Abstract
Al2O3-TiCN-Fe composites were successfully prepared by hot pressing using natural ilmenite, aluminum, and carbon. The process of aluminothermic reduction of ilmenite and the influence of carbon on the phase evolution, synthetic products, microstructure, and properties were investigated using x-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and mechanical analysis. The XRD results showed that the reduction process of the FeTiO3-2Al system was a gradual deoxygenation process. In the FeTiO3-2Al-xC systems, carbon participates in the reduction reaction to form TiN, which gradually transforms to TiCN. The carbon content had a great influence on the presence of titanium oxides during the reaction. In addition, the grain size of Al2O3, TiCN, and Fe phases became smaller as the carbon content was increased. When the carbon content was 0.88 mol, the synthesized products achieved comprehensive mechanical properties, i.e., bending strength and Vickers hardness of 375 MPa and 15.5 GPa, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ilmenite has attracted interest from many researchers worldwide because of its abundant reserves and richness in two important metal elements, i.e., Fe and Ti. Different types of composite materials can be obtained by chemical reactions between ilmenite and different reducing agents. Research studies have focused on carbon reduction,1,2 metal reduction,3,4 and combined reduction of metal and carbon.5,6,–7 Among these reductions, the chemical reaction between ilmenite, aluminum, and carbon has been investigated extensively to prepare Al2O3-TiC-Fe or Al2O3-TiCN-Fe composites in a nitrogen-containing atmosphere because Al2O3-TiC/TiCN-Fe ceramics have excellent mechanical properties, such as resistance to high temperature, wear, corrosion, and oxidation.8,9
Various techniques can be used to manufacture Al2O3-TiC/TiCN-Fe composites. Among these, in situ techniques have received extensive attention,10,11,–12 including mechanical alloying, reactive sintering, microwave heating, or self-propagating high-temperature synthesis. Reinforcement can be synthesized by the reaction in composites prepared by such in situ techniques, which then nucleate and grow spontaneously. Thus, the reinforcement surface is not polluted, the matrix and reinforcement show good compatibility, and the resulting material exhibits high interfacial bonding strength. In situ synthesis methods simultaneously eliminate the cumbersome pretreatment reinforcement process, simplifying the preparation process. These features lead to better mechanical properties compared with composites processed using traditional, ex situ approaches.13,14,–15
Several studies have explored the FeTiO3-Al-C system, mostly10,16,17 focusing on preparation of Al2O3-TiC/TiCN-Fe composite powders, but the preparation, microstructure, and properties of such composites have not been investigated in depth. In some studies,18,19,–20 Al2O3-TiC/TiCN-Fe composites were synthesized through the FeTiO3-Al-C system, but the phase evolution during the formation process has not yet been thoroughly investigated. In addition, many investigations21,22,–23 have focused on synthesis of Al2O3-TiC composite by TiO2, mainly investigating the preparation of powders instead of materials. The reaction mechanism has not been further studied, and the reaction sequences, aluminum reduction processes, and effect of C on the reaction process, microstructure, and properties remain unclear.
In the work presented herein, Al2O3-TiCN-Fe composites were synthesized by hot pressing using in situ reaction between natural ilmenite, aluminum, and carbon. The reaction process and mechanism of the FeTiO3-2Al-xC systems during hot-pressing processes were explored using the carbon-free FeTiO3-2Al system and carbon-containing FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C systems. The influence of carbon on the phase evolution, microstructure, and properties of the product was also studied.
Experimental Procedures
Material Synthesis
Ilmenite (average particle size approximately 158.26 μm; Panzhihua Mineral, Panzhihua, China), aluminum (> 99% purity, < 80 μm), and graphite powder (> 99% purity, < 30 μm) were used as raw materials. Table I presents the chemical composition of ilmenite. Aluminum and graphite powder were used as the reducing agent. The mixtures were prepared in accordance with Eq. 1:
A vertical planetary ball mill (QM-3SP4; Nanjing T-Bota Scietech Instruments & Equipment Co., Ltd., Nanjing, China) was used to mill the mixture of ilmenite, aluminum, and graphite. Steel balls with different diameters (6 mm and 10 mm) were used in the milling operation. The ball-to-powder ratio was maintained at 12:1. Ball milling was performed at room temperature in air condition.
