Abstract
The leaching behavior of lead and silver from lead sulfate residues in NaCl-CaCl2-NaClO3 media was studied systematically. The results indicate that Cl− concentration, NaClO3 concentration, leaching time and temperature had significant effects on lead and silver leaching, whilst agitation speed and the liquid–solid ratio had a secondary effect on silver leaching but little effect on lead leaching. The Ca2+ concentration had an important effect on \( {\text{SO}}_{4}^{2 - } \) removal but little effect on lead and silver leaching. The optimum parameters for lead and silver leaching were: initial Cl− concentration: 207.5 g/L; NaClO3 concentration: 12 g/L; Ca2+ concentration: 1.3 times of stoichiometric quantity; temperature: 90 ± 2 °C; time: 1 h; liquid–solid ratio: 8:1–10:1; and agitation speed: 450 rpm. The lead and silver leaching were > 98% and 95%, respectively. The removal ratio of \( {\text{SO}}_{4}^{2 - } \) was > 98%. The leaching kinetics of lead follow the shrinking core model of mixed control, and the apparent activation energy was 13.4 kJ/mol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amounts of lead sulfate hazardous residues (LSHR) are produced in zinc hydrometallurgical processes. They have a high value in comprehensive recovery and utilization due to the contained lead, silver, copper, zinc, cadmium and other valuable metals. Unfortunately, the residues are usually stockpiled until the recovery of valuable metals from the residues becomes economic. This not only wastes resources but also causes environmental pollution due to the presence of toxic substances such as lead, cadmium and other elements in the residues. With the decrease of mineral resources and the increasingly strict control of environmental protection, the residues are becoming more and more important as a secondary source of metals and as a target for remediation. Recently, some techniques have been studied to treat the residues. The main techniques are to form agglomerates, which are fed into blast furnaces, or to dry and then feed them into intensive smelting furnaces such as Top-blown Submerged Lancing Technology,1 the Oxygen Bottom-blowing Process2 and Kivcet.3 There are some disadvantages to these pyrometallurgical processes, such as emissions of lead and SO2 off-gas, which are quite difficult to control, high energy consumption, and complex furnace design. Therefore, some hydrometallurgical processes have been studied to treat the residues.
Lead sulfate can be leached in sodium carbonate, ammonium carbonate or sodium hydroxide solutions to transform it into lead carbonate or lead hydroxide, including complex compounds, such as Pb3(CO3)2(OH)2, NaPb2(CO3)2(OH) and Pb4(SO4)(CO3)2(OH)2.4,5,6,7,8,–9 The formation of these compounds increases the reagent consumption and makes the subsequent processing for lead/silver extraction and the use of the final products more challenging. The products are not suitable for pyrometallurgical extraction of lead, while the high cost of sodium carbonate or sodium hydroxide makes the processes unattractive.
Ammonia-ammonium-sulfate or ammonium-acetate leaching has also been studied for lead recovery from lead-bearing residues. This process leaves most of the silver in the resultant residues. It has a high reagent cost and further processing is required for silver extraction from the leach residues. Hence, the processes are not attractive.
Sodium chloride can be used as a lixiviant for lead sulfate and silver sulfate. Reactions (1) and (2) depict the reactions between chloride ions and lead sulfate/silver sulfate:
In the presence of high chloride concentrations, PbCl2 subsequently converts to \( {\text{PbCl}}_{3}^{ - } \) and \( {\text{PbCl}}_{4}^{2 - } \) with high solubility as shown in the following reactions (3) and (4):
Similarly, \( {\text{AgCl}}_{2}^{ - } \), \( {\text{AgCl}}_{3}^{2 - } \) and \( {\text{AgCl}}_{4}^{3 - } \) can be formed as reaction (5) in the brine leaching processes:
Some researchers10,11,12,13,14,15,–16 have applied sulfuric acid leaching to transfer zinc into solution and make lead-enriched residues. Further leaching with brine (using NaCl) dissolved the lead and silver. The recoveries of lead and silver were > 80% and 60–80%, respectively. These studies indicate that the recovery of silver was not high and the negative effect of sulfate ion on the subsequent processes for the extraction of lead and silver was not considered. Sinadinović et al.17 investigated the kinetics of lead leaching from lead sulfate in calcium chloride and magnesium chloride solutions. Lead leaching was a diffusion and chemically controlled reaction while the sulfate ion could be precipitated as an insoluble calcium sulfate from the leachate in reaction (6):
Apart from the above, a biological conversion of anglesite into galena was developed by Schröder-Wolthoorn et al.18 The formation of PbS was confirmed by the increased Pb:O ratio in the sludge (1:0.1) relative to the Pb:O ratio in the PbSO4-containing residue (1:3.3).
