Abstract
Liquid-state interfacial reactions of p-type (Bi,Sb)2Te3 thermoelectric (TE) material with Sn and Sn-3.0 wt.%Ag-0.5 wt.%Cu (SAC305) solders, respectively, were examined at 250°C on a commercial highly orientated (Bi,Sb)2Te3 substrate with a (110) soldering plane. For the reactions with Sn, the initial reaction phase was the porous SnTe intermetallic compound (IMC). Then, a dense SnTe layer with many tiny cracks formed between the porous phase and TE substrate. With longer aging time, the dense SnTe gradually changed to the porous phase due to Sb dissolution. In the subsequent stage, the reaction phase zone included porous SnTe, an alternating layer microstructure of SnTe and liquid solder, and SnTe/Sn3Sb2 alternating layers from the solder to TE. The IMC growth was extremely fast, being approximately 15 μm/min, and nearly linear with aging time, suggesting reaction control. For the reactions with SAC305, a similar microstructure of porous SnTe and the dense layer was observed. Compared with the reactions with Sn, however, the IMC growth rate was greatly suppressed by ~ 80%. This can be attributed to the Ag-rich phase layer between the dense SnTe and (Bi,Sb)2Te3. Ag was verified to be the key element for dramatically changing the interfacial reaction behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among various thermoelectric (TE) materials, bismuth telluride-based alloys, including p-type (Bi,Sb)2Te3 and n-type Bi2(Te,Se)3, are the most common commercial material systems due to their high ZT values (figures of merit) at room temperature.1,2,3,4,5 They have been widely used in various thermoelectric devices, including for thermoelectric refrigeration and green power conversion from waste heat. Bi2Te3-based alloys exhibit highly anisotropic thermoelectric properties owing to their rhombohedral layered structure (in space group R3m).6,7 The thermoelectric performance of Bi2Te3 along the directions perpendicular to the c-axis is much better than that along the parallel orientation.7 Most commercial TE materials are single-crystalline or highly oriented polycrystalline.8,9,10 Additionally, because of the weak van der Waals-like bonds between the atomic Te-Te planes along the c-axis, cleavage planes frequently appear in the direction perpendicular to the c-axis.10,11
Thermoelectric devices or modules are composed of many pairs of p-type and n-type Bi2Te3-based pellets. They are generally designed to be connected electrically in series and thermally in parallel. A large number of p-type and n-type TE elements must be assembled into a TE module by soldering. Therefore, there exist many solder joints in a TE module. Sn is the major constituent element in most lead-free solders. A thin diffusion barrier layer with a thickness of several micrometers is usually deposited on the soldering surface of the TE pellet to prevent rapid interfacial reactions with the molten solder during the soldering process.12,13,14,15 However, Sn or other elements in the solder may react with the lateral surfaces of the TE pellets where no barrier layer is deposited due to the capillary effect. Areas with poor coating quality may also come into direct contact with the molten solder. Furthermore, after long-term product use, Sn may still diffuse through the barrier layer to react with TE materials. Fundamental knowledge and information on the interfacial reactions between solders and TE materials are thus important to evaluate the reliability of TE modules. Hence, studies on the related interfacial reactions and growth of intermetallics (IMCs) have attracted increasing interest and attention from the research community. Although some studies on these topics have been reported in literature,14,16,17,18,19,20,21 the mechanisms underlying various interfacial phenomena require further exploration.
Chen et al.16 investigated the interfacial reactions in Sn/p-type (Bi0.25Sb0.75)2Te3 couples at 250°C. Laboratory-made polycrystalline (Bi0.25Sb0.75)2Te3 was used to perform the reactions. In the initial stage, the dominant reaction phase was the SnTe phase with nanosize pores. The overall IMC growth rate was extremely fast. The reaction phase exhibited a significant microstructural evolution with the formation of periodic alternating layers of Sn3Sb2 and SnTe. Those researchers further examined the interfacial reactions of Sn with various n-type Bi2(Te,Se)3 systems, including Bi2Te3, Bi2Se3, and Bi2(Te1−xSex).17 With addition of only 2 at.% Se into the Bi2(Te1−xSex), the IMC growth rate was dramatically reduced.
