Abstract
The room temperature mechanical behavior of the fully bainitic steel grade 20CrMoVTiB410 was studied in the as-quenched and tempered conditions. The hardenability response of the steel during heat treatment was assessed. In the as-quenched condition itself, the steel exhibited a good combination of strength, ductility and toughness. Tempering the quenched steel till to 550°C, showed uniform mechanical properties. Tempering at 650°C showed secondary hardening behaviour, where the highest strength and least impact toughness was observed. Tempering at 700°C showed a sharp decrease in strength but with significant enhancement of toughness. The properties obtained were correlated with the microstructure and phase analysis was established using optical, scanning electron microscope, transmission electron microscope and x-ray diffraction techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bainitic steel grades based on Cr-Mo and Cr-Mo-V are extensively used for high-temperature applications in the power plant sector.1 The steel grade 20CrMoVTiB410 is a popularly used bainitic steel for high-temperature bolting applications, due to its technically superior stress relaxation behaviour.2–5 The steel develops a completely bainitic structure over a wide range of cooling rates. Our company produces and supplies this steel grade for high-temperature bolting applications under as-quenched and tempered conditions at large tonnage levels for power plant customers. The steel designed for high-temperature applications has Cr, Mo and B which ensures that bainitic nose is broadened in the time-temperature-transformation diagram. The steel also has low carbon content to ensure that the bainite start temperature is high. The steel also has a small addition of Ti which promotes fine grains during melting and deformation by the formation of fine Ti(C,N) precipitates. This also fixes the nitrogen in the ferrite which improves the steel toughness. The steel further contains a small quantity of B which delays the proeutectoid ferrite formation, which in turn enhances the steel hardenability. The steel has significant quantity of V (0.6–0.8%) which enables precipitation of alloy carbides at high-temperature tempering or in service conditions.6–8
There is an interest in the automotive industry, where air-cooled bainitic steels are being developed which have properties competing with those of micro-alloyed steels. The steel in the as air-cooled or as-quenched conditions shows an upper bainitic microstructure and has a strength higher than that of control-cooled microalloyed steels. The present steel was found to display a wide range of cooling rates in which bainite tends to form and it was felt that the thermal power plant bolting steel can be promoted for use as bainitic steels for room-temperature applications. Instead of air cooling, the present steel was examined in oil-quenched condition, where an upper bainitic microstructure could be obtained. In addition, the steel was tempered after quenching at various tempering temperatures to study the impact of microstructural changes and their effects on mechanical properties.
Experimental Procedure
The steel was melted as per composition in an electric arc furnace followed by the ladle refining and vacuum degassing route. Steel was cast in a 4 metric ton ingot with the bottom pour uphill casting technique. The steel chemistry was determined using an ARL 3460 spectrometer and the gas content in the steel (O, N, H) was analysed using a LECO gas analyser. The cast ingot was hot-stripped and charged in the hot condition in a soaking pit at 1260°C and held for 6 h. The homogenised ingot from the soaking pit was directly hot rolled to a final product of a 150-mm-diameter cross-section bar with a reduction ratio of about 25. The 150 mm diameter bar was used for further laboratory scale heat treatment processing.
A sample of the bar was cut and normalised at 990°C and subjected to a Jominy end quench test as per ASTM A255. Nine samples of 150 mm diameter and 150 mm length were heat treated in a laboratory scale muffle furnace. Two samples were loaded each time with spacers for uniform heating. Samples were hardened by austenising at 990°C and soaking for 3 h followed by oil quenching. The as-quenched hardness was evaluated in a 15 mm slice of a sample from the hardened bar. The hardness was measured at three different locations (centre, surface and mid-radius. R/2) using a Brinell hardness machine at a load of 3000 kgf with a 10 mm steel ball as per ASTM E10. The hardened samples were tempered at varying temperatures from 100 to 700°C soaking for 5 h at each temperature. The room temperature mechanical properties were evaluated at various tempering temperatures. The samples were tested at the mid-radius (R/2) location and the testing was carried out using a Shimadzu tensile testing machine and the test sample conformed to the ASTM E8 round sample of gauge diameter 12.5 mm and gauge length 50 mm. Impact properties were evaluated at different tempering temperatures using a standard FIE impact testing machine. Samples for the impact test were prepared as per ASTM E23. The microstructure was evaluated at R/2 using an optical microscope and scanning electron microscope (SEM) using an Oxford instrument at various tempering temperatures. The etchant used was 4% nital reagent. The prior austenite grain size was determined by oxidising the grain boundaries by heating the samples in a furnace as per ASTM E112. Transmission electron microscope (TEM) studies were carried out on the as-quenched and tempered sample (700°C) using a JEOL 200 kV machine. An x-ray diffraction (XRD) study was carried out on a Philip Xpert machine with a Cu kα filter.
Results and Discussion
This ingot of cast and hot-rolled steel with a 25:1 reduction ratio is usually heat-treated by austenitising at 970–990°C followed by water quenching and tempering between 680 and 720°C for power plant applications. In the present study, a detailed investigation has been carried out on the heat-treatment response of a fully bainitic 20CrMoVTiB410 steel in the as-quenched and subsequently tempered condition.
