°C under air atmosphere and the reactions were observed. As seen in Fig. 4a, the powders without a passivation agent partially became red-hot, like burning charcoal, in about 30 s, and the red rapidly spread to the surrounding powders; while the color of the powders after cooling turned black. The sample that was treated with ITDT also became red-hot and combusted, as shown in Fig. 4b, but the start time was prolonged to around 1 min 28 s. For the powders that were treated with phosphoric acid and with the synergistic treatment of ITDT and phosphoric acid, no red-hot combustion phenomenon was observed, as shown in Fig. 4c and d. This indicates that both the naked powders without a protective film and the powders which were coated with a single ITDT organic film showed poor oxidation resistance at high temperature, whereas the phosphate film significantly improved the oxidation resistance. For comparison, the original coarse powders before refinement (D50 = 138 μm) were heat-treated under the same conditions, and the results were as shown in supplementary Fig. S1. The apparent color of the raw powders turned form bright silver-gray before heating to burnt yellow after heating, due to the oxidation of the powder surface, but no burning was observed. It can be concluded that the refined Nd–Fe–B powders, which were under the synergistic coating protection of ITDT and phosphoric acid, possess the anti-combustion and anti-oxidation performance just like the original coarse powders.
TG-DSC measurements were conducted on the Nd–Fe–B magnetic powders with different passivation agents after 3 h of ball milling, and the results are shown in Fig. 5. The original coarse powder before refinement was also tested for comparison. As seen from the TG curves in Fig. 5a, the naked powders and ITDT-treated powders started to gain weight at around 100°C. The naked powders had rapid oxidative weight gain around 200°C, and this indicates that the powders began to combust. For the ITDT-treated powders, the weight rapidly increased at around 260°C, which was higher than for the naked powder, indicating that ITDT reduced and delayed the oxidation of powder. The powder treated with phosphoric acid showed nearly no oxidation weight gain at a temperaturę lower than 125°C, and the TG curve was relatively smooth, indicating that the phosphate film which wrapped around the Nd–Fe–B powder had a good effect on oxidation resistance. The weight increase of the synergistically treated powder was very gentle, showing nearly no oxidation weight gain at a temperature lower than 200°C. Even exposed in air atmosphere at a temperature higher than 200°C, for the synergistically coated powders, the oxidation weight increase was small, showing remarkably better oxidation resistance than the other samples and being closest to the coarse powders. In the DSC curves shown in Fig. 5b, the naked and ITDT-coated powders showed exothermic peaks around 100°C, indicating that the powders underwent a rapid oxidative exothermic process. For the phosphoric acid-treated powders and the synergistically coated powders, no rapid exothermic phenomenon was observed; in particular, the curve of the synergistically coated powders was quite smooth, being closest to the coarse powders. This indicates that the synergistic coating is the optimal method for improving the oxidation resistance of the refined powders at a high temperature, being consistent with previous TG results.
Two groups of Nd–Fe–B magnetic powders were passivation-ball-milled (one group for 30 min and the other for 3 h) and then heat-treated at different temperatures for 1 h in air atmosphere. Then, the oxygen contents were measured. To exclude the factor of the oxygen contained in phosphoric acid and ITDT, the increase in the value of oxygen content (Δ oxygen content) was considered, as seen in Fig. 6. Usually, when the environmental temperature was higher, the powder oxidation was more severe and the value of Δ oxygen content was higher. As shown in Fig. 6a, for the sample which was ball-milled for 30 min without protective agents, the powders showed an obvious increase in oxygen content when the temperature was higher than 100°C, and it burned when the temperature was higher than 175°C with the oxygen content rapidly increasing. For the powders milled for 30 min with ITDT addition, the oxygen content rapidly increased at a temperature higher than 200°C. By contrast, the powder samples treated with phosphoric acid and synergistic addition showed the noticeably lower value of Δ oxygen content even at a temperature as high as 300°C, exhibiting good oxidation-resistant performance. When the particle size was finer, the activity was higher. The situations for the samples ball-milled for 3 h are shown in Fig. 6b. The powder samples without protective agents and with ITDT addition both showed obvious increases in oxygen contents after 50–100°C and burned at 150–300°C with rapid increases in oxygen content. In sharp contrast, the oxygen increases were relatively low for the two powders that were treated with phosphoric acid and with synergistic addition. Specifically, in an environment higher than 200°C, the synergistically modified sample exhibited the lowest value of Δ oxygen content, indicating an excellent oxidation resistance performance.