Because the thermoelectric performance of Bi2Te3-based systems is strongly anisotropic, commercial TE materials are usually made by unidirectional crystal growth approaches such as the Bridgman method or zone-melting method. Ye et al.18 found that the unidirectional crystal structure of p-type (Bi,Sb)2Te3 had a strong effect on its interfacial reactions with Sn. The IMC growth also exhibited strong anisotropy. In our recent study,19 the solid-state interfacial reactions of commercial p-type (Bi,Sb)2Te3 with Sn and Sn-Ag-Cu solders were examined. The SnSb and SnTe IMCs formed rapidly and showed the linear growth in aging time.
Although the liquid-state Sn/(Bi,Sb)2Te3 interfacial reactions had been examined previously,16 the IMC growth kinetics has not been investigated yet and the related mechanisms underlying the reactions and microstructural evolution remain unclear, requiring further investigation and observations. In addition, the (Bi,Sb)2Te3 substrate used in that work was a polycrystalline material, rather than single-crystal or highly oriented material. However, the interfacial reactions and IMC growth might be affected by the crystal orientation. Better understanding of the liquid-state reactions between solders and Bi2Te3-based materials will help the development and improvement of the assembly technology for such devices.
In this study, we conducted further liquid-state interfacial reactions of Sn and Sn-3.0 wt.%Ag-0.5 wt.%Cu solders with commercial (Bi,Sb)2Te3 with high crystalline orientation. The contact plane (1 1 0) is along the c-axis. The progress of the microstructural evolution of the formed IMCs was carefully observed at different reaction durations. The correlated formation and evolution mechanism are further discussed and interpreted. The corresponding IMC growth kinetics was investigated in detail, being found to be linear in aging time. In comparison with pure Sn, the IMC growth was greatly reduced in the reactions with SAC305. The reason for the retarding effect of Ag on the IMC growth is also discussed.
Experimental Procedures
Commercial p-type (Bi,Sb)2Te3 with dimensions of 4 mm × 4 mm × 2 mm (Kryotherm, Russia) was used to conduct the interfacial reactions with pure Sn and Sn-3.0 wt.%Ag-0.5 wt.%Cu solders, respectively. In our prior study,19 the commercial (Bi,Sb)2Te3 pellet was confirmed to have a composition of Bi-30 at.%Sb-60 at.%Te. In addition, x-ray diffraction (XRD) analysis revealed only one strong diffraction peak, corresponding to (1 1 0) plane of (Bi,Sb)2Te3, suggesting that the material was highly orientated polycrystalline or nearly single-crystal. Accordingly, the soldering plane (4 mm × 4 mm) had a preferred orientation of (1 1 0), which is along the c-axis. Figure 1 illustrates the crystalline orientation of the p-type (Bi,Sb)2Te3. SAC305 solder was prepared from the high-purity constituent elements (99.99 wt.%) that were sealed in an evacuated quartz tube, followed by homogenization in a furnace at 900°C for 3 days, then quenching in water. The SAC solder ingot was cut into discs with thickness of 2 mm. The solder alloy and (Bi,Sb)2Te3 substrate were polished to ensure the flatness of the joining surface.
Both the TE substrate and the solder disc were rinsed with rosin mildly activated (RMA) flux. The TE substrate was placed on the solder alloy, then they were placed on a hot plate at 250°C for soldering. Liquid-state interfacial reactions were carried out in the furnace at 250°C for different reaction durations. The aging times were quite short, so the aging time was precisely controlled to reduce errors, being measured from the occurrence of solder melting.
After reacting for a period of time, the samples were removed from the furnace and quenched immediately. They were mounted in epoxy resin, then metallographically ground and polished to expose the cross-sectional interface. To obtain clear images of the interfacial morphology, Sn-etching solution was used to slightly etch the solder of the samples. The interfacial results were observed by scanning electron microscopy (SEM). Electron probe microanalysis (EPMA, JXA-8200; JEOL) was used to analyze the composition of the formed IMC phases. The IMC layer thickness was measured using an image analyzer. The average value of the layer thickness was determined from at least seven measurements at different locations.