In the present work, the leaching behavior of lead and silver from LSHR in NaCl-CaCl2-NaClO3 media has been studied. The literature on this process was inadequate. The process is based on the formation of complex chlorides of lead and silver and insoluble calcium sulfate. It not only improves lead and silver recovery but also avoids the negative effect of the sulfate ions. It has important practical and scientific significance in saving resources and protecting the ecological environment. A lead hydrometallurgical plant in Yunnan Province, China, is now applying this process. In the present paper, optimization of seven key parameters, namely Cl− concentration, NaClO3 concentration, Ca2+ concentration, leaching time, temperature, agitation speed and liquid-to-solid ratio were selected to verify the process performance. Furthermore, the leaching kinetics of lead at different temperatures was analyzed.
Materials and Methods
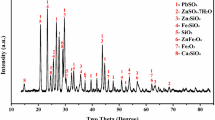
The LSHR used in this study were from a zinc hydrometallurgical plant in Yunnan, China. The residues were washed with 0.5–1 g/L sulfuric acid solution and then with water, then dried, ground, and used for all the experiments. The samples were screened using Tyler Sieves and a mechanical vibrator. About 90% of the materials were between 58 µm and 75 µm. This fraction was used in all the experimental work. The XRD pattern and SEM/EDS tests for LSHR were analyzed by a Rigaku D/MAX-rA diffractometer (CuKα radiation; 50 kV, 40 mA) and a Nova NanoSEM 450 with Oxford-Max50 EDS. The results are shown in supplementary Figs. S1 and S2, respectively. Supplementary Fig. S1 shows that the main lead phase was PbSO4. Supplementary Fig. S2(a) shows that the color of most of the particles with rough surfaces was white and off-white. Combining supplementary Fig. S1, supplementary Fig. S2(b), (c) and (d), shows that the phase composition of most of the particles was PbSO4. Supplementary Table SI shows the elemental assay fractions of spots a, b and c in Fig. S2(a). The chemical composition was determined by atomic absorption spectroscopy (AAS) and the titrimetric method given in supplementary Table SII. The contents of lead and silver were 18.02% and 0.0524%, respectively. Calcium chloride, sodium chloride and sodium chlorate were of analytical grade and supplied by Tianjin Chemical Reagent.
The leaching experiments were conducted in a 2-L beaker. Heating was provided by an electric mantle and the temperature of the slurry was adjusted by a temperature controller within ± 2 °C. The slurry was agitated by a two-blade impeller driven by a variable speed motor. Typically, a known amount of lixiviant solution was first heated to the desired temperature. Then 100 g (dry) LSHR was slowly fed into the solution with agitation. The reaction time was recorded with time zero corresponding to the moment when the entire lead sulfate residue had been added. After completion of leaching, the slurry was immediately vacuum-filtered. The filter cake was washed with hot deionized water to remove soluble salts. The filtrate was collected and the volume was recorded. The leach residues were dried in an air oven at 110 °C for 24 h. A sub-sample of leach residues was taken to analyze for Pb, Ag and S. The leaching efficiencies (a) of lead and silver were calculated by Eq. 1:
where mo and mt are the weights of raw materials and of leach residues in g, respectively, w0,i and wt,i are the lead or silver weight fraction in the raw materials and the leach residues in wt.%, respectively, and i is lead or silver.
In the kinetic experiments, the procedure was partly different from the previous leaching experimental procedure. The main differences are as follows: 5 g LSHR were mixed with 1 L brine solution for observing the kinetic experimental rules of a high liquid to solid ratio. In the experiments, about 20 mL of leachate were sampled each time, and their volumes recorded before analysis for lead. The influence of the sampling was taken into account when the mass balance results were calculated. The leaching rate of lead was calculated by Eq. 2:
where V is the initial volume of solution in L, Vi is the sample volume in L, Ci is the Pb concentration in g/L when sampled, and i is the number of the sample.
