Abstract
Fluoride molten-salt electrolytic slag is an important secondary resource for recovery of rare earth elements. In this study, the recovery of rare earths from this source was systemically investigated using borax roasting followed by hydrochloric acid leaching. Increasing the roasting temperature, reaction time and borax dosage promoted the recovery of rare earth elements. The roasting process kinetics was controlled by interfacial chemical reaction, with an activation energy of 159.02 kJ mol−1. The leaching experiments demonstrated that increasing the leaching temperature, time, hydrochloric acid concentration and liquid/solid ratio improved the rare earth recovery. The optimum conditions were identified as roasting the slag at 700°C for 60 min with a borax mass dosage of 38 wt.% and subsequently leaching the resulting rare earth-containing residues in 4 mol L−1 HCl at 60°C at a liquid/solid ratio of 5:1 for 40 min. These conditions gave a rare earth recovery exceeding 97%. This work proposes a novel technical route for efficiently and economically recovering rare earths from this source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth (RE) elements are widely used in photoelectric, magnetic and alloy materials because of their unique properties.1,2,3 In recent years, demand for RE metals and alloys has grown rapidly with increasing consumption of NdFeB and other permanent magnetic materials. In modern industrial practices, RE metals are mainly produced by molten-salt electrolysis using RE chlorides (RECl3), fluorides (REF3) or oxides (RE2O3) as raw materials;4 however, the RE yield is usually < 95%, resulting in the enrichment of residual RE in the fluoride molten-salt electrolytic slag (FMSES). It is estimated that about 5000 tons of RE oxides are lost via this route every year in China.5 The utilization of secondary RE-containing resources has become a popular research topic in recent years owing to the low content and uneven distribution of RE in ores.6,7,8,9,10,11 Efficient and economical recycling from FMSES is therefore of crucial importance for sustainable rare earth production.
FMSES is usually characterized by its high RE content in the form of fluorides and oxides together with graphite powder (from graphite electrolytic cells) and iron oxide (from entrainment of steel tools or equipment) as impurities. RE oxides can be readily recovered by acid treatment, while RE fluorides hardly react with either hydrochloric or sulfuric acids. The current methods of recovering RE from fluorides are by NaOH digestion or by roasting. When digested in concentrated NaOH solution, the RE fluorides in FMSES convert to insoluble RE(OH)3 and soluble NaF at a certain temperature, allowing the separation of RE3+ and F− by their difference in solubility. According to the research of Chen12 and Sun,13 the reaction temperature is a key factor for the recovery of REF3, with this route giving a final RE recovery of only about 90% under optimized conditions. In the roasting method, the separation of RE and fluorine through roasting has been investigated extensively in the treatment of bastnaesite.14,15 Additives are required to convert RE fluoride to the respective oxide: recovery of the RE is achieved in a subsequent washing or leaching process. There are many papers concerning the reactions of REF3 with NaOH16,17 and Ca(OH)218 in the roasting process, as demonstrated by Eqs. 1 and 2. However, use of NaOH would seriously corrode the furnace lining, while Ca(OH)2 needs a higher roasting temperature (about 950°C) to obtain sufficient recovery. Recently, Liang et al.19 recovered RE from a lanthanum-containing electrolytic slag by adding Na2SiO3 to the roasting process: the recovery reached about 99% in the leaching process, but a roasting temperature as high as 850°C was still required.
It is critical to the efficient recovery of RE from FMSES that the RE fluorides can be converted to RE oxides at lower temperatures. Boron salts have successfully been used as defluorination agents in the treatment of fluoride-containing magnetic tailings.20 Roasting FMSES with the addition of borax, followed by subsequent hydrochloric acid leaching, was studied in this article with the aim of reducing production cost by converting RE fluorides in FMSES at a lower temperature. The effects of roasting temperature, roasting time, borax dosage, leaching temperature, leaching time, hydrochloric acid concentration and the liquid-to-solid ratio (L/S ratio, mL/g) on the RE recovery were systemically investigated. The FMSES conversion mechanism in the roasting process and the optimum conditions for RE recovery were clarified to provide theoretical and technical support for the economic and efficient utilization of FMSES.
Experimental
Materials
Analytical-grade reagents were used, including borax, sodium hydroxide and hydrochloric acid. Deionized water was used in solution preparation and for washing. Samples of FMSES were obtained from a plant located in Longnan, Jiangxi Province, China, crushed by a ball mill and sieved into granules (< 150 μm). The compositions of the FMSES and RE distribution are presented in Table I.
An x-ray diffraction (XRD) pattern of the FMSES is presented in Fig. 1. The RE elements were mainly present as fluorides (NdF3 or PrOF); graphite powder and Fe2O3 were present as impurities. Analysis of the FMSES is shown in Table I, indicating that the total REO content was 38.62 wt.%. The main RE elements were neodymium, praseodymium and lanthanum.