Results and Discussion
Figure 2a shows a backscattered electron image (BEI) micrograph after the Sn/(Bi,Sb)2Te3 interfacial reaction at 250°C for only 20 s. Only one uniform IMC layer was formed with a thickness of ~ 5 μm in the interfacial zone. The growth of the IMC phase was very fast, and it exhibited a porous structure. Large numbers of fine irregular voids with size of several hundred nanometers appeared within the IMC layer. Because the sample was slightly etched by Sn-etching solution for better observation, it was suggested that the voids were filled with liquid solder before etching. The reaction was also conducted for 10 s. A similar porous microstructure was observed, and the IMC layer was approximately 2.5 μm thick.
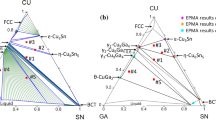
The reaction was further conducted for 1 min, as shown in Fig. 2b. The uniform IMC layer was ~ 22 μm in thickness. Obviously, the reaction phase showed two different microstructures. The 7-μm-thick IMC layer near the (Bi,Sb)2Te3 exhibited a dense structure with some tiny cracks, as seen in the magnified inset image. On the opposite side, the IMC phase adjacent to the solder revealed a porous structure. The composition of the dense phase was determined by EPMA to be Sn-36.1%Te-8.7%Sb-2.1%Bi (at.%) and the porous layer consisted of Sn-37.6%Te-2.6%Sb-0.7%Bi (at.%). The ternary Sn-Sb-Te phase diagram22 suggests that both of them correspond to the SnTe phase, but the dense SnTe possessed higher Sb solubility than the porous phase. The analyzed compositions of the reaction phases were close to the results in the previous study by Chen et al.16 on liquid-state reactions and those in our prior research on solid-state interfacial reactions.19 Additionally, the reaction phases were also demonstrated to be the SnTe phase using XRD analysis in previous studies.16,19 Because it was known that the dominant IMC phase was SnTe, we did not further use XRD to characterize the reaction phase in this work.
The composition of the (Bi,Sb)2Te3 contained 10% Bi and 30% Sb (at.%), but the Bi and Sb contents of the porous IMC were relatively lower. Accordingly, the formation of the porous structure in the initial reaction (Fig. 2a) could be due to the dissolution of a lot of Sb and Bi from the (Bi,Sb)2Te3 into the solder. A similar reaction was performed for 30 s. The thickness of the overall IMC was ~ 11 μm, and its microstructure was similar to that after the 1-min reaction. Consequently, the formation of the dense IMC took place between 20 s and 30 s.
After reacting for 3 min, the IMC layer was greatly thickened to about 50 μm. As shown in the right image in Fig. 2c, three regions with different microstructures were identified, including the porous zone, the dense layer with tiny cracks, and one thin dense layer without cracks, from the solder side to (Bi,Sb)2Te3. Moreover, after aging for 10 min, the SnTe phase rapidly and uniformly increased to reach ~ 163 μm in thickness, as depicted in Fig. 2d. The SnTe phase was almost porous structured (~ 100 μm), while the thin SnTe layer near the (Bi,Sb)2Te3 was relatively denser. In the magnified inset image in Fig. 2d, the dense layer without cracks was about 8 μm. As presented in Fig. 2b–d, a dense SnTe zone with many tiny cracks was present. It is worth noting that these tiny cracks were almost aligned with the interface. The initiation of the tiny cracks was suggested to be associated with the compressive stress induced by the fast growth and volume expansion of the brittle SnTe phase. With respect to the formation mechanism of cracks, further explanation is discussed below.
When the reaction was prolonged to 30 min, the IMC layer became extremely thick, reaching ~ 457 μm, as shown in Fig. 3a. Figure 4 shows the growing thickness of the whole IMC as a function of reaction time. These results clearly indicate that the fast IMC growth in the Sn/(Bi,Sb)2Te3 reactions was nearly linear in aging time. The IMC microstructure and growth behavior are consistent with the previous study.16 However, the IMC growth rate (15 μm/min) in this study was slower than that in the previous study (27 μm/min). This growth difference likely resulted from the different (Bi,Sb)2Te3 substrates. In this study, we used commercial p-type substrates with preferred orientation of (110), while the samples in the previous study were laboratory-made polycrystalline (Bi,Sb)2Te3.