Magnetic Properties
In order to characterize the anti-oxidation effect of the refined magnetic powder after surface modification and the influence on the magnetic properties, the Nd–Fe–B powders passivation-ball-milled for 3 h were heat-treated in air atmosphere for 1 h at different temperatures. The magnetic properties were characterized using demagnetization curves, and the results are shown in Fig. 7. The sample of the raw coarse powder before refinement was also tested for comparison. As seen in Fig. 7a, the optimal magnetic properties of the refined powders were obtained from sample coated with synergistic addition of both ITDT and phosphoric acid (Br = 0.75 T, Hcj = 633 kA/m), and the ITDT-coated powders also exhibited good magnetic properties (Br = 0.75 T, Hcj = 624 kA/m), indicating that adding ITDT during the milling process can effectively protect the magnetic properties of the powders. Figure 7b and c shows that the magnetic properties of the synergistically coated powders were effectively protected in the environments of 100°C and 200°C. However, the magnetic properties of the ITDT-coated powders were well-protected at 100°C, but the powders combusted at 200°C, causing a rapid drop in remanence Br and coercivity Hcj. Although the initial magnetic properties of phosphoric acid-treated samples were not high, their properties were well maintained even after being heated at 200°C in air atmosphere, indicating that the added phosphoric acid played a good role in improving the high-temperature oxidation resistance of the magnetic powders. This suggests that the organic protective film formed by ITDT alone on the powder surface had an excellent protective performance at room temperature. However, its effect of high-temperature oxidation resistance was poor, and oxidation and combustion occurred with an increase in the temperature when exposed to air atmosphere. The high-temperature oxidation resistance of the single phosphate film was obvious; however, the protective effect on the magnetic properties during ball milling at room temperature was relatively weak. Under the synergistic effect of phosphoric acid and ITDT, the refined powders with the combined protective film showed the best initial performance, closest to that of the raw coarse powders, and exhibited excellent oxidation resistance performance in high-temperature environments.
Typical and extensively used binders in bonded magnets are thermoplastic binders, such as polyamides (PA, with a melting point of about 180°C) and polyphenylene sulfide (PPS, with a high melting point up to 280°C).35,36 To judge the oxidation resistance at a higher temperature in practical application, the magnetic compounds with Nd–Fe–B powders and PPS resin were prepared and injected into a mold forming 10.0 × 10.0 × 10.0 mm magnetic cubes for testing. The cube with the original coarse powder was not obtained because of the low melt flow performance. In addition, both of the samples using the naked powders and the ITDT-coated powders burned during the injection-molding process. That was because these two types of powders oxidized and combusted at high temperature, and then the mixed PPS resin was ignited. So, the bonded magnetic cubes of them were not obtained. In contrast, for the phosphoric acid-treated and the synergistically treated samples, the injection-molding process was very smooth. The demagnetization curves of the obtained bonded Nd–Fe–B magnetic cubes were measured using a B-H analyzer, as shown in Fig. 7d. The synergistically treated samples exhibited both a higher coercivity and a higher remanence than the sample treated with phosphoric acid alone, indicating that the additions of phosphoric acid and ITDT showed synergistic effects on high-temperature oxidation resistance, and that the refined Nd–Fe–B magnetic powders possess excellent high-temperature oxidation resistance. Thus, synergistic modification with phosphoric acid and ITDT is of great significance for the maintenance of the magnetic properties of the Nd–Fe–B powder and the final energy product of magnets.
Conclusion
During the refinement process of the Nd–Fe–B powders, the synergistic treatment using ITDT and phosphoric acid significantly improved the high-temperature oxidation resistance of the magnetic powders. Both ITDT and phosphoric acid had protective effects on the crystalline structure of Nd–Fe–B during the ball-milling process, and the synergistic protection was optimum. The ITDT protective film showed excellent protective performance for the magnetic properties at room temperature, but showed poor high-temperature oxidation resistance. The phosphate film showed an excellent oxidation resistance at high temperatures, but its protective effect on the magnetic properties is relatively weak during the ball-milling process. The H atom from phosphoric acid enabled the reaction with ITDT after the release of isopropanol, and a dense synergistic protective layer formed on the powder surface with the phosphate–ITDT film intertwining and complementing each other. The insoluble protective layer covers the surface of the magnetic powders, thereby preventing the erosion of oxygen. Thus, the combined film formed by the synergistic addition of phosphoric acid and ITDT enables the refined powders to achieve a good initial magnetic performance and excellent oxidation resistance at high temperatures.
