Abstract
In this study, a new technique was proposed for the economical and environmentally friendly recovery of valuable metals from copper smelting slag while simultaneously upgrading nickel laterite through a co-reduction followed by wet magnetic separation process. Copper slag with a high FeO content can decrease the liquidus temperature of the SiO2-Al2O3-CaO-MgO system and facilitate formation of liquid phase in a co-reduction process with nickel laterite, which is beneficial for metallic particle growth. As a result, the recovery of Ni, Cu, and Fe was notably increased. A crude Fe-Ni-Cu alloy with 2.5% Ni, 1.1% Cu, and 87.9% Fe was produced, which can replace part of scrap steel, electrolytic copper, and nickel as the burden in the production of weathering steel by an electric arc furnace. The study further found that an appropriate proportion of copper slag and nickel laterite in the mixture is essential to enhance the reduction, acquire appropriate amounts of the liquid phase, and improve the growth of the metallic alloy grains. As a result, the liberation of alloy particles in the grinding process was effectively promoted and the metal recovery was increased significantly in the subsequent magnetic separation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Weathering steel (WS) has been widely used in bridges, vehicles, pylons, containers, warships, and other large structural applications because of its good corrosion resistance and life-cycle cost.1 It contains a small amount of alloying elements, such as Cu, Ni, Cr, P, Mn, and Si. Copper and nickel are the main alloying elements that most affect the corrosion resistance of the WS.1 , 2 In the process of producing weathering steel, the required amounts of electrolytic copper and electrolytic nickel are added to adjust the chemical composition of the WS. However, the price of electrolytic copper and nickel has increased gradually and remained high in recent years, leading to higher WS production costs.
Copper smelting slag always contains Fe and Cu, and sometimes it contains Ni, which can be considered an important secondary resource for utilization.3 These metals remain in slag through mechanical entrapment in the form of matte and chemical dissolution of the oxides into fayalite or magnetite.4 Recently, many treatment processes have been proposed, with the goal of reusing slag, including chemical leaching,5 bio-leaching,6 magnetic roasting,7 selective agglomeration-reverse flotation,8 slag modification-physical separation,9 , 10 and direct reduction-magnetic separation.11 , 12 Despite the advantages of these methods, the recovery of metals is not ideal because the copper-bearing minerals (metallic copper and copper sulfide) closely associate with fayalite and magnetite. Nickel laterite is a complex and refractory ore, which is difficult to beneficiate by traditional physical separation processes.13 The direct reduction process to upgrade this type of refractory ore has been a topic of metallurgical interest. In the reduction process, additives, such as Na2CO3, CaSO4, Na2SO4, and Na2S, are used to improve the nickel recovery by promoting the growth of metallic particles.14 Most investigators believe that these additives are helpful for decreasing the liquidus temperature of the slag and generating more liquid phase (Fe-FeS solution) during the reduction process.14,15,16,17 Liquid phase can promote mass transfer and improve alloy particles growth.18 Therefore, generation of the liquid phase is one of the key factors in achieving large metallic particles. Some reports also note that the liquidus temperature of the SiO2-CaO-MgO-Al2O3 system notably decreases when the FeO content increases.19,20,21 Generally, the FeO content of nonferrous slag, such as copper slag, is quite high.
In this article, an innovative technique is presented for effectively promoting the growth of metallic particle size by co-reduction of copper smelting slag and nickel laterite, utilizing the high FeO content of the copper slag. This new process can recover valuable metals, such as Fe, Ni, and Cu, from copper smelting slag in an economically and environmentally friendly way, as well as upgrade the nickel laterite. In addition, the product obtained from the new process can be used as the raw material, replacing a part of sponge iron, electrolytic copper, and electrolytic nickel for producing weathering steel by an electric arc furnace.
Experiments
Materials
The chemical compositions of the copper smelting slag and nickel laterite are shown in Supplemental Table 1. As is shown, the MgO content of nickel laterite was as high as 15.8%. However, the presence of MgO raises the slag liquidus temperature during the reduction process. The copper slag and nickel laterite were crushed and ground to a size of 80 wt.% passing 0.074 mm.
Soft coal had a fixed carbon content of 52.1% on an air-dried basis (FCad), a volatile matter content of 30.4% on a dry ash-free basis (Vdaf), and an ash content of 4.5% on an air-dried basis (Aad); a 0.6% S content was used as the reductant. In addition, the ash in it had a liquidus temperature of 1376°C.
