Abstract
Composites of Al-Si-Mg (A356) alloy with silicon carbide particles were synthesized in-house and foamed by melt processing using titanium hydride as foaming agent. The effects of the SiCP size and content, and foaming temperature on the stability and quality of the foam were explored. It was observed that the foam stability depended on the foaming temperature alone but not on the particle size or volume percent within the studied ranges. Specifically, foam stability was poor at 670°C. Among the stable foams obtained at 640°C, cell soundness (absence of/low defects, and collapse) was seen to vary depending on the particle size and content; For example, for finer size, lower particle contents were sufficient to obtain sound cell structure. It is possible to determine a foaming process window based on material and process parameters for good expansion, foam stability, and cell structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultralow-density (0.2–0.8 g/cm3) aluminum-based closed cell metal foams are of interest due to their high structural stiffness per unit weight and high energy absorption per unit transmitted stress. Furthermore, they have lower thermal conductivity and better mechanical damping compared with their parent metal.1 In general, methods to prepare aluminum foams involve melting. Presence of oxides and/or particles is essential in the melt to facilitate liquid foam evolution and survival until solidification.2 The inert ceramic particles in aluminum alloy metal matrix composites (MMC) can act as “foam stabilizers”, enabling production of closed cell MMC foams.
In MMC foams manufactured by melt processing, ceramic particles act as primary foam stabilizers, although oxides may further increase foam stability,3 specifically in air-purged foams.3,4,5 MMC foams have also been prepared by mixing titanium hydride (TiH2) into mechanically stirred Al alloy–ceramic particulate melts.6,7,8,9 Another technique involves mixing TiH2 powder into the MMC melt, quickly solidifying the melt into a foamable precursor, and heating it to produce the MMC foam.10 In foams to which foaming agent is added externally, oxides (which may form during impeller stirring) may marginally contribute to foam stability over and above the particles. On the other hand, oxides play a significant role in powder compact-melted MMC foams.
As stated above, nonair (e.g., hydrogen gas using TiH2)-purged MMC foams are principally stabilized by ceramic particles. Therefore, particle characteristics such as particle material, size, and volume percent (vol.%) are expected to influence the foaming tendency and foam stability. However, limited work exists in this regard for MMC foams. The effects of silicon carbide particle (SiCP) parameters (1–20 µm; 8–14.5 vol.%) on foam stability have been studied for air-purged foams.5 The SiC contents to obtain a stable foam were increased at coarser particle size. Similar particle size and vol.% combinations were used to produce stable foams by introduction of a blowing agent in Al-Si-Mg/SiCP,6,7,8,11 including commercial A356-SiC composites.9 Gui et al.6 reported foam densities in the range of 0.4–0.8 g/cm3 in A356-20vol.%SiCP composite foams prepared at 640°C. Work on submicron-sized particles (generated in situ) with Al-4.4Cu-2TiB2 composites showed much lower contents to be sufficient for obtaining higher foam expansions.12 Overall, it is observed that, in particle-containing Al alloy foams, the particle content necessary for high foaming tendency and foam stability reduces with decreasing particle size.
The objective of the present work is to study the role of the particle size and content, and foaming temperature on the foamability (the capacity of the melt to foam and reach larger expansions), foam stability (the capacity of the expanded liquid foam to remain intact until solidification), and the soundness of the cells (the quality of the resultant solidified foam) in Al-Si-Mg/SiCP composite-based foams. The ceramic particle size and contents were varied to the same levels usually present in aluminum alloy-based MMCs.
Materials and Methods
Materials
A356 alloy (Al-6.9 Si-0.28 Mg, wt.%) and SiC particles were used in this work. An extra 0.7 wt.% Mg was added to the A356 alloy for easy particle incorporation during composite preparation. The liquidus temperature of the modified A356 alloy (615°C) remains unchanged with additional Mg content.13 Particle sizes (d50) of 6 µm, 9 µm, 13 µm, and 17 µm were used in different quantities (5 vol.%, 10 vol.%, 15 vol.%, and 20 vol.%). The particles were heat treated (at 1100°C for 2 h in air) prior to their addition, to improve the introduction and dispersion of SiC in the molten aluminum. TiH2 powder (d50 of 11 μm) was used as foaming agent at 1.5 wt.% of melt.