In the liquid-state reactions, the IMC growth front was at the IMC/substrate interface, since the liquid Sn was the dominant diffusion species. Accordingly, the dense SnTe layer was suggested to be the newly formed IMC phase, while the porous SnTe phase was formed relatively earlier. As the reaction progressed, Sn atoms rapidly diffused to react with the (Bi,Sb)2Te3 and then form the new IMC layer. The originally formed dense SnTe layer would move towards the solder matrix. From the observed results, the thickness of the dense SnTe layer did not increase significantly during the whole reaction process, demonstrating that the dense SnTe phase formed near the (Bi,Sb)2Te3 side should gradually transform to the porous structure. Moreover, the Sb content of the dense SnTe layer was much higher than that of the porous one. As discussed above, the porosity formation was due to Sb dissolution from the SnTe into the liquid solder. From the prior study,19 both the SnTe and SnSb phases were formed in the solid-state Sn/(Bi,Sb)2Te3 reactions. In the liquid-state reactions, however, no SnSb phase was observed. Based on the mass balance, this implies that Sb of (Bi,Sb)2Te3 should be dissolved and enter the liquid solder.
The IMC microstructure after 30 min of reaction shown in Fig. 3a and b was significantly different from that after 10 min of reaction. The interfacial zone showed three distinctly different regions. In addition to the porous SnTe phase, the inner two regions revealed an alternating layer structure. The gray phase of the alternating dark/grey layers was the primary IMC phase, i.e., SnTe phase. As shown in Fig. 3b, the composition of the dark phase (point A) near the TE substrate was Sn-39.8%Sb-3.8%Te-0.7%Bi (at.%), which was identified to be Sn3Sb2. In the middle region, the dark phase (point B) revealed it was slightly etched. The composition was Sn-11.5%Sb-0.7%Te-1.8%Bi (at.%), indicating that it was the liquid Sn phase with high Sb solubility before quenching. As a result, the reaction zone consisted of porous SnTe, alternating layers of liquid solder/SnTe, and alternating layers of SnTe/Sn3Sb2.
The formation of the alternating SnTe/Sn3Sb2 layers can be ascribed to the supersaturation–nucleation mechanism.16,23,24 At the initial reaction stage, the Sn diffused towards (Bi,Sb)2Te3 and only SnTe was formed. The atomic ratio of Te to Sb in the dense SnTe phase was about 4. Based on mass balance, the Sb of the substrate was excess and would dissolve into the liquid Sn, which had Sb solubility as high as ~ 7 at.%.25 As the reaction progressed, when the Sb of the liquid solder gradually reached saturation, the Sb dissolution rate decreased, thus the excess Sb accumulated near the (Bi,Sb)2Te3. The supersaturated Sb would lead to nucleation and formation of Sn3Sb2. When the excess Sb was depleted due to the Sn3Sb2 formation, the dominant IMC again changed to become SnTe. This can reasonably explain the formation of the alternating layer structure. The middle region of the SnTe/solder alternating layer is now further discussed. The alternating SnTe/Sn3Sb2 layers gradually moved away from the TE substrate. Careful inspection of Fig. 3b reveals that the Sn3Sb2 of the alternating layer gradually disappeared, which is due to the dissolution into the liquid solder. In contrast, the SnTe phase stably existed at the interface. As a result, the formation of the liquid solder/SnTe alternating layers (the middle zone) can be explained based on two reasons: the Sn3Sb2 dissolution of the SnTe/Sn3Sb2 alternating layers and the microcrack propagation of the SnTe phase.