Limestone containing 60.4% CaO, 37.5% loss on ignition (LOI), and a particle size less than 0.074 mm was used to adjust the basicity (the mass ratio of CaO/SiO2) of the mixture.
Experimental Methods
The ground copper slag, nickel laterite, and limestone were mixed thoroughly for balling. Different proportions of copper slag and laterite were used and tested to obtain the proper proportion. The green balls were made in a disc pelletizer, measuring 800 mm in diameter, having a 200 mm rim depth, and rotating at 38 rpm. Green balls (diameter of 8–12 mm) were dried at 110°C for 2 h in a drying oven until their mass was unchanged. Approximately 80 g of dry pellets and the required amounts of coal were mixed and loaded into an alumina crucible that was heated at 1250°C for 60 min under nitrogen atmosphere. After reduction, the crucible was removed from the furnace, and the reduced pellets were cooled to ambient temperature under the protection of nitrogen in preparation for the subsequent grinding (80 wt.% passing 0.043 mm) and wet-magnetic separation process under the condition of 0.08-T magnetic field intensity. The detailed method of magnetite separation is shown in a previous Ref. 9. Subsequently, the compositions of both the dry magnetic products (crude alloy) and non-magnetic products were analyzed using chemical methods.
The soft melting characteristics, including the deformation temperature (Td), sphere temperature (Ts), and flow temperature (Tf), of mixtures having varying proportions of copper smelting slag and nickel laterite (mass copper/mass nickel slag) were determined by a horizontal tube furnace as shown in Supplementary Figure 1. The detailed method is reported in the literature.21 , 22
The phase composition of samples was investigated using an x-ray diffractometer (XRD, RIGAKU D/Max 2500, Japan, radiation: Cu Ka, tube current and voltage: 250 mA). The microstructures of the reduced pellets were revealed using a Leica DMLP optical microscopy and a scanning electron microscope (SEM, JEOL, JSM-6490LV). The SEM images were recorded using the backscatter electron mode while operating in low-vacuum mode at 0.5 Torr and 20 keV. Energy dispersive spectroscopy (EDS, JEOL, JSM-6490LV) was used to determine the chemical composition of the phases in the reduced pellets. FactSage 7.0 was utilized to calculate the liquidus temperature and determine the phase diagram (SiO2-Al2O3-CaO-MgO and FeO-SiO2-Al2O3-CaO-MgO).
Results and Discussion
Thermodynamic Analysis
The dominant reactions probably occurred in the co-reduction roasting of copper and iron compounds with coal and are presented as Eqs. 1–7. The standard free energy (ΔG θ) of these reactions was calculated under one atmospheric pressure by FactSage™ 7.0 and HSC 5.0 software, and the results are shown in Supplemental Figure 2. The standard free energy variation for the all reactions is negative over the temperature range of 1000–1300°C, indicating those reactions are thermodynamically possible.
XRD patterns of the laterite reduction samples with varying amounts of copper slag [(F-forsterite (Mg2SiO4), A-augite (Ca(Mg,Fe)Si2O6), M-monticellite (CaMgSiO4), and I-iron (Fe)], reduction at 1250°C for 60 min with a basicity of 0.3 and a C/Fe mass ratio of 0.82: (a) XRD patterns of the reduced pellets; (b–e) standard XRD patterns of forsterite, monticelite, augite, and iron)
Soft Melting Characteristics
As mentioned above, a certain amount of the liquid phase is required to ensure sufficient aggregation and growth of the metal particles during the reduction roasting. For the reduction of pellets with only laterite ore, it is very difficult to generate the liquid phase at reduction temperature because of its high silica and magnesia contents. However, FeO can lower the liquidus temperature. As seen in Supplemental Figure 3, the liquidus temperature decreased from 1280°C [Supplemental Figure 3(a)] to 1200°C [Supplemental Figure 3(b)] with the addition of 10 wt.% FeO to the SiO2-Al2O3-CaO-MgO system. Based on this, it is inferred that copper slag can, because of its high FeO content, reduce the liquidus temperature of the mixture in the case of co-reduction with nickel laterite.