MMC Synthesis and Foaming
The procedure for foam manufacture involved preparing Al-Si-Mg/SiCP composite by molten metal mixing (stir-casting) and subsequent foaming. Experiments were carried out in a cylindrical clay-bonded graphite crucible (inner diameter ~ 100 mm) in an electrical resistance-heated laboratory furnace (20 kW, internal dimensions 200 mm × 200 mm × 465 mm) with melt weight of 1.3 kg. Heat-treated SiCP was added to A356 melt at 725°C under impeller stirring. The impeller was connected to an arrangement containing a motor with a variable-frequency drive. Stirring was continued for an additional 5 min after particle addition. The liquid composite was then cooled to the foaming temperature. In view of the SiC particles settling in the stagnant melt during MMC cooling (which took 60–75 min), stirring was initiated a few minutes prior to TiH2 addition. When TiH2 was dispersed into the melt, it dissociated, releasing hydrogen bubbles over the next 3–4 min, leading to melt expansion in the vertical direction inside the crucible. At the end of expansion, which was confirmed visually, the crucible containing the liquid foam was carefully taken out of the furnace and cooled under forced air. During cooling, in some cases, the liquid foam collapsed inside the crucible.
Foam heights were measured (a) at the end of expansion (liquid foam height), and (b) after cooling to room temperature (solidified foam height). These heights were normalized by the original melt height, and are called total expansion and net expansion, respectively. Based on literature14 and our previous study15 with various temperatures (630–670°C), two foaming temperatures, viz. 640°C and 670°C, were chosen, corresponding to the highest and lowest net expansion, respectively. However, it is possible that the highest and lowest net foam expansions may occur at a different set of temperatures for different combinations of particle size and vol.%.
The cylindrical foam ingots were sectioned longitudinally along the ingot axis by electrodischarge sawing. Cell sizes were measured on optically scanned foam sections by individual measurements on random straight lines perpendicular to the foaming direction. Microstructural examination of the cell structures was carried out by optical microscopy.
Results
Effect of Temperature on Foam Stability
Total and net expansion data along with macrosections of foam ingots are shown in Fig. 1. Good foamability was observed at 670°C for various SiCP sizes and contents, as shown by their total expansions in Fig. 1a. However, all foams collapsed severely during cooling, resulting in poor foam stability. Although a certain amount of net expansion was recorded (not shown in Fig. 1a), it was of little practical value due to severe foam distortion. The decay started from the top portion of the foam which got densified, under which the middle portion buckled, losing its shape (Fig. 1a). Thus, the deleterious effect of high foaming temperature with respect to foam collapse was dominant, masking possible effects of particle characteristics.
Foam expansion (total expansion = net expansion + collapse) data at 670°C (a) and 640°C (b) for different SiCP size/vol.% combinations. The optical image on the left side shows collapsed foam made at 670°C. The right-side optical image shows typical foam prepared at 640°C (magnified image shows the foam quality). The vol.% of SiC is indicated on top of each bar. White arrow indicates foaming direction
On the other hand, at 640°C, good total/net expansions were obtained, indicating good foamability and stability. Although some foam collapse was observed (Fig. 1b), it did not buckle or distort the foams, and thus preserved their integrity. Therefore, these foams were further characterized.
Foam Quality
Foam ingots prepared at 640°C were free from large cracks (i.e., extending across several cell diameters) and/or abnormally large cells. They exhibited uniform cell size (“isotropy” of cell geometry) along the major portion of the foam ingot (Fig. 1b). In the top portion, the cells were finer than in the rest of the ingot. These finer cells possibly formed during the initial foaming stage when fine bubbles nucleate and rise upwards by buoyancy with limited growth. In the bottom portion, an unfoamed dense part exists, which was taken into account to calculate the total and net expansions.