Data Availability
Raw data generated and analyzed in this research are available from the corresponding author on reasonable request.
References
W. Xi, W. Liu, M. Yue, D. Zhang, Q. Lu, H. Xu, H. Zhang, Q. Wu, and Y. Li, IEEE Trans. Magn. 54, 1. https://doi.org/10.1109/TMAG.2018.2839605 (2018).
W.Q. Liu, R.J. Hu, M. Yue, Y.X. Yin, and D.T. Zhang, J. Magn. Magn. Mater. 435, 187. https://doi.org/10.1016/j.jmmm.2017.04.009 (2017).
P.S. Muljadi, Suprapedi Energy Procedia 68, 282. https://doi.org/10.1016/j.egypro.2015.03.257 (2015).
M.P. Paranthaman, C.S. Shafer, A.M. Elliott, D.H. Siddel, M.A. McGuire, R.M. Springfield, J. Martin, R. Fredette, and J. Ormerod, JOM 68, 1978. https://doi.org/10.1007/s11837-016-1883-4 (2016).
B.M. Ma, J.W. Herchenroeder, B. Smith, M. Suda, D.N. Brown, and Z. Chen, J. Magn. Magn. Mater. 239, 418. https://doi.org/10.1016/S0304-8853(01)00609-6 (2002).
O. Gutfleisch, M.A. Willard, E. Brück, C.H. Chen, S.G. Sankar, and J.P. Liu, Adv. Mater. 23, 821. https://doi.org/10.1002/adma.201002180 (2011).
M.A.R. Önal, S. Dewilde, M. Degri, L. Pickering, B. Saje, S. Riaño, A. Walton, and K. Binnemans, Green Chem. 22, 2821. https://doi.org/10.1039/D0GC00647E (2020).
B. Ma, A. Sun, X. Gao, X. Bao, J. Li, and H. Lang, J. Magn. Magn. Mater. 457, 70. https://doi.org/10.1016/j.jmmm.2017.11.097 (2018).
K.Y. Xiao, H. Xu, Z.W. Guo, and W. Zheng, Solid State Phenom. 278, 63. (2018).
N. Jones, Nature 472, 22. https://doi.org/10.1038/472022a (2011).
H.K. Park, J.H. Lee, J. Lee, and S.K. Kim, Sci. Rep. 11, 3792. https://doi.org/10.1038/s41598-021-83315-9 (2021).
Y. Yang, R. Ren, Y. Wang, L. Fang, Y. Chen, Q. Gao, L. Yang, J. Liu, X. Fang, and B. Dong, IEEE Trans. Magn. 56, 1. https://doi.org/10.1109/TMAG.2020.3027438 (2020).
D. Plusa, B. Slusarek, M. Dospial, U. Kotlarczyk, and T. Mydlarz, J. Alloys Compd. 423, 81. https://doi.org/10.1016/j.jallcom.2005.12.051 (2006).
F. Yang, X. Zhang, Z. Guo, S. Ye, Y. Sui, and A.A. Volinsky, J. Alloys Compd. 779, 900. https://doi.org/10.1016/j.jallcom.2018.11.335 (2019).
B. Ma, A. Sun, X. Gao, X. Bao, and J. Li, J. Magn. Magn. Mater. 467, 114. https://doi.org/10.1016/j.jmmm.2018.07.061 (2018).
M. Wu, Y. Li, X. Wang, L. Chen, and Y. Mu, J. Rare Earth 35, 1221. https://doi.org/10.1016/j.jre.2017.08.005 (2017).
M.J. Kramer, L.H. Lewis, L.M. Fabietti, Y. Tang, W. Miller, K.W. Dennis, and R.W. McCallum, J. Magn. Magn. Mater. 241, 144. https://doi.org/10.1016/S0304-8853(01)00955-6 (2002).