As shown in the inset image in Fig. 3b, many microcracks were present in the SnTe, but no cracks were found within the Sn3Sb2. Based on the Joint Committee on Powder Diffraction Standards (JCPDS) crystallographic database, the densities of SnTe, Sn3Sb2, and (Bi,Sb)2Te3 were calculated to be 6.46 g/cm3, 6.89 g/cm3, and 6.878 g/cm3, respectively. The formation of tiny and larger cracks could be caused by the compressive stress induced by the volume expansion during formation of brittle SnTe. In Fig. 2b–d, the dense SnTe layer with tiny cracks could be caused for a similar reason. The compressive stress induced by the SnTe formation inevitably resulted in cracking in the IMC phase.
When large numbers of microcracks were connected and propagated within the IMC phase, the resulting larger cracks and channels greatly facilitated the flow of liquid Sn and accelerated the Sb dissolution in the SnTe phase. The dissolution of the SnTe phase further caused the occurrence of recrystallization. Consequently, the dissolution–recrystallization process led to the refinement of the SnTe grains and the formation of porous structure.26,27 A similar microstructural transformation was also observed in the Sn-0.7 wt.%Cu/Ni reaction.28 Figure 5a shows the top-view microstructure of the porous SnTe phase after deep etching. The SnTe phase was composed of fine equiaxed grains with grain size of less than 500 nm. Obviously, a lot of microvoids were distributed in the SnTe phase. Figure 5b shows a cross-sectional image of the porous phase. The propagation of microvoids and cracks produced channels that increased the interfacial reaction rate.
The interfacial reactions between p-type (Bi,Sb)2Te3 and SAC305 solder were further examined at 250°C. Figure 6a shows the formation of one uniform and dense SnTe layer with thickness of ~ 6 μm after aging for 1 min. When the reaction was carried out for 3 min, the whole IMC layer grew thicker to ~ 14 μm. Compared with the Sn/(Bi,Sb)2Te3 reaction, the IMC growth with SAC305 solder was significantly suppressed. The IMC near the solder started to change to a porous structure. With prolonged reaction time, the porous structure became the major microstructure of the SnTe phase. Figure 6b shows the interfacial result after aging for 10 min. The SnTe phase distinctly exhibited the two different microstructures. The entire reaction layer was approximately 40 μm thick, and the porous layer was as thick as ~ 30 μm. Unlike the reactions with Sn, there were no significant tiny cracks in the dense phase. This is likely because the IMC growth was relatively slower and thus compressive stress was not greatly induced. The composition of the porous IMC was Sn-35.9%Te-0.4%Bi-1.6%Sb-0.9%Ag-0.2%Cu (at.%), while that of the dense IMC was Sn-35.1%Te-3.2%Bi-12.4%Sb-0.5%Ag-0.03%Cu (at.%). Similar to the Sn/(Bi,Sb)2Te3 results, the porous SnTe phase also had a lower Sb content. Accordingly, the Sb dissolution of the SnTe phase might be the main cause for the formation of the porous structure.
For the reactions with SAC305 solder, the IMC growth was much slower than that with pure Sn. For comparison, the average thickness of the SnTe layer as a function of aging time in the reaction with SAC305 solder is also illustrated in Fig. 4. Similarly, the IMC growth also followed a linear relationship with aging time, suggesting that the IMC growth was likely reaction controlled. From the linear fitting slope, the growth rate was 3.1 μm/min, around one-fifth of that for the reaction with pure Sn. In our prior study,19 the growth of the SnTe phase was strongly inhibited in the solid-state reactions with SAC305. This can be attributed to the formation of a thin Ag-rich phase layer between the dense SnTe and (Bi,Sb)2Te3.
Furthermore, elemental mapping of the reaction couple was performed after 10 min at 250°C. Figure 7a–d shows the BEI micrograph and element maps of Ag, Bi, and Sb, respectively. The Ag mapping results clearly show that one thin and irregular Ag-rich phase layer with thickness of ~ 1 μm was distributed at the interface between the SnTe and (Bi,Sb)2Te3. The mapping color of the Ag-rich was similar to that of the Ag3Sn phase in the SAC solder. However, we cannot further confirm the species of the IMC phase because it was too thin to precisely determine its composition by EPMA. The thin Ag-rich phase might retard the elemental diffusion of (Bi,Sb)2Te3 and suppress the nucleation and formation of the SnTe phase. Additionally, the Bi mapping revealed that the dense SnTe layer had higher Bi solubility than the porous layer. As shown in Fig. 7d, the Sb solubility of the dense IMC layer was much higher than that of the porous layer. Notably, Sb element was present in the solder matrix. From these results, it is evident that the Sb of the IMC layer dissolved into the liquid solder, and thus it gradually transformed into a porous structure.