Procedures
The roasting experiments were performed in a muffle furnace at a preset temperature, in which well-mixed FMSES and borax (particle size < 150 μm) were placed in a corundum crucible and roasted in air for a certain duration. The roasted product was cooled to ambient temperature in air and then washed twice in a 2 mol L−1 NaOH solution at 85°C for 60 min, using an impeller speed of 200 r min−1 and L/S ratio of 5:1 to separate the RE and fluoride. In the washing step, unreacted RE fluorides can further convert to RE hydroxides by reacting with NaOH in solution.21 The filtered residue was washed in hot water, dried at 50°C for 24 h and then leached in a HCl solution. The leaching experiments were conducted in a beaker heated using a water bath. After filtering, the RE contents of the solution and the leach residue were analyzed by Ganzhou Accurate Testing & Technology Co., Ltd. The total amount of rare earth oxides (TREO) was determined by the oxalate gravimetric method (GB/T 14635-2008, China), with the analytical error of < 0.4 wt.%. RE element analyses were conducted by inductively coupled plasma emission spectrometer (ICP, 725-OES, Agilent Technologies, USA) according to the national standard of China (GB/T 18882.1-2008, China), with the analytical error of < 0.7 wt.%. Solid samples were characterized by x-ray diffractometry (Empyrean, PANalytical B.V., The Netherlands) with Cu Kα radiation and a scanning speed of 10° per minute to determine the phase transformation qualitatively in the roasting process.
Method
Rare Earth Recovery
Owing to the complex composition of FMSES, it was difficult to directly determine the conversion of RE fluorides during the roasting process. Therefore, after fluoride removal, the roasted product was dissolved in hydrochloric acid solution (hydrochloric acid 5 mol L−1, 200 r min−1, L/S ratio 5:1, 85°C, 60 min), and the RE contents in the solution and residue were determined. The RE recovery in the combined roasting and hydrochloric acid leaching process was calculated according to Eq. 3:
where λ is the RE recovery, %; m is the mass of leaching residue, g; ω is the RE content in leaching residue, %; M represents the mass of FMSES, g; and ε is the RE content in FMSES, %.
Kinetic Analysis of Roasting Process
A kinetic analysis of the FMSES roasting process was performed using the Kissinger method.22,23 The activation energy of the roasting process was estimated using multiple differential scanning calorimetry (DSC) measurements. A 10-mg mixture of FMSES and borax (particle size < 150 μm) was placed in a corundum crucible and detected with DSC (DIAMOND TG/DTA6300, PerkinElmer, USA) at different heating rates. High-purity nitrogen (> 99.999%) was used as the protective gas at a flow rate of 20 mL min−1. Accordingly, the activation energies were obtained from Eq. 4:
where Ea is the activation energies of the roasting process, J mol−1; R is the gas constant, 8.314 J (mol K)−1; Tm is the temperature at the peak valley on the DSC curve, K; β is the heating rate, °C min−1.
Results and Discussion
Roasting Process
The effect of roasting temperature on the RE recovery was first investigated with a roasting duration of 60 min. The results are shown in Fig. 2a. Elevated roasting temperature was of benefit to the RE recovery, which rapidly increased from 73% to 92% with an increase in the roasting temperature from 500°C to 600°C. This indicated that conversion of the RE fluorides can be accelerated at higher temperatures. With a further increase of roasting temperature to 750°C, the RE recovery slowly increased from about 92% to 97%, but did not improve significantly further beyond a certain value. Thus, considering both the RE recovery and the energy consumption, 700°C was adopted for optimizing the roasting process.
Figure 2b presents the effect of roasting time on the RE recovery at 700°C. As is customary, prolonged roasting time favored the RE recovery. It increased from 90.8% to 96.7% with an increase in the roasting time from 10 min to 40 min. The recovery improved slightly further, to about 98% after 80 min. Therefore, an appropriate roasting time was chosen as 60 min, which was considered adequate to achieve a complete conversion of the RE fluorides.
Figure 2c shows the effect of borax dosage on the RE recovery at 700°C for a reaction time of 60 min. The results indicate that increasing the borax dosage facilitated the RE recovery. When the borax dosage increased from 15% to 38% (relative to the mass of FMSES), the RE recovery increased from 85% to 97%. With further increase of borax dosage, the RE recovery increased only slightly. The RE fluorides reacted almost completely at a borax dosage of 38%, so this value was adopted in the later experiments.