J. Slapnik, I. Pulko, R. Rudolf, I. Anžel, and M. Brunčko, Addit. Manuf. 38, 101745. https://doi.org/10.1016/j.addma.2020.101745 (2021).
M.P. Paranthaman, V. Yildirim, T.N. Lamichhane, B.A. Begley, B.K. Post, A.A. Hassen, B.C. Sales, K. Gandha, and I.C. Nlebedim, Materials 13, 3319. https://doi.org/10.3390/ma13153319 (2020).
M. Pallapa, and J.T.W. Yeow, Smart Mater. Struct. 24, 025007. https://doi.org/10.1088/0964-1726/24/2/025007 (2014).
B. Pawlowski, and J. Töpfer, J. Mater. Sci. 39, 1321. https://doi.org/10.1023/B:JMSC.0000013891.38980.1c (2004).
P. Sebayang, T. Sudiro, D. Aryanto, D. Hasibuan, and Z. Sitorus, J. Phys.: Conf. Series 1091, 012017. https://doi.org/10.1088/1742-6596/1091/1/012017 (2018).
T. Xie, S. Mao, C. Yu, S. Wang, and Z. Song, Vacuum 86, 1583. https://doi.org/10.1016/j.vacuum.2012.03.019 (2012).
J. Li, S. Mao, K. Sun, X. Li, and Z. Song, J. Magn. Magn. Mater. 321, 3799. https://doi.org/10.1016/j.jmmm.2009.07.039 (2009).
H. Chen, J. Zheng, L. Qiao, Y. Ying, L. Jiang, and S. Che, Adv. Powder Technol. 26, 618. https://doi.org/10.1016/j.apt.2015.01.011 (2015).
W. Liu, Y. Yang, Y. Meng, and J. Wu, Mater. Trans. 44, 1159. https://doi.org/10.2320/matertrans.44.1159 (2003).
W.Z. Qin, J. He, and J.H. Meng, Adv. Mater. Res. 535–537, 1314. (2012).
B. Ma, A. Sun, Z. Lu, C. Cheng, and C. Xu, J. Magn. Magn. Mater. 416, 150. https://doi.org/10.1016/j.jmmm.2016.05.013 (2016).
J. Zheng, M. Jiang, L. Qiao, J. Sheng, J. Li, and L. Jiang, Mater. Lett. 62, 4407. https://doi.org/10.1016/j.matlet.2008.07.046 (2008).
R. Han, Y. Shao, X. Quan, K. Niu, Polym. Compos., 1 (2021). Doi: https://doi.org/10.1002/pc.25984.
N.W. Elshereksi, M. Ghazali, A. Muchtar, C.H. Azhari, Dent. Mater. J., 539 (2017). Doi: https://doi.org/10.4012/dmj.2016-014.
X. Wang, Q. Zhu, X. Liu, and B. Hou, Coatings 11, 152. https://doi.org/10.3390/coatings11020152 (2021).
C. Jiang, Z. Gao, H. Pan, and X. Cheng, Electrochem. Commun. 114, 106676. https://doi.org/10.1016/j.elecom.2020.106676 (2020).
X. Ding, L.-F. Xue, X.-C. Wang, K.-H. Ding, S.-L. Cui, Y.-C. Sun, and M.-S. Li, J. Magn. Magn. Mater. 416, 247. https://doi.org/10.1016/j.jmmm.2016.04.048 (2016).
D. Brown, B.-M. Ma, and Z. Chen, J. Magn. Magn. Mater. 248, 432. https://doi.org/10.1016/S0304-8853(02)00334-7 (2002).
P. Sardjono and R. Ramlan, Key Eng. Mater. 855, 34. (2020).
Acknowledgements
The authors acknowledge the researchers (Liqiang Jiang, Senqi Shen, and Junjie Zhou) of Hangzhou Haisheng Technology Company for the assistant in the experimental of preparing bonded magnets.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Zheng, J., Cheng, X. et al. Surface Modification and Refinement of Nd–Fe–B Magnetic Powder Using ITDT and Phosphoric Acid. JOM 73, 3941–3949 (2021). https://doi.org/10.1007/s11837-021-04850-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-04850-4