After milling, about 20 g of the mixture was placed into a graphite crucible in a hot-pressing sintering furnace, which was evacuated to vacuum then filled with flowing nitrogen. The atmosphere flow rate was 1 L/min. The samples were sintered at different temperatures (600–1400°C) for 30 min under pressure of 20 MPa to investigate the synthesis process.
Material Characterization
The as-milled powders and sintered samples were characterized by XRD analysis (D/MAX-1200; Rigaku Denki, Beijing, China) using Cu Kα radiation in the range 10°–90°. Peak positions were taken from the International Center for Diffraction Data database. Raw XRD data were refined and analyzed using the MDI Jade 6.0 program (Materials Data Incorporated, Livermore, CA, USA). Silicon powder (SRM640; National Institute of Standards and Technology, Gaithersburg, MD, USA) was used as external standard to correct for instrumental broadening. The experimental errors were reduced as much as possible by curve fitting. The sintered specimens were investigated by scanning electron microscopy (SEM, VEGA II LMU; Tescan, Brno, Czech Republic).
Mechanical Performance Testing
The sintered specimens were cut into bars to measure the flexure strength. The final dimensions of these samples were 3 mm × 3 mm × 30 mm. The flexure strength of the samples was measured by a three-point bending method with a support roller span length of 20 mm and crosshead speed of 0.5 mm/min. The Vickers hardness (VH) was measured using Vickers indentation at load of 20 kg with dwell time of 15 s.
Results and Discussion
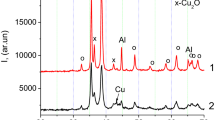
Figure 1 shows the XRD patterns of ilmenite and as-milled FeTiO3-2Al-xC (x = 0 mol, 0.25 mol, 0.5 mol, 0.75 mol, 0.88 mol, and 1 mol) powders. Figure 1a shows the XRD pattern of ilmenite powder. The only phases revealed in the XRD patterns were FeTiO3, TiO2, and Fe2Ti3O9, suggesting that, in addition to FeTiO3, TiO2 and Fe2Ti3O9 phases were present in this kind of ilmenite. Presence of Fe2Ti3O9 also indicates a serious degree of weathering of ilmenite. In addition, the ilmenite powder contained element impurities such as Ca, Mg, Si, Mn, and Al. The specific chemical composition is presented in Table I. These impurity elements were not clearly shown by XRD due to their low concentrations. Figure 1b shows the XRD pattern of as-milled FeTiO3-2Al powder. The XRD profiles indicated that the milled powders contained FeTiO3, TiO2, Fe2Ti3O9, and Al phases. No new peaks were present, suggesting that reactions between FeTiO3, TiO2, Fe2Ti3O9, and Al did not occur on any significant scale during milling. The XRD patterns of the as-milled FeTiO3-2Al-xC powders as a function of the carbon content are shown in Fig. 1c, d, e, f and g. The only phases shown by the XRD patterns were FeTiO3, TiO2, Fe2Ti3O9, Al, and C. The intensity of the carbon peaks increased significantly with increase in the carbon content.
Influence of Carbon Content on the Reaction Process
To clarify the influence of the carbon content on the aluminothermic reduction process of ilmenite during the hot-pressing process, the carbon-free FeTiO3-2Al and carbon-containing FeTiO3-2Al-xC systems were investigated. The XRD results for each system after sintering at different temperatures are shown in Fig. 2, and all the phases present at each temperature from 600°C to 1400°C are listed in Table II.
XRD patterns of reaction process (a) FeTiO3-2Al, (c) FeTiO3-2Al-0.5C, and (d) FeTiO3-2Al-0.75C; (b) ΔG-T curves of reactive process in the FeTiO3-2Al system; enlarged XRD patterns showing the transition from TiN to TiCN with increasing temperature in the (e) FeTiO3-2Al-0.5C and (f) FeTiO3-2Al-0.75C system
Figure 2a shows the XRD patterns of as-milled FeTiO3-2Al powder sintered at different temperatures for 0.5 h under pressure of 20 MPa and nitrogen atmosphere. No new phases appeared after heating the sample to 600°C, suggesting that no reaction occurred during heating to this temperature. The intensity of the TiO2 peaks increased when the sample was heated to 700°C, suggesting that reaction of Fe2Ti3O9, FeTiO3, and Al forming TiO2, Al2O3, and Fe occurred after melting of Al. The other product of the ilmenite reduction was iron. Unfortunately, the main peaks for iron were coangular with aluminum peaks and could not be deconvoluted, which is primarily due to the low intensity of the iron peaks that were expected to be present. In addition, two intermediate alloy phases appeared, i.e., Al3Ti and AlFe3. Many studies have reported an Al3Ti phase, which was synthesized by the reaction of TiO2 and Al.21,22,24 Another intermediate alloy phase, AlFe3, was formed by the reaction of residual aluminum with newly formed iron.