The optimized conditions for the roasting process were at a temperature of 700°C, reaction time of 60 min and a borax mass dosage of 38%. An XRD analysis of the roasted product in optimized conditions is displayed in Fig. 3. Compared with Fig. 1, the diffraction peaks of the RE fluorides were significantly reduced after roasting, while those of RE oxides (Nd2O3, Pr6O11) and Pr(BO2)3 appeared. Additionally, the characteristic peaks of graphite and Fe2O3 were still visible, owing to the low roasting temperature and short roasting duration. Furthermore, the difference in fluorine contents before and after roast in the solid samples under optimized conditions was < 0.14%, indicating that fluorine does not enter the gas phase in the form of F2 or HF. Meanwhile, unreacted RE fluorides may exist in the roasted product, such as some LaF3 in Fig. 3, which can convert to RE hydroxide in the subsequent NaOH washing step by Eq. 7.21
Thus, the conversion of RE fluorides with borax in the roasting step probably occurred according to Eqs. 5 and 6. Based on the thermodynamic data from resources24 and,25 the Gibbs energy change of Reaction (5) was calculated as − 2368.06 kJ mol−1 at 700°C, and it was − 1964.54 kJ mol−1 while using Na2B4O7. Because of similar properties of rare earth elements, Reaction (6) can also occur thermodynamically at 700°C despite lacking thermodynamic data for PrOF. Furthermore, molten B2O3 can promote the roasting reaction because of its low melting point of ~ 450°C.26 This also can be confirmed by no B2O3 phase being detected by XRD in the roasted product.
To further study the FMSES conversion mechanism in the roasting process, a kinetic analysis was carried out using the Kissinger method.22,23 The resulting DSC curves at different heating rates are shown in Fig. 4. The exothermic peak appears at about 620°C under various heating rates, indicating that the reactions happen in a temperature range of 550–675°C, yielding the obtained temperatures (Tm) representing the valley of the curves at different heating rates. Combining these data with the corresponding heating rates (β) gave the plot shown in Fig. 5, from which the activation energy of the reaction in the roasting process can be obtained from the slope of the best-fitting line. The slope of the fitted linear relationship between \( - R\ln \frac{\beta }{{T_{m}^{2} }} \) and \( T _{m}^{ - 1} \) was 159.02 × 103, indicating that the apparent activation energy (Ea) of FMSES reacting with borax in the roasting process was 159.02 kJ mol−1. Therefore, it was inferred that the roasting process is controlled by an interfacial chemical reaction, which is in agreement with the above experimental results.
Leaching Process
The leaching experiments were conducted with the roasting product obtained under optimized conditions. The influences of leaching temperature, leaching time and hydrochloric acid concentration on the RE recovery were studied.
The effect of leaching temperature is shown in Fig. 6a. Increasing the leaching temperature increased the RE recovery, which reached 97% at 60°C. It did not obviously increase further at higher temperatures > 50°C. Consequently, 60°C was selected as an appropriate leaching temperature.
The effect of leaching time on the RE leaching recovery is described in Fig. 6b. The RE recovery increased moderately with an increase of leaching time from 10 min to 50 min, reaching about 97% in 40 min. Thus, the leaching time of 40 min was chosen.
The hydrochloric acid concentration is usually the governing factor of the RE recovery in industrial practice. The effect of hydrochloric acid concentration on the RE recovery is presented in Fig. 6c. As predicted, hydrochloric acid concentration had a significant influence: the RE recovery was 96% in 4 mol L−1 HCl. Higher HCl concentrations did not further accelerate the leaching process, owing to the sufficient HCl for the leaching reaction.
The effect of the L/S ratio on the RE recovery is shown in Fig. 6d. The recovery increased from 41% to 97% with an increase of the L/S ratio from 2:1 to 5:1. This can be attributed to the lack of sufficient hydrochloric acid to recover RE and to the limited solubilities of RE chlorides in the solution.26 Moreover, no obvious improvement of RE recovery was achieved with a further increase of the L/S ratio to 6:1. Thus, an appropriate L/S ratio of 5:1 was assigned to the leaching process.
Proposed Process Flow Sheet
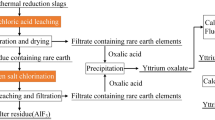
Based on the discussion above, a process flow sheet for treatment of FMSES was developed, as described in Fig. 7. RE elements can be recovered efficiently from FMSES by borax roasting followed by hydrochloric acid leaching. The optimum conditions found in this study were roasting of the FMSES at 700°C for 60 min with a borax dosage of 38% and subsequently leaching the roasted RE-containing calcine in 4 mol L−1 hydrochloric acid solution at 60°C for 40 min with a L/S ratio of 5:1. Under these conditions, the RE recovery exceeded 97% with graphite and iron oxides remaining in the residues. The resulting RE-containing solution can be processed to further separate the individual RE elements.