To clarify the suppression effect caused by Ag or Cu, interfacial reactions of (Bi,Sb)2Te3 with Sn-3.5 wt.%Ag and Sn-0.7 wt.%Cu were conducted at 250°C for 10 min. As seen in Fig. 8a, the reaction phase layer in the Sn-3.5 wt.%Ag case was about 30 μm thick. Compared with the pure Sn case (~ 160 μm), it is clear that the Ag addition greatly retarded the IMC growth. In contrast, in the Sn-0.7 wt.%Cu reaction (Fig. 8b), it was as thick as ~ 200 μm. Moreover, the microstructure and thickness of the reaction phase in the Sn-3.5 wt.%Ag case were similar to those of the SAC305 reaction. In the Sn-0.7 wt.%Cu case, the alternating layer structure and cracks were observed within the reaction layer, which was similar to the Sn/(Bi,Sb)2Te3 reaction. These results clearly demonstrate that Ag was indeed the key element for changing the interfacial reaction behavior. In contrast, Cu had no significant influence on the reactions.
In Fig. 8a, the Ag-rich phase was relatively thicker and was further analyzed by EPMA. The analyses were conducted at more than five different sites, and the results were highly consistent. It was determined to be 37.5%Ag-18.4%Sb-37.2%Te-5.7%Bi-1.2%Sn (at.%). The composition ratio of the elements was Ag:Sb:Te:Bi = 6:3:6:1. Notably, the Ag-rich phase had almost the same element ratios as the p-type (Bi,Sb)2Te3. Based on the Ag-Sb-Te phase diagram,29 however, it cannot be identified as the possible corresponding binary or ternary equilibrium phases. In the Ag-Sb-Te system, only one ternary compound, AgSbTe2, is thermodynamically stable. Based on the composition ratio, the Ag-rich phase can be expressed as Ag2SbTe2 or Ag6BiSb3Te6. However, a phase with nominal composition of Ag2SbTe2 does not exist in the Ag-Sb-Te system. Therefore, it is suggested that the Ag-rich phase was a metastable phase. Such formation of metastable phases often occurs in certain interfacial systems; For example, metastable CoSn4 was formed in the Sn/Co-P reaction and Sn/Co-Ga reaction.30,31 Further transmission electron microscopy (TEM) analysis would be required for phase identification in the future.
The IMC growth kinetics can be described by the equation
where x is the IMC thickness, k is the growth rate constant, t is the reaction time, and n is the time exponent. The time exponent is determined by the slope of the natural logarithm plot of ln(x) versus ln(t). Based on the IMC growth results in Fig. 4, the corresponding kinetics parameters for the Sn/(Bi,Sb)2Te3 and SAC305/(Bi,Sb)2Te3 reactions were determined and are presented in Table I. The time exponents are 0.90 and 0.83 for the reactions of (Bi,Sb)2Te3 with pure Sn and SAC305, respectively. This again confirms that the IMC growths were linear with aging time.
For the reactions with Sn, the linear IMC growth remained even though the IMC was as thick as ~ 450 μm. This can be attributed to the fact that the porous structure and tiny cracks offered fast diffusion paths for Sn. IMC microstructures are closely related to the IMC growth mechanism. A typical example is found in the liquid-state Zn-Sn/Ni interfacial reactions.32 The porous Ni5Zn21 phase in Zn-20 wt.%Sn/Ni exhibited linear growth. In contrast, the growths of the dense Ni5Zn21 in the Zn-Sn/Ni (30 wt.% to 50 wt.% Sn) reactions were parabolic in aging time. The porous structure is favorable for the diffusion of the dominant diffusion species. In contrast, the dense phase retards the diffusion and thus results in a tendency towards diffusion-limited, parabolic growth.