With a further temperature increase to 800°C, the amount of TiO2 phase obviously increased, as shown by the corresponding increase of the relative peak intensities of the TiO2, Al2O3, and Fe phases. Thus, the reaction between Fe2Ti3O9, FeTiO3, and Al continued from 700°C to 800°C. The ilmenite disappeared at this temperature, but the intermediate alloy phases, Al3Ti and AlFe3, were still present in the XRD patterns.
When the temperature was increased to 900°C, the intensity of the TiO2 phase weakened, the intensity of the Al2O3 phase increased significantly, and the Ti3O5 phase appeared in the XRD patterns, suggesting that the reaction between TiO2 and Al to form Ti3O5 and Al2O3 had already occurred. The peaks of the intermediate alloy phases, Al3Ti and AlFe3, became weak and then disappeared at 1000°C, which is due to the reaction between TiO2 and these two intermediate alloy phases, as shown in Eqs. 2 and 3. The standard Gibbs free energy of formation for the reactions in Eqs. 2 and 3 is negative.
When the materials were heated to 1000°C, the intensity of the peaks corresponding to Ti3O5 and Al2O3 phases increased significantly while those for TiO2 phase disappeared. When the temperature was increased from 1000°C to 1100°C, two significant changes in the XRD pattern were observed, i.e., the Ti3O5 and Fe phases disappeared, and the peaks of Ti2O3, TiO, Fe2Ti, and Fe3Ti3O phases appeared. The appearance of Ti2O3, TiO, Fe3Ti3O, and Fe2Ti can be assigned to the reaction between Ti3O5, Al, and Fe because of the disappearance of Ti3O5 and Fe, as shown in Eqs. 4–7. Thermodynamic data for Fe3Ti3O could not be found, thus the Gibbs free energy of the reaction generating Fe3Ti3O could not be obtained accurately. However, Fe3Ti3O can be approximately considered as (Fe2Ti)1.5·(Ti1.5O). Ti1.5O lies between TiO and Ti in the order of valence, thus the Gibbs free energy of the reaction that generates Fe3Ti3O can be estimated as between − 310 kJ and − 412 kJ by Eqs. 5 and 7. As can be seen from Eqs. 4–7, the standard Gibbs free energy of these reactions is negative. In addition, Ti2O3 could also be obtained by the reaction between Ti3O5 and Al, as shown in Eq. 8. Although the energy of formation of Eq. 4 is more negative than that of Eq. 8, formation of Ti2O3 via Eq. 8 required 1/3 mol Ti3O5 compared with 4/3 mol Ti3O5 Al for Eq. 4. Khoshhal et al.3 considered that the formation of Fe2Ti in the FeTiO3-2Al system is due to aluminothermic reduction of FeTiO3 and TiO2. However, in this work, the disappearance of Ti3O5 and Fe proves that the formation of Fe2Ti was due to the reaction between Ti3O5, Al, and Fe.
When the temperature was increased from 1100°C to 1300°C, no new phases appeared and the intensity of the TiO and Fe3Ti3O phases strengthened, accompanied by weakening of the Ti2O3 phase. Thus, Ti2O3 was further reduced to TiO and Fe3Ti3O by Al as the temperature was increased. In the FeTiO3-2Al system, the Al content was theoretically sufficient to reduce all of the ilmenite to Ti. Al could not be found by XRD, probably due to its low content; however, Al was still present in the system. At the temperature of 1300°C, the final product was composed of Al2O3, Fe3Ti3O, Fe2Ti, and TiO phases. The presence of Fe3Ti3O and TiO phases indicates that it was difficult for Al to reduce ilmenite to Ti completely. Figure 3 shows the thermodynamic process of ilmenite reduction by Al. As seen from Fig. 2b, in the process of aluminothermic reduction of ilmenite, the formation of TiO2, Ti3O5, Ti2O3, and TiO can occur due to the negative ΔG°. However, the reaction by which TiO was reduced by Al, forming Ti, is difficult to carry out above 1000°C because of the positive ΔG°.