Conclusion
This work proposes a novel technical route for recovering RE from FMSES, featuring borax roasting followed by hydrochloric acid leaching. The RE fluorides react with borax to form RE oxides in the roasting step. Increasing the roasting temperature, reaction time and borax dosage promoted the RE recovery. A kinetic analysis showed that the roasting process was controlled by interfacial chemical reaction. The optimized conditions of the roasting process were a temperature of 700°C for 60 min with a borax dosage of 38 wt.%.
The effects of leaching temperature, leaching time and hydrochloric acid concentration on the RE recovery were also studied. The optimum conditions for the leaching step of the roasted FMSES were a 4 mol L−1 hydrochloric acid solution at 60°C, leaching time of 40 min and a L/S ratio of 5:1. The RE leaching recovery exceeded 97%. This work will contribute to the development of a novel and more efficient treatment of FMSES for RE recovery, providing technical and theoretical support for the feasible recovery of RE from various FMSES-type residues.
References
P. Bakke, K. Pettersen, and H. Westengen, JOM 55, 46 (2003).
T. Du, Chin. J. Nonferrous Met. 6, 15 (1996).
J.S. Rao, H.J. Li, H.S. Xue, and J. Cent, South Univ. Technol. 17, 28 (2010).
H.M. Zhu, Rare Earth Metal Production by Molten Salt Electrolysis (New York: Springer, 2014), pp. 1765–1772.
Z.H. Chen, Adv. Mater. Ind. 6, 31 (2018).
B.N. Deng, G.H. Li, J. Luo, Q. Ye, M.X. Liu, M.J. Rao, Z.W. Peng, and T. Jiang, JOM 70, 2869 (2018).
C.R. Borra, B. Blanpain, Y. Pontikes, B. Koen, and T.V. Gerven, JOM 68, 2958 (2016).
M.D. Liu, Z.X. You, Z.W. Peng, X. Li, and G.H. Li, JOM 68, 567 (2016).
Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B. Steenari, T.V. Gerven, P.T. Jones, and K. Binnemans, J. Sustain. Metall. 3, 122 (2017).
K. Binnemans, P.T. Jones, B. Blanpain, T.V. Gerven, Y. Yang, A. Walton, and M. Buchert, J. Clean. Prod. 51, 1 (2013).
J.W. Darcy, H.M.D. Bandara, B. Mishra, B. Blanplain, D. Apelian, and M.H. Emmert, JOM 65, 1381 (2013).
D.Y. Chen, Y.H. Ou, L.C. Liu, and G. Xie, Jiangxi Metall. 25, 4 (2005).
D.J. Sun, Chinese Patent No. CN104818390A (2015).
P. Cen, W.Y. Wu, and X. Bian, Metall. Mater. Trans. B 48, 1539 (2017).
Y.K. Huang, T.A. Zhang, Z.H. Dou, J. Liu, and F.F. Tang, Rare Met. 35, 649 (2016).
Y.B. Lu and S.D. Zhu, Chinese Patent No. CN105256156A (2016).
D.Y. Hu, L.C. Li, and R.G. Zhang, Chinese Patent No. CN104843761A (2015).
J. Lin, Q.P. He, and C.X. Li, Chinese Patent No. CN101956078A (2011).
Y. Liang, Y.K. Li, L.Y. Xue, and Z. Yu, J. Clean. Prod. 177, 567 (2018).
Y. Li, H.X. Lei, and X.W. Huang, J. Chin. Soc. Rare Earths 32, 604 (2014).
G.X. Xu, Rare Earths, 2nd ed., Vol. 1 (Beijing: Metallurgical Industry Press Co., Ltd., 2005), p. 367.
H.E. Kissinger, Anal. Chem. 29, 1702 (1957).
P. Budrugeac and E. Segal, J. Therm. Anal. Calorim. 88, 703 (2007).
A. Roine, HSC Chemistry, vers. 9.0. (Pori: Outotec Research Oy, 2016). http://www.outotec.com/products/digital-solutions/hsc-chemistry/. Accessed 25 June 2019.
M.W. Chase, J.L. Curnutt, A.T. Hu, H. Prophet, A.N. Syverud, and L.C. Walker, J. Phys. Chem. Ref. Data 3, 311 (1974).
J.A. Dean, Lange’s Handbook of Chemistry, 2nd ed. (Beijing: China Science Publishing & Media Ltd., 2003), pp. 3.45–3.47.
Acknowledgements
The authors are grateful to the National High Technology Research and Development Program of China (863 Program, Grant No. 2012AA061901) and the National Natural Science Foundation of China (Grant No. 51774155) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Wei, T., Xiao, M. et al. Rare Earth Recovery from Fluoride Molten-Salt Electrolytic Slag by Borax Roasting-Hydrochloric Acid Leaching. JOM 72, 939–945 (2020). https://doi.org/10.1007/s11837-019-03732-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03732-0