Results and Discussion
Leaching of Lead and Silver
Effect of Cl−, NaClO3 Concentration
A mixture of sodium chloride and calcium chloride was tried as a lixiviant for lead and silver. The lead and silver were dissolved by chloride to form complexes. The effect of the total Cl− concentration over the range 116.6–262.1 g/L on the leaching of lead and silver were studied in the following conditions: initial Ca2+ concentration 15 g/L; temperature 90 ± 2 °C; time 1 h; liquid-to-solid ratio 10:1; and agitation speed 450 rpm. The results in Fig. 1 show that the leaching efficiency of lead increased sharply from 70.4% to 95.4% with an increase of total Cl− concentration from 116.6 g/L to 146.9 g/L. This increased efficiency is mainly attributed to the increase of chloride ions in the solution. The leaching rate of lead was more than 95% when the total Cl− concentration was above 146.9 g/L, and it increased up to 98% when the total Cl− concentration was 207.5 g/L. The slight decrease at the higher concentration may be due to the formation of sodium jarosite, which may have covered the leach residues surface and hindered the reactions between the lixiviant and the solid particles.19 Therefore, the optimum total Cl− concentration appeared to be around 207.5 g/L for the leaching of lead. On the other hand, the leaching efficiency of silver was only about 80%. This may be because some of silver in the LSHR was in a reduced form, such as elemental silver or silver sulfide. For leaching of elemental silver and silver sulfide in chloride media, an oxidant is required. Reactions (7) and (8) depict the main reactions between silver, silver sulfide and sodium chlorate. Thus, the effect of NaClO3 concentration on the leaching of lead and silver was investigated using the total Cl− concentration of 207.5 g/L, keeping all other parameters constant (Fig. 2).
As shown in Fig. 2, the leaching rate of silver increased from 81.5% to 95.8% as the NaClO3 concentration increased from 1 g/L to 12 g/L while the leaching rate of lead was about 98%. A further increase in NaClO3 concentration up to 15 g/L had no significant effect on the leaching of lead and silver. Hence, all further experiments were performed by using the total Cl− concentration of 207.5 g/L and the NaClO3 concentration of 12 g/L.
Effect of Ca2+ Concentration
The presence of sulfate ion adversely affects the subsequent extraction of lead and silver from the leachate. In order to avoid the negative effect of the sulfate ions, CaCl2 was selected as a constituent of the lixiviant. It not only provides Cl− for the complexing of lead and silver but also provides Ca2+ for the generation of gypsum, as in reaction 6.
Figure 3 shows the effect of Ca2+ concentration on lead and silver leaching and the removal ratio (D) of \( {\text{SO}}_{4}^{2 - } \) from the leachate under the optimum conditions as before: a temperature of 90 ± 2 °C, time 1 h, liquid-to-solid ratio 10:1, and agitation speed 450 rpm. D was calculated by Eq. 3:
where ws and wt,s are the total S content in the raw materials and the leach residues in wt.%, respectively.
Figure 3 shows that the Ca2+ concentration had no significant effect on the lead and silver leaching. However, D increased sharply from 75.2% to 92.5% as the Ca2+ concentration increased from 5 g/L to 10 g/L. Then, the growth of D was slowed and reached more than 98% when the Ca2+ concentration was above 30 g/L. Thus, it appears that the majority of the \( {\text{SO}}_{4}^{2 - } \) can be removed from the leachate and transferred into the leach residue when 1.3 times of the stoichiometric quantity of calcium was fed into the lixiviant. Supplementary Fig. S3 and Fig. 4 show the XRD and SEM/EDS of the leach residues, respectively. The main phase of lead sulfate in the raw materials disappeared and new phases of gypsum were generated. Supplementary Table SIII shows the element fractions of spots a, b and c in Fig. 4(a).
Effect of Leaching Time
The effect of leaching time from 0.5 to 3 h on the lead and silver leaching was studied at the optimum conditions determined previously: a temperature of 90 ± 2 °C, liquid-to-solid ratio 10:1, and agitation speed 450 rpm. The results shown in Fig. 5 prove that a retention time of 1 h was sufficient for the lead and silver leaching. A shorter retention time caused a significantly lower extraction efficiency. Therefore, a retention time of 1 h was used in further experiments.
Effect of Leaching Temperature
The leaching of metals is often positively affected by the temperature. One reason is because the activation energy of most chemical reactions decreases with an increase of the reaction temperature, so that the effective collision frequency of the reactant molecules increases with a decrease of activation energy. Another reason is because the diffusion coefficient increases with an increase of the reaction temperature.