The porous structure of the SnTe phase enhanced the diffusion of liquid solder. For the reactions with pure Sn, the Sn atoms of solder diffuse across the porous SnTe and the dense SnTe layer, react with Te atoms of (Bi,Sb)2Te3, and then produce the new SnTe phase at the dense SnTe/(Bi,Sb)2Te3 interface. The excess Bi and Sb in the dense SnTe would diffuse outwards and dissolve into the molten solder. For the Sn-Ag solder, Ag atoms also diffuse towards the (Bi,Sb)2Te3 to form the Ag-rich phase. As mentioned above, the Ag-rich phase had almost the same element ratios as (Bi,Sb)2Te3 and the Sn content was very limited. These results suggest that Ag could have stronger affinity with (Bi,Sb)2Te3 than Sn. However, the Ag-rich phase could be a metastable phase with nominal composition of Ag6BiSb3Te6, which only appeared during the rapid and nonequilibrium interfacial reaction process. On the other hand, the linear SnTe growth suggested that the SnTe formation was dominated by reaction, rather than diffusion. As a result, the Ag-rich phase layer might severely influence the chemical reaction between Sn and Te or retard the nucleation of SnTe, rather than block the Sn diffusion. This can reasonably explain why the addition of Ag strongly inhibits the SnTe growth.
Conclusion
This study investigated the interfacial reactions of commercial p-type (Bi,Sb)2Te3 with Sn and SAC305 solders at 250°C. The soldering plane of the highly orientation TE material was (110), which was perpendicular to the c-axis. In the liquid-state Sn/(Bi,Sb)2Te3 reactions, SnTe was the major reaction phase at the initial stage. The SnTe phase had two distinctly different microstructures, viz. porous and dense phases. The dense to porous phase transition occurred due to Sb dissolution–recrystallization structural transformation. Additionally, there were many tiny cracks within the dense phase layer, resulting from compressive stress induced by the volume expansion during the fast SnTe growth. As the reaction progressed, the IMC microstructure changed to three different zones: porous SnTe, SnTe/solder alternating layers, and SnTe/Sn3Sb2 alternating layers. The fast and linear IMC growth can be attributed to the porous structure, which favors convection and diffusion of liquid solder to react with (Bi,Sb)2Te3. The reactions with SAC305 solder were also conducted. Similarly, the SnTe phase was formed with porous and dense structures. Elemental mapping demonstrated that one very thin Ag-rich phase layer was present between the SnTe and (Bi,Sb)2Te3. The SnTe growth was greatly suppressed due to the hindrance of the Ag-rich layer. In the Sn-3.5 wt.%Ag/(Bi,Sb)2Te3 reaction, it was verified that the addition of Ag to the solder effectively retarded the IMC growth. The Ag-rich phase with nominal composition of Ag6BiSb3Te6 was not an equilibrium phase.
References
G.J. Snyder and E.S. Toberer, Nat. Mater. 7, 105 (2008).
H. Alam and S. Ramakrishna, Nano Energy 2, 190 (2013).
H.J. Goldsmid, Materials 7, 2577 (2014).
W. He, G. Zhang, X. Zhang, J. Ji, G. Li, and X. Zhao, Appl. Energy 143, 1 (2015).
S. Fan, J. Zhao, J. Guo, Q. Yan, J. Ma, and H.H. Hng, Appl. Phys. Lett. 96, 182104 (2010).
Y. Hosokawa, K. Tomita, and M. Takashiri, Sci. Rep. 9, 10790 (2019).
C.V. Manzano, B. Abad, M. Munoz Rojo, Y.R. Koh, S.L. Hodson, A.M. Lopez Martinez, X. Xu, A. Shakouri, T.D. Sands, T. Borca-Tasciuc, and M. Martin-Gonzalez, Sci. Rep. 6, 19129 (2016).