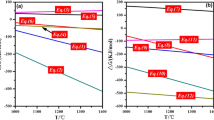
Figure 1 illustrates the effect of the proportion of copper slag on the soft melting characteristics of the mixture. The liquidus temperature of the mixture, when blended with different proportions of copper slag (the main oxide composition of the mixture is shown in Supplemental Table 2), was calculated using FactSage 7.0, and the result is shown in Fig. 1a. With the addition of copper slag to the mixture, the liquidus temperature decreased significantly. Additionally, the characteristic fusion temperatures (TD, TS, and TF) of the mixtures with varying amounts of copper slag were determined, as shown in Fig. 1b. For the mixture in absence of copper slag, the deformation temperature (TD), softening temperature (TS), and fluid temperature (TF) were 1240°C, 1365°C, and 1420°C, respectively. With an increase in the copper slag proportion in the mixture, the characteristic fusion temperatures of the mixture clearly decreased, which confirmed that the addition of copper slag improved the soft melting characteristics of the pellets during the co-reduction roasting process.
Phase Transformation
Figure 2 shows that the primary phases in the pellets that were reduced with only laterite ore were forsterite (Mg2SiO4), augite (Ca(Mg, Fe)Si2O6), monticellite (CaMgSiO4), and iron (Fe). With the addition of copper slag, the peak intensity of the augite (Ca(Mg,Fe)Si2O6) was gradually increased, while that of the forsterite (Mg2SiO4) was decreased and even vanished. These results indicate that, with an increase in the copper slag proportion, the content of forsterite (Mg2SiO4) decreased significantly in the reduced pellets. Conversely, more augite (Ca(Mg,Fe)Si2O6) was generated during the co-reduction roasting with copper slag.
Metallic Particle Growth Behavior
The microstructures of the reduced pellets with different proportions of copper slag are shown in Fig. 3 and reveal the metallic particle growth behavior. As seen, the metallic particles are dispersed and their size is very small (shown in Fig. 3a). However, by adding 10% copper slag, the pellet structure became more compact and some molten phases appeared in the pellets. By further increasing the proportion of copper slag, more liquid was generated, which agreed well with the results of the softening-melting tests (Fig. 1). The liquid phase is responsible for the rapid grain coarsening, where smaller particles will go into solution preferentially and precipitate on larger particles, accelerating the transport rate as the metal ions diffuse through liquids faster than through solids. Therefore, the average size of metallic particles was greater than 50 μm when 40% copper slag was added, while the particle size was less than 10 μm, under the same reduction conditions, in the absence of copper slag (pellets with nickel laterite alone).
Metal Recovery
Generally, the large metallic particles responded well to the subsequent physical separation processes because of sufficient mineral liberation from the non-magnetic gangue by grinding.
Supplemental Figure 4 shows the results of the magnetic separation of the reduced pellets that had copper slag contents ranging from 0% to 70%. With the increase of the copper slag proportion to 40%, the iron, nickel, and copper recoveries improved significantly from 38.3% to 69.0%, 40.9% to 86.1%, and 56.5% to 80.5% [as shown in Supplemental Figure 4(a)], respectively. There were also sharp increases in the iron and copper contents from 66.0% to 84.6% and 0.4% to 0.9% [as shown in Supplemental Figure 4(b)], respectively. In addition, the nickel content decreased slightly from 6.9% to 4.5% because of the increase of iron content in the alloy. Thereafter, the recovery indexes were all constant. However, whether this alloy containing Fe, Ni, and Cu can be used as a material for preparing weathering steels depends not only on the nickel and copper contents, but also on the Ni/Cu mass ratio. Typically, the Ni/Cu mass ratio in weathering steels varies from 1 to 2.5.
Taking overall consideration of the metal recoveries, metal grade, and Ni/Cu mass ratio in the alloy, the appropriate proportion of copper slag is 60%. Under this condition, the Fe-Ni-Cu alloy containing 2.5% Ni, 1.1% Cu, and 87.9% Fe can be prepared via co-reduction followed by the wet magnetic separation process. The corresponding recoveries of Ni, Cu, and Fe were 87.2%, 78.7%, and 69.0%, respectively.
Element Distribution Characteristics
The distribution characteristics of the Fe, Ni, Cu, Mg, and Si elements in the pellets reduced with 40% copper slag are shown in Supplemental Figure 5. In the reduced pellets, most of the elemental iron had been enriched in the metallic iron phase, and it was not distributed with non-metallic elements, such as Si and Mg. Based on the variable trends of the EDS line scanning of the Fe, Cu, and Ni, it is inferred that the Fe, Cu, and Ni cations in the molten slag were reduced to the metallic phase in the co-reduction process, and then the metallic nickel and copper transferred into the iron phase to form an Fe-Ni-Cu alloy through solid solution.