Figure 6 shows the effect of leaching temperature from 45 °C to 90 °C on lead and silver leaching under the optimum conditions as before and a liquid-to-solid ratio 10:1, and agitation speed 450 rpm. This indicates that the leaching rate of lead and silver increased from 87.6% to 97.7% and from 78.5% to 91.3% when the temperature increased from 45 °C to 75 °C respectively. While the leaching rate of silver increased up to 95.8% with a further increase in temperature to 90 °C, this increase in temperature had no significant effect on the lead leaching. Considering both the effect on lead and silver leaching, we chose a temperature of 90 °C for all further experiments.
Effect of Agitation Speed
Agitation plays an important role in leaching processes. It can improve the diffusion of reactant molecules which is a key step of the liquid–solid reaction. The results of agitation, as shown in Fig. 7, under the optimum conditions as before and a liquid-to-solid ratio 10:1, indicate that the effect of agitation speed on silver leaching was more than that of lead leaching. Leaching rate of silver increased from 90.1% to 95.8% as the agitation speed increased from 250 rpm to 450 rpm. A further increase in agitation speed up to 550 rpm had no significant effect on silver leaching while some slurry was splashed out of the beaker. Agitation speeds between 250 rpm and 550 rpm had no effect on the leaching rate of lead which remained at about 98%. So an agitation speed of 450 rpm was chosen.
Effect of Liquid-to-Solid Ratio
Generally, the liquid-to-solid ratio has an effect on the viscosity of the pulp and the consumption of leaching reagent, so that it affects the extraction efficiency and subsequent treatment. Considering the saturation solubility of lead and silver in the chloride media, the experimental conditions were designed at 6:1, 8:1, 10:1 and 12:1, keeping all the other parameters constant at their optimum value (supplementary Fig. S4). It appears that the leaching rate of lead is barely influenced, whilst the leaching rate of silver increased slowly with an increase in the liquid-to-solid ratio. Considering the negative effect of a large quantity of leachate in further processing, a liquid–solid ratio ranging from 8:1 to 10:1 was considered reasonable and was adopted in subsequent experiments.
Leaching Kinetics of Lead
The experimental kinetic data were analyzed on the basis of the shrinking core model.20 The integrated rate equation for lead dissolution is expressed in Eq. 4 assuming no precipitate layer covers to the unreacted core. Equation 5 shows the integrated rate equation considering insoluble products (CaSO4 or sodium jarosite) hinders diffusion as the reaction proceeds:
where α is the leaching rate in %, k is the apparent rate constant,and t is time in min.
Therefore, there is a linear relationship between \( 1 - (1 - \alpha )^{1/3} \), \( 1 - \frac{2}{3}\alpha - (1 - \alpha )^{2/3} \) and t. Also, from k at various temperatures, the apparent activation energy can be obtained from the Arrhenius Eq. 6:
Figure 8(a) shows the relationship between temperature and time on the leaching rate of lead, keeping all other optimum parameters constant. In order to clarify the quantitative relationship between k and temperature, the data were analyzed in terms of Eqs. 4 and 5 as plotted in Fig. 8(b) and (c), respectively. The linear regression equations between \( 1 - (1 - \alpha )^{1/3} \), \( 1 - \frac{2}{3}\alpha - (1 - \alpha )^{2/3} \) and t for different temperatures are listed in supplementary Table SIV. They indicate that a better linear relationship is obtained between \( 1 - (1 - \alpha )^{1/3} \) and t (r2 > 0.97) than between \( 1 - \frac{2}{3}\alpha - (1 - \alpha )^{2/3} \) and t (r2 > 0.86). The relationship between lnk and 1/T is given in Fig. 8(d) which shows a straight line from which the apparent activation energy can be derived as Ea = 13.4 kJ/mol according to Eq. 6. A kinetic equation for the effect of leaching temperature on lead leaching can be derived:
It was established that the leaching kinetics of lead from LSHR in NaCl-CaCl2-NaClO3 media follows the shrinking core model of mixed control relatively well. It seems that the fit is significantly better at the lower temperatures.
Conclusion
Leaching of lead and silver from lead sulfate hazardous residues in NaCl-CaCl2-NaClO3 media provided a high leaching rate of lead and silver, a short flow sheet, ecological friendliness and low energy consumption. The experimental results show that Cl− concentration, NaClO3 concentration, leaching time and temperature had significant effects on the leaching of lead and silver, whilst the agitation speed and liquid–solid ratio had secondary effects on the leaching of silver but had little effect on the leaching of lead. Ca2+ concentration had a minor impact on the leaching of lead and silver but an important effect on the removal of \( {\text{SO}}_{4}^{2 - } \) from the leachate.