(a) XRD patterns of FeTiO3-2Al-xC powders sintered at 1400°C for 0.5 h; (b) Enlarged XRD patterns showing the effect of carbon content on the TiCN peak position; (c) Backscattered electron micrographs of sintered samples; (d) Bending strength and VH of sintered samples; (e) Elemental line scan spectra of the 0.5C sample
Figure 2c shows the XRD patterns of FeTiO3-2Al-0.5C powder sintered at different temperatures for 0.5 h under pressure of 20 MPa and nitrogen atmosphere. Compared with the FeTiO3-2Al system, there was not much change before the temperature was raised to 800°C. When the temperature was increased to 800°C, the Al peaks disappeared and two intermediate alloy phases appeared, i.e., Al3Ti and Al13Fe4. The intermediate alloy Al13Fe4 phase was formed by the reaction of Al and the freshly formed Fe. When the temperature was increased to 900°C, the intensity of the TiO2 phase became weaker, and Ti3O5 peaks appeared. In addition, the Al13Fe4 phase disappeared and another Al5Fe2 phase appeared. When the temperature was raised to 1000°C, the TiN and Ti2O3 phases and another AlFe phase appeared, and the peaks of the Al5Fe2 phase disappeared. At 1000°C, the AlFe phase disappeared completely. During this process, there were two notable changes. The first change was in the Al-Fe phase, such as Al13Fe4, Al5Fe2, and AlFe. Richards et al.25 calculated that the intermediate alloy Al-Fe phase had negative Gibbs free energy at 700°C; therefore, thermodynamic formation of these Al-Fe phases is completely available. From the perspective of the reaction process, the Al-Fe phase would participate in the reaction to reduce TiO2 when there was no Al in the system, as shown in Eqs. 9–15. The estimated data can be obtained according to the available thermodynamic data at 700°C. As seen from Eqs. 9–15, the Gibbs free energy of the reactions in which Al-Fe is involved is negative. Khoshhal et al.3,5,26 also detected the Al5Fe2 phase in a study of the FeTiO3-2Al and FeTiO3-2Al-C systems and confirmed that the Al5Fe2 phase as a reducing agent would reduce TiO2. However, the Al13Fe4 and AlFe phases were not detected in their research. The second change was the formation of TiN. The TiN phase was formed by the reaction between Ti3O5 and C under a nitrogen atmosphere. According to the reduction process of ilmenite by Al in the FeTiO3-2Al system under nitrogen atmosphere, it is impossible to form TiN by aluminothermic reduction of the titanium oxides. In addition, many studies27,28,–29 have observed that Ti3O5 is easily converted to TiN in presence of C under nitrogen atmosphere.
When the temperature was increased from 1100°C to 1400°C, the intensity of Ti2O3 and C peaks weakened, and TiN was gradually transformed to the TiCN phase with increasing temperature. The titanium oxides completely disappeared at the temperature of 1400°C, and the final product was composed of Al2O3, TiCN, and Fe phases.