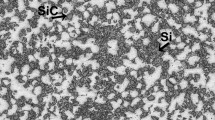
According to the features described above, foams with varying quality (in terms of defects) were obtained depending on the SiCP size and content. Within the range of SiCP parameters studied (Fig. 2), it was observed that a lower particle size range required lower minimum vol.%, and vice versa, to obtain good foam quality. For SiC finer than 10 µm size, only 5–10 vol.% particle content was needed, while 15–20 vol.% was required for coarser SiC particles. Foams obtained within these combinations (Fig. 2) exhibited fewer defects, whereas deviation from these SiCP vol.%/size combinations resulted in lower expansion, collapse, and/or inferior foam quality (Fig. 3). As shown in Fig. 3, the defects were mostly cell wall rupture (punctured cells) and small cracks (joined punctured cells). Foams prepared with 5 vol.% of coarser particles showed these defects on larger scale. In comparison, 17 µm/15% foam exhibited lesser defects and better cell wall connectivity (Fig. 3).
The SiCP distribution was random within the cell walls and did not show any preference for segregation to the gas–metal interface (Fig. 2). In case of finer SiCP, particle agglomerates could be seen in the cell wall region. The mean ± standard deviation cell wall thickness (in microns) was 33 ± 15, 32.8 ± 17, 35.3 ± 16, and 36.5 ± 15 for 6 µm/5%, 9 µm/5%, 13 µm/15%, and 17 µm/20% SiCP foams, respectively.
Discussion
High viscosity and low surface tension are two important characteristics in melt foaming with respect to bubble nucleation and resistance to liquid drainage. Presence of particles in aluminum alloy melt enhances the viscosity and seems to reduce the surface tension.3 In the present case, presence of magnesium also decreased the surface tension of the matrix alloy.
Scatter in foam expansion data prevents the proposal of detailed explanations regarding the effect of SiC parameters on the total expansion. Figure 4 shows the expansion data versus the number of SiC particles present in the melt, calculated based on the known average particle size and content and assuming sphericity. Note that the total expansion initially increased with increase in the number of particles then remained somewhat constant, before decreasing thereafter. This can be explained based on the assumption that a certain minimum number of particles are required to surround bubbles for their survival in the melt. It is generally believed that, if a significant fraction of the particles are situated at gas–metal interface, a stabilizing effect occurs.16 However, our metallographic evidence consistently showed (Fig. 2) that particles/clusters were distributed within the cell walls. This arrangement may enhance the local viscosity,10 and the foam could be sustained by this particle configuration. However, in the absence of the minimum number of particles, bubble collapse occurs during foam expansion, resulting in reduced total expansion. Complex oxides of Al and Mg, which might form due to the high synthesis temperatures of the composites, could also aid foam survival.
The net foam expansion is numerically equal to the total foam expansion minus the amount of collapse. With the higher melt superheating (55°C) at 670°C, strong liquid contraction is expected, coupled with higher diffusion rates of hydrogen. The observed foam collapse can be attributed to faster diffusion of hydrogen gas from bubbles to the foam surface through the melt, and its eventual escape to the atmosphere. This may also happen through coarsening of fine bubbles (present in the top portion of the foam, Fig. 1), which are prone to collapse. These effects result in loss of excess pressure within the bubbles and consequent collapse, thereby leading to densification in the upper region of the foam (Fig. 1a). This local densification produces a secondary effect of buckling in the middle portion of the foam, which solidifies last. In other parts of the foam ingot, there was no evidence of abnormal cells or cell coalescence (Fig. 1a). The fact that no foam could survive at 670°C shows that the foam decay was dominated by temperature, rather than by the SiC particle parameters, which influence the melt viscosity and surface tension.