Optimum process operating parameters were established as follows: initial Cl− concentration: 207.5 g/L; NaClO3 concentration: 12 g/L; Ca2+ concentration: 1.3 times of stoichiometric quantity of calcium; temperature: 90 ± 2 °C; time: 1 h; liquid-to-solid ratio: 8:1–10:1 and agitation speed: 450 rpm. Under these experimental conditions, the leaching rates of lead and silver were more than 98% and 95%, respectively, and the removal ratio of \( {\text{SO}}_{4}^{2 - } \) from the leachate was more than 98%.
It was established that the leaching kinetics of lead from LSHR in NaCl-CaCl2-NaClO3 media follows the shrinking core model of mixed control and the apparent activation energy was determined as 13.4 kJ/mol.
Leaching of lead and silver from lead sulfate hazardous residues in NaCl-CaCl2-NaClO3 media is feasible. A hydrometallurgical lead plant with an annual output of 30,000 tonnes lead has been found. In the future, extraction of lead from the leachate needs further study to improve the process.
References
J. Hoang, M.A. Reuter, R. Matusewicz, S. Hughes, and N. Piret, Mine. Eng. 22, 742 (2009).
J.M. Yao and D.B. Li, China Nonferrous Metall. 2, 30 (2015).
G.F. Chen and D.Q. Wang, Lead Metallurgy (Beijing: Metallurgical Industry Press, 2000), pp. 90–99.
Y. Gong, J.E. Dutrizac, and T.T. Chen, Hydrometallurgy 28, 399 (1992).
J.E. Dutrizac and T.T. Chen, Acta Metall. Slovaca 1, 5 (1998).
L.C. Ferracin, A.E. Chácon-Sanhueza, R.A. Davoglio, L.O. Rocha, D.J. Caffeu, A.R. Fontanetti, R.C. Rocha-Filho, S.R. Biaggio, and N. Bocchi, Hydrometallurgy 65, 137 (2002).
N.K. Lyakov, D.A. Atanasova, V.S. Vassilev, and G.A. Haralampiev, J. Power Sources 171, 960 (2007).
D. Atanasova, J. Univ. Chem. Tech. Metall. 2, 262 (2008).
M. Şahin and M. Erdem, Hydrometallurgy 153, 170 (2015).
R. Raghavan, P.K. Mohanan, and S.C. Patnaik, Hydrometallurgy 48, 225 (1998).
R. Raghavan, P.K. Mohanan, and S.R. Swarnkar, Hydrometallurgy 58, 103 (2000).
M.X. Liao and T.L. Deng, Mine. Eng. 17, 17 (2004).
A. RuŞen, A.S. Sun, and Y.A. Topkaya, Hydrometallurgy 93, 45 (2008).
F. Farahmand, D. Moradkhani, M.S. Safarzadeh, and F. Rashchi, Hydrometallurgy 95, 316 (2009).
M.Y. Ye, G.J. Li, P.F. Yan, L. Zheng, S.H.Y. Sun, ShS Huang, H.F. Li, Y. Chen, L.K. Yang, and J.L. Huang, Sep. Purif. Technol. 183, 366 (2017).
M.Y. Ye, P.F. Yan, S.H.Y. Sun, D.J. Han, X. Xiao, L. Zheng, ShS Huang, Y. Chen, and S.H.W. Zhuang, Chemosphere 168, 1115 (2017).
D. Sinadinović, Ž. Kamberović, and A. Šutić, Hydrometallurgy 47, 137 (1997).
A. Schröder-Wolthoorn, S. Kuitert, H. Dijkman, and J.L. Huisman, Hydrometallurgy 94, 105 (2008).
Z.H. Guo, F.K. Pan, X.Y. Xiao, L. Zhang, and K.Q. Jiang, Trans. Nonferrous Met. Soc. China 20, 2000 (2010).
X.W. Yang and D.F. Qiu, Hydrometallurgy, 2nd ed. (Beijing: Metallurgical Industry Press, 2011), pp. 142–161.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (No. 51764026; No. 51364019; No. 51564029). The authors gratefully acknowledge Prof. David B. Dreisinger of The University of British Columbia and Prof. Chunmei Tan of the Yunnan Copper group for English editing.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Jin, B., Song, Q. et al. Leaching Behavior of Lead and Silver from Lead Sulfate Hazardous Residues in NaCl-CaCl2-NaClO3 Media. JOM 71, 2388–2395 (2019). https://doi.org/10.1007/s11837-019-03472-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03472-1