M. Wang, Z. Tang, T. Zhu, and X. Zhao, RSC Adv. 6, 98646 (2016).
A. Krishna, N. Vijayan, B. Singh, K. Thukral, and K.K. Maurya, Mater. Sci. Eng. A 657, 33 (2016).
I.T. Witting, T.C. Chasapis, F. Ricci, M. Peters, N.A. Heinz, G. Hautier, and G.J. Snyder, Adv. Electron. Mater. 5, 1800904 (2019).
X. Yan, B. Poudel, Y. Ma, W.S. Liu, G. Joshi, H. Wang, Y. Lan, D. Wang, G. Chen, and Z.F. Ren, Nano Lett. 10, 3373 (2010).
T.Y. Lin, C.N. Liao, and A.T. Wu, J. Electron. Mater. 41, 153 (2012).
C.-F. Lin, N.Y. Hau, Y.-T. Huang, Y.H. Chang, S.-P. Feng, and C.-M. Chen, J. Alloys Compd. 708, 220–230 (2017).
W.-C. Lin, Y.-S. Li, and A.T. Wu, J. Electron. Mater. 47, 148 (2018).
S.-W. Chen, Z.-W. Liu, H.-S. Chu, and Z.-Y. Huang, J. Alloys Compd. 7031, 111 (2018).
S.-W. Chen, H.-J. Wu, C.-Y. Wu, C.-F. Chang, and C.-Y. Chen, J. Alloys Compd. 553, 106 (2013).
S.-W. Chen, C.-Y. Wu, H.-J. Wu, and W.-T. Chiu, J. Alloys Compd. 611, 313 (2014).
S. Ye, J.-D. Hwang, and C.-M. Chen, Metall. Mater. Trans. A 46, 2372 (2015).
C.-H. Wang, M.-H. Li, C.-W. Chiu, and T.-Y. Chang, J. Alloys Compd. 767, 1133 (2018).
H. Zhang, H.Y. Jing, Y.D. Han, L.Y. Xu, and G.-Q. Li, J. Alloys Compd. 576, 424 (2013).
H.-J. Wu, A.T. Wu, P.-C. Wei, and S.-W. Chen, Mater. Res. Lett. 6, 244 (2018).
F. Xu, F. Zhang, C. Yuan, R. Wang, H. Chen, Y. Ouyang, and X. Tao, Guangxi Sci. 24, 361 (2017).
S.-K. Lin, M.-Y. Tsai, P.-C. Tsai, and B.-H. Hsu, Sci. Rep. 4, 4557 (2015).
M. He, X. Su, F. Yin, J. Wang, and Z. Li, Scripta Mater. 59, 411 (2008).
S.-W. Chen, C.-C. Chen, W. Gierlotka, A.-R. Zi, P.-Y. Chen, and H.-J. Wu, J. Electron. Mater. 37, 992 (2008).
J. Yang, Q. Zhang, J.Y. Lee, and H.P. Too, J. Colloid Interface Sci. 308, 157 (2007).
G. Xi, K. Xiong, Q. Zhao, R. Zhang, H. Zhang, and Y. Qian, Cryst. Growth Des. 6, 577 (2006).
C.-H. Wang and S.-W. Chen, Acta Mater. 54, 247 (2006).
H.-J. Wu and S.-W. Chen, Acta Mater. 59, 6463 (2011).
C.-H. Wang, S.-E. Huang, and C.-W. Chiu, J. Alloys Compd. 619, 474 (2015).
C.-H. Wang and K.-T. Li, J. Mater. Sci. 51, 7309 (2016).
C.-H. Wang, H.-H. Chen, and P.-Y. Li, Mater. Chem. Phys. 136, 325 (2012).
Acknowledgements
Funding support of this research by the Ministry of Science and Technology of Taiwan under Project No. MOST 108-2221-E-194-036 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Ch., Li, Mh., Chiu, Cw. et al. Liquid-State Interfacial Reactions of Sn and Sn-Ag-Cu Solders with p-Type (Bi,Sb)2Te3 Thermoelectric Material. JOM 72, 3558–3566 (2020). https://doi.org/10.1007/s11837-020-04288-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-020-04288-0