Figure 2d shows the XRD patterns of FeTiO3-2Al-0.75C powder sintered at different temperatures for 0.5 h under pressure of 20 MPa and nitrogen atmosphere. Compared with the FeTiO3-2Al-0.5C system, the reaction process of the FeTiO3-2Al-0.75C system was almost similar to that of the FeTiO3-2Al-0.5C system. However, there were some major changes in the reduction process of the FeTiO3-2Al-0.75C system. First, the formation temperature of TiN in the FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C systems was 1000°C and 900°C, respectively, indicating that enrichment of carbon tends to decrease the formation temperature of TiN during the sintering process. Second, the last intermediate titanium oxide that appeared in the FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C systems was Ti2O3 and Ti3O5, respectively, indicating that Ti3O5 was not further reduced to Ti2O3 but directly converted to TiN in the FeTiO3-2Al-0.75C system. Many studies29,30,–31 have reported that Ti2O3 is very unstable in the carbothermal reduction process under nitrogen-containing atmosphere. However, it can be seen from study of the FeTiO3-2Al-0.5C system that Ti2O3 could be stably present in the nitrogen atmosphere. Third, a new (Fe, C) phase appeared at 700°C in the FeTiO3-2Al-0.75C system, due to the carbon solution in the iron. Carbon was dissolved in iron and diffused with iron as a transport medium, which favors the carbothermal reduction process. Once formation of TiC or TiN started, the fraction of (Fe, C) decreased as TiCN or TiN increased, which implies the onset of a reaction between (Fe, C) and reduced TiO2.32
The study of the FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C systems showed that the reduction process of ilmenite was preceded by three main stages. First, FeTiO3 and Fe2Ti3O9 were reduced by Al to form TiO2 and Fe. TiO2 was then reduced by Al to Ti3O5 or Ti2O3. Finally, Ti3O5 (or Ti2O3) was reduced by carbon to the cubic phase of TiN under nitrogen atmosphere, then the TiN phase gradually transformed to TiCN. This finding is in line with previous research29,33,34 which identified that the reaction sequence of TiO2 reduced by carbon was TiO2 → TinO2n−1 → Ti3O5 → TiCxNyO1−x−y under nitrogen atmosphere.
Figure 2e and f show enlarged XRD patterns from Fig. 2c and d in the range of 41.7° to 42.8°, revealing the transition from TiN to TiCN with increasing temperature in the FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C system, respectively. As the temperature is increased, the TiN peaks shift to the left to smaller angle, indicating formation of TiCN. This peak shift corresponds to an increase in lattice parameter. The lattice parameters of the TiCN phase are presented in Table III.
Influence of Carbon Content on Phase, Microstructure, and Properties of Synthesis Products
Figure 3a shows the XRD patterns of the milled FeTiO3-2Al-xC powders sintered at 1400°C for 0.5 h under pressure of 20 MPa and nitrogen atmosphere. After sintering at 1400°C, titanium oxide could not be detected by XRD, indicating that all of the titanium oxides were reduced to TiCN. The phases present in the final synthetic product were Al2O3, TiCN, and Fe.
Figure 3b shows the influence of the carbon content on the peak position of the TiCN phase, and Table IV presents the TiCN lattice parameter. As the carbon content was increased, the peak position of the TiCN phase shifted to the left and the lattice parameter of the TiCN phase also increased. On the one hand, in the process of carbon reduction of Ti3O5 (or Ti2O3) in nitrogen atmosphere, carbon and nitrogen atoms could gradually displace oxygen atoms of Ti3O5 (or Ti2O3) at high temperatures to form TiCxNyO1−x−y, causing the peak position to shift further to the left.35,36 On the other hand, at given reaction temperature, when the carbon content is increased, more carbon will participate in the reduction reaction and thus more oxygen atoms will be displaced by carbon and nitrogen atoms.2,37 Consequently, the carbon and nitrogen content in the cubic phase increased, which is related to a significant increase in the lattice parameter because the TiC lattice parameter (0.4322 nm) is significantly higher than those of TiN (0.4242 nm) or TiO (0.4172 nm for TiC0.006O0.987N0.007).38 The results obtained in this work agree with this observation.
Backscattered electron images of sintered samples prepared from the FeTiO3-2Al-xC systems are shown in Fig. 3c. The three different microstructure colors represent three different phases, i.e., Al2O3, TiCN, and Fe. These three phases were interlaced, and their distribution characteristics were not obvious. When the carbon content was low, the Al2O3, TiCN, and Fe phases clustered together and looked very coarse, especially the TiCN and Fe phases. With higher carbon content, the phases were observed to become smaller and the TiCN and Fe distribution was more uniform.