On the other hand, lower foaming temperature delays outgassing, saving the foam from buckling before solidification, resulting in greater foam stability. At 640°C, the collapse was seen to be much smaller or nil across a range of SiC characteristics (Fig. 1b). It was seen (Fig. 4) that the net expansion was low at the lowest number of particles, increasing to high values for intermediate number of particles, and finally dropping for very large number of particles. When there were very few particles, liquid drainage through plateau borders is expected to be high due to the low melt viscosity, resulting in drawing of liquid from cell walls to the plateau borders. This can result in cell wall ruptures. Cell wall rupture is evident (Fig. 3) for a given coarser particle size with decreasing vol.% (i.e., with decreasing number of particles). Excessive cell wall thinning is resisted with high fraction of particles, thus keeping pace with the expansion. The largest number of particles in the present case corresponds to the smallest particle size for which particle agglomeration was observed (Fig. 2), resulting in a lower effective number of particles. The particle size has implications for the critical cell wall thickness beyond which breakage occurs. The smaller the particles, the lower the critical thickness. Due to particle agglomeration, the minimum cell wall thicknesses are similar in the present case. The defects in the foam ingots may also be attributed to the expansion, which may still be occurring during foam solidification.17 During this expansion, cell wall thinning and rupture (cell coalescence) may remain as a defect on account of the lack of melt redistribution due to the increased amount of solid phase.
Conclusion
Al-Si-Mg alloy MMCs containing various sizes and contents of SiCP could be foamed by melt route using TiH2. Good foam expansion (foamability) was achieved using all experimental parameters. Irrespective of the particle size or vol.% of the composite, the foam stability depended on the foaming temperature. Specifically, at 670°C, the foams were unstable and prone to severe collapse. The stable foams obtained at 640°C showed varying quality depending on the SiCP size and content. Although the cell morphology (size and shape) was similar, the cell soundness (absence of/low defects, and collapse) varied. For finer SiCP, a lower quantity was sufficient to obtain good foam quality. Thus, for a given system, the foaming temperature, particle size, and particle content can be tailored to obtain foams with good stability and quality.
References
M.F. Ashby, A.G. Evans, N.A. Fleck, L.J. Gibson, J.W. Hutchinson, and H.N.G. Wadley, Metal Foams—A Design Guide (Woburn, MA: Butterworth-Heinemann, 2000), p. 4.
J. Banhart, Adv. Eng. Mater. 8, 781 (2006).
N. Babcsán, D. Leitlmeier, and H.P. Degischer, Materialwiss. Werkstofftech. 34, 22 (2003).
D. Leitlmeier, H.P. Degischer, and H.J. Flankl, Adv. Eng. Mater. 4, 735 (2002).
W. Deqing and S. Ziyuan, Mater. Sci. Eng. A 361, 45 (2003).
M.C. Gui, D.B. Wang, J.J. Wu, G.J. Yuan, and C.G. Li, Mater. Sci. Eng. A 286, 282–288 (2000).
S. Yu, Y. Luo, and J. Liu, Mater. Sci. Eng. A 487, 394–399 (2008).
N.V. Ravi Kumar, N. Ramachandra Rao, B. Sudhakar, and A.A. Gokhale, Mater. Sci. Eng. A 527, 6082 (2010).
N.V. Ravi Kumar, N. Ramachandra Rao, and A.A. Gokhale, Mater. Sci. Eng. A 598, 343 (2014).
V. Gergely and B. Clyne, Adv. Eng. Mater. 2, 175 (2000).
W. Jiejun, L. Chenggong, W. Dianbin, and G. Manchang, Compos. Sci. Technol. 63, 569 (2003).
S. Bhogi, J. Nampoothiri, K.R. Ravi, and M. Mukherjee, Mater. Sci. Eng. A 685, 131 (2017).
F.C.R. Hernandez, M.B. Djurdjevic, W.T. Kierkus, and J.H. Sokolowski, Mater. Sci. Eng. A 396, 271 (2005).
C.C. Yang and H. Nakae, J. Alloys Compd. 313, 188 (2000).
N.V. Ravi Kumar and A.A. Gokhale, Trans. Indian Inst. Met. 65, 753 (2012).
S.W. Ip, Y. Wang, and J.M. Toguri, Can. Metall. Q. 38, 81 (1999).
M. Mukherjee, F. Garcia-Moreno, and J. Banhart, Scr. Mater. 63, 235 (2010).
Acknowledgements
The authors sincerely thank the Defense Research and Development Organization for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi Kumar, N.V., Gokhale, A.A. Role of Temperature and SiCP Parameters in Stability and Quality of Al-Si-Mg/SiC Foams. JOM 70, 823–828 (2018). https://doi.org/10.1007/s11837-018-2825-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2825-0