Effect of the Chemical Composition on Bainite Formation
The composition of the steel studied is shown in Table I. The presence of Ti in the steel ensures the formation of fine cuboidal Ti(C,N) inclusion in the molten steel, which enables a finer grain size during solidification and hot deformation stages. The steel has a low carbon content as well as elements that promote the formation of bainite, such as Cr, Mo and B. These elements also promote hardenability. The residual impurity levels are low. The bainite start temperature was estimated to be 563.4°C using Eq. 1.8
It can be seen that C, Cr and Mo significantly decrease the bainite start temperature. In addition, the low carbon in the steel promotes a lower carbide fraction. Alloying with about 1% Mo shifts the bainite nose, and alloying with ~0.002%B delays the ferrite nucleation tendency by the boron moving to the prior austenite grain boundaries.8
Effect of Hardenability
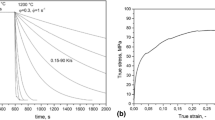
The hardenability of the steel evaluated using the Jominy end quench studies is shown in Fig. 1. The steel exhibits excellent hardenability and the hardness was consistently maintained over a thickness of 100 mm Jominy distance without a fall in hardness. This implies that the microstructure of the steel is uniform throughout the 100 mm cross-section. The high hardenability in the steel is due to the presence of Cr, Mo, B and V in the steel. Based on the Jominy end quench hardenability data and for the oil-quenched condition (quench severity of 0.35), the hardening depth profile (U-curve) was evaluated as shown in Fig. 2.
The variation in hardness with the bar cross-section in the as-quenched condition is shown in Fig. 2. It can be seen that the highest hardness of 41 HRC is retained through thicknesses up to 75 mm diameter. For bar diameters between 100 mm and 170 mm, the core hardness is 40 HRC while the surface hardness is 41 HRC, which demonstrates excellent hardenability.
Microstructure and Mechanical Properties in the As-Quenched Condition
The 150 mm diameter bar steel was evaluated for its performance in the heat treated condition which involved austenitizing at 990°C followed by oil quenching. In this condition, the carbides associated with the alloying elements Cr, Mo and V are completely dissolved in the austenitic matrix. The ferrite stabilising elements like Cr, Mo and V shift the AC3 temperature (temperature above which complete austenite forms) to a higher temperature range and the eutectoid point shifts to lower carbon levels.5 The as-quenched microstructure shows a fully bainitic microstructure with a complete absence of a martensitic structure, as shown in Fig. 3a–c. TEM studies of the as-quenched microstructure showed a completely upper bainitic microstructure without martensite even at the surface zones. The as-quenched hardness obtained in a 150 mm cross-section sample varies from 40 HRC to 42 HRC (centre: 40HRC, mid-radius: 41 HRC, surface 42 HRC) with a fully bainitic matrix.
The formation of the bainite on oil quenching may be explained with the continuous cooling transformation (CCT) diagram of the steel5 as shown in Fig. 4. It can be seen that the alloying elements in the steel have shifted the (ferrite + carbide) transformation curve to the right and that there is a widening of the bainitic nose. This has led to the formation of the bainite phase over a wide range of cooling rates. The critical cooling rates to produce a martensitic structure is very high (100°C/s). It is difficult to produce martensite in this steel even at the most severe cooling rates.5 Probably, thin sections of the steel with water or brine quenching may give martensite. Alloying elements Cr, Mo, Ti, V and B are all bainite-promoting elements and they have a tendency to retard nucleation and the growth process of pearlite.
CCT diagram of 20CrMoVTiB4105
This is corroborated by the fact that the highest hardness in the as-quenched condition showed 41 HRC. The steel was completely upper bainitic with dispersion of carbides within the bainitic ferrite as observed in optical, SEM and TEM micrographs in Fig. 3a–c. Figure 3c shows bainitic laths in the matrix along with interlath upper bainitic carbides. Such bainitic carbide (Fe3C) formation in the as-quenched condition is not observed in other alloy steels subjected to martensitic transformation.
The mechanical properties of the steel in the as-quenched condition with a bainitic microstructure, as in Fig. 3a–c, showed an ultrahigh-strength range with good ductility and impact toughness as shown in Table II. The yield strength to the tensile strength ratio in the as-quenched steel with bainitic microstructure is 0.91, which is attributed to the work hardening of the fine upper bainite structure. The prior austenite grain size in the steel conforms to ASTM grain size No. 9–9.5, as shown in Fig. 5a, b. The fine grain size may be attributed to the grain refinement caused by the Ti(C,N) precipitate shown in Fig. 5b. The Ti(C,N) precipitate forms in the liquid state and refines the grain size during solidification and hot working.6 The fine austenite grain size and the formation of fine bainite within the finer austenite grains leads to ultrahigh-strength levels with good ductility and impact toughness in the steel. Thus, the steel has potential applications even in the as-quenched condition due to its bainitic microstructure and ultrahigh-strength properties with reasonable impact toughness, unlike other alloy steels with martensitic transformation which need to be tempered. However, the cost associated with 1% Mo and 0.75% V in the steel is a point of concern for people working on air-cooled bainitic steel for automotive applications. The next stage of the research should be focused on the same steel with reduced Mo and V levels in which it may be possible to still achieve an acceptable range of mechanical properties.
Tempering of As-Quenched Bainitic Steel
In a manner similar to the tempering of the alloy