The bending strength and VH of samples sintered at 1400°C for 0.5 h under pressure of 20 MPa are shown in Fig. 3d. The VH value was observed to increase with increase in the carbon content. On the one hand, the grain size decreased as the carbon content was increased, resulting in an increase in hardness. On the other hand, according to the reduction process, the carbon was insufficient to completely reduce the oxygen in the TiCN when the carbon content was small, which resulted in TiCN’s high oxygen content. The bending strength increased as the carbon content was increased, reaching a peak when the carbon content was 0.88 mol. When the carbon content was increased to 1 mol, the bending strength dropped sharply, due to residual carbon in the material. The ilmenite contained a little impurity which was difficult to be reduced by carbon; therefore, 1 mol of carbon was not completely consumed during the reaction. Under nitrogen atmosphere, the amount of carbon used in the synthesis of TiCN by carbothermal reduction was less than that of TiC, as shown in Eq. 1. This is confirmed by some studies1,37 showing that high carbon content will lead to carbon residue in the material after synthesis of TiCN. When the carbon content was 0.88 mol, the synthetic products achieved comprehensive mechanical properties, i.e., bending strength and VH values of 375 MPa and 15.5 GPa, respectively.
Backscattered electron images and elemental line scan spectra of the 0.5C specimens are shown in Fig. 3e. The elemental line scan spectra confirm that the light-grayish particles are TiCN, because the distribution of the corresponding elements overlapped (such as Ti, C, and N), while the dark-gray particles were Al2O3 and the white particles were Fe. In addition, the scanning results reveal the distribution of impurities, which were difficult to detect using XRD. The distribution curve of the Mg element can be seen to overlap with a part of the Al2O3 curve, suggesting that Mg impurity is generally distributed in Al2O3. Tang et al.6 detected MgAl2O4 phase during the reduction process of the FeTiO3-1Al-2.5C system because of the high content of Mg in ilmenite. In this work, the Mg and Al2O3 impurities were distributed together, but did not form a Mg-Al-O ternary phase because of low content of Mg impurities in ilmenite. The distribution characteristics of Si and Mn impurities were not obvious, according to their line scan curve distribution; most of them were dissolved in iron with a small part distributed on the phase boundary.
Conclusion
Al2O3-TiCN-Fe composites were synthesized by chemical reactions between natural ilmenite, aluminum, and carbon via a hot-pressing process. The aluminothermic reduction of ilmenite in the carbon-free FeTiO3-2Al system was a gradual deoxygenation process. A series of intermediate titanium oxides were generated by the reduction of aluminum, i.e., TiO2, Ti3O5, Ti2O3, TiO, Fe3Ti3O, and Fe2Ti, according to the order of reduction. In the carbon-containing FeTiO3-2Al-xC systems, TiN is generated with carbon involved in the reaction process, which will gradually transform to TiCN with increased temperature. The synthesis temperature of TiN decreased with increase of the carbon content in the system, being 1000°C and 900°C in the FeTiO3-2Al-0.5C and FeTiO3-2Al-0.75C system, respectively. Carbon could play an obvious role in grain refinement, and the grain size decreased and the distribution of TiCN and Fe became more uniform as the carbon content was increased. The system with 0.88 mol carbon resulted in comprehensive mechanical properties after sintering at 1400°C, i.e., bending strength and Vickers hardness of 375 MPa and 15.5 GPa, respectively.
References
K.H. Wu, G.H. Zhang, and H.P. Gou, Vacuum 151, 51 (2018).
E. Ahmadi, A. Fauzi, H. Hussin, N. Baharun, K.S. Ariffin, and S.A. Rezan, Int. J. Min. Met. Mater. 24, 444 (2017).
R. Khoshhal, M. Soltanieh, and M.A. Boutorabi, J. Alloys Compd. 628, 113 (2015).
N.J. Welham, J. Alloys Compd. 274, 303 (1998).
R. Khoshhal, M. Soltanieh, and M.A. Boutorabi, Int. J. Refract. Met. Hard Mater. 45, 53 (2014).
A.T. Tang, S.M. Liu, and F.S. Pan, Prog. Nat. Sci. 23, 501 (2013).
N.J. Welham, T. Kerr, and P.E. Willis, J. Am. Ceram. Soc. 82, 2332 (1999).
Z. Yin, C. Huang, B. Zou, H. Liu, H. Zhu, and J. Wang, Ceram. Int. 39, 8877 (2013).
Z. Yin, C. Huang, B. Zou, H. Liu, H. Zhu, and J. Wang, Mater. Sci. Eng. A 577, 9 (2013).
M. Razavi, A.H. Rajabi-Zamani, M.R. Rahimipour, R. Kaboli, M.O. Shabani, and R. Yazdani-Rad, Ceram. Int. 37, 443–449 (2011).