Conclusion
-
1.
An Fe-Ni-Cu alloy containing 2.5% Ni, 1.1% Cu, and 87.9% Fe can be prepared by co-reduction at 1250°C for 60 min with the addition of 60 wt.% copper slag and at a basicity of 0.3, followed by wet magnetic separation. The corresponding recoveries of Ni, Cu, and Fe were 87.2%, 78.7%, and 69.0%, respectively.
-
2.
Copper slag, as an activating agent, can facilitate the development of the melting phase in the co-reduction process with nickel laterite and improve the transport rate of the metallic ions, thereby accelerating the aggregation of metallic particles. Hence, the metallic particles grow significantly, and the magnetic separation of the alloy phase from the gangue phases is enhanced. As a result, the metal recovery increased with the addition of copper slag to the mixture.
References
M. Morcillo, I. Díaz, B. Chico, H. Cano, and D. de la Fuente, Corros. Sci. 83, 6 (2014).
M. Morcillo, B. Chico, I. Díaz, H. Cano, and D. De la Fuente, Corros. Sci. 77, 6 (2013).
M. Sánchez and M. Sudbury, J. Min. Metall. B 49, 161 (2013).
B. Gorai, R.K. Jana, and Premchand, Resour. Conserv. Recy. 39, 299 (2003).
I. Muravyov, V. Fomchenko, V. Usoltsev, A. Vasilyev, and F. Kondrateva, Hydrometallurgy 119, 40 (2012).
S. Panda, S. Mishra, D.S. Rao, N. Pradhan, U. Mohapatra, S. Angadi, and B.K. Mishra, Korean J. Chem. Eng. 32, 667 (2015).
K. Mawejaa, T. Mukongob, and I. Mutomboc, J. Hazard. Mater. 164, 856 (2009).
A. Sarrafi, B. Rahmati, and H.R. Hassani, Miner. Eng. 17, 457 (2004).
Z.Q. Guo, D.Q. Zhu, J. Pan, and T.J. Wu, Metals 86, 6 (2016).
Z.Q. Guo, D.Q. Zhu, J. Pan, and F. Zhang, JOM 69, 2341 (2016).
X.L. Zhou, D.Q. Zhu, J. Pan, and T.J. Wu, Int. ISIJ 55, 1347 (2015).
B. Su Kim, S. Ki Jo, D. Shin, J.C. Lee, and S.B. Jeong, Int. J. Miner. Process. 43, 143 (2015).
R.R. Moskalyk and A.M. Alfantazi, Miner. Eng. 15, 593 (2002).
M. Jiang, T. Sun, Z. Liu, J.Liu. Kou, and N.S. Zhang, Int. J. Miner. Process. 32, 123 (2013).
M.J. Rao, G.H. Li, X. Zhang, J. Luo, Z.W. Peng, and T. Jiang, Separa. Sci. Technol. 51, 1727 (2016).
D.Q. Zhu, Y. Cui, K. Vining, S. Hapugoda, J. Douglas, J. Pan, and G.L. Zheng, Int. J. Miner. Process. 1, 106 (2012).
G.H. Li, T.M. Shi, M.J. Rao, T. Kiang, and Y.B. Zhang, Miner. Eng. 19, 32 (2012).
R.M. German, P. Suri, and S.J. Park, J. Mater. Sci. 39, 44 (2009).
H. Tsuji, ISIJ Int. 52, 1000 (2012).
Y. Kobayashi, H. Todoroki, and H. Tsuji, ISIJ Int. 35, 51 (2011).
J. Luo, G.H. Li, Z.W. Peng, M.J. Rao, Y.B. Zhang, and T. Jiang, JOM 68, 3015 (2016).
GB/T 219-2008, Determination of Fusibility of Coal Ash (National Standards of the People’s Republic of China, 2008); (in Chinese)
Acknowledgements
The authors wish to express their thanks to the National Key Technology R&D Program of China (No. 2013BAB03B04) for the financial support of this research and would like to thank the Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources of Hunan Province, which supplied us the facilities and funds to complete the experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Z., Pan, J., Zhu, D. et al. Co-reduction of Copper Smelting Slag and Nickel Laterite to Prepare Fe-Ni-Cu Alloy for Weathering Steel. JOM 70, 150–154 (2018). https://doi.org/10.1007/s11837-017-2641-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2641-y