J. Pourhosseini, M. Zakeri, M.R. Rahimipour, E. Salahi, and GhR Pourhosseini, Mater. Sci. Technol. Lond. 26, 1132 (2010).
M. Kholghy, S. Kharatyan, and H. Edris, J. Alloys Compd. 502, 491 (2010).
T. Nukami and M.C. Flemings, Metall. Mater. Trans. A 26, 1877 (1995).
H. Soda, Q. Xia, A. McLean, A.K. Pramanick, and G. Motoyasu, Mater. Sci. Eng. A 216, 61 (1996).
B.S.S. Daniel, V.S.R. Murthy, and G.S. Murty, J. Mater. Sci. Technol. 68, 132 (1997).
P.E. Willis, N.J. Welham, and A. Kerr, J. Eur. Ceram. Soc. 18, 701 (1998).
M. Razavi, M.R. Rahimipour, T. Ebadzadeh, and S.S.R. Tousi, Bull. Mater. Sci. 32, 155 (2009).
Z.G. Zou, C.Q. Yin, Y. Wu, and X.M. Li, Key Eng. Mater. 280–283, 1501 (2007).
Z.G. Zou, Y. Wu, C.Q. Yin, and X.M. Li, J. Wuhan Univ. Technol. Mater. Sci. Ed. 22, 706 (2007).
Z.G. Zou, J.L. Li, and Y. Wu, Key Eng. Mater. 280–283, 1103 (2005).
S. Alamolhoda, S. Heshmati-Manesh, and A. Ataie, Adv. Powder Technol. 23, 343 (2012).
Y.F. Shen, Z.G. Zou, Z.G. Xiao, K. Liu, F. Long, and Y. Wu, Mater. Sci. Eng. A 528, 2100 (2011).
V.P. Kobyakov, N.V. Sachkova, and M.A. Sichinava, Inorg. Mater. 46, 1396 (2010).
J.J.S. Dilip, B.S.B. Reddy, S. Das, and K. Das, J. Alloys Compd. 475, 178 (2009).
R.W. Richards, R.D. Jones, P.D. Clements, and H. Clarke, Int. Mater. Rev. 39, 191 (1994).
R. Khoshhal, M. Soltanieh, and M.A. Boutorabi, Int. J. Refract. Met. Hard Mater. 52, 17 (2015).
D.P. Xiang, Y. Liu, M.J. Tu, Y.Y. Li, and W.P. Chen, Int. J. Refract. Met. Hard Mater. 27, 111 (2009).
H. Kwon and S. Kang, J. Am. Ceram. Soc. 92, 272 (2009).
A. Jha and S.J. Yoon, J. Mater. Sci. 34, 307 (1999).
L.M. Berger, J. Hard Mater. 3, 3 (1992).
L.M. Berger, J. Mater. Sci. Lett. 20, 1845 (2001).
N.J. Welham and J.S. Williams, Metall. Mater. Trans. B 30, 1075 (1999).
W.Y. Li and F.L. Riley, J. Eur. Ceram. Soc. 8, 345 (1991).
R.M. Ren, Z.G. Yang, and L.L. Shaw, Mater. Sci. Eng. A 286, 65 (2000).
H.P. Gou, G.H. Zhang, and K.C. Chou, Metall. Mater. Trans. B 46B, 48 (2015).
S.A. Rezan, G. Zhang, and O. Ostrovski, ISIJ Int. 52, 363 (2012).
H.P. Gou, G.H. Zhang, X. Yuan, and K.C. Chou, ISIJ Int. 56, 744 (2016).
G. Neumann, R. Kieffer, and P. Ettmayer, Monatsh. Chem. 103, 1130 (1972).
Acknowledgements
The authors acknowledge financial support from The Ministry of Education “Chunhui Plan” Cooperative Research Project (16201417), the Key Research Project of Xihua University (Z1520102), Xihua University Key Laboratory Open Fund Project (SZJJ2014-061), and Scientific Research Project in Sichuan Province Department of Education (15201444).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Chen, M. & Xiao, X. Influence of Carbon Content on Aluminothermic Reduction of Ilmenite During Hot Pressing. JOM 71, 1822–1830 (2019). https://doi.org/10.1007/s11837-019-03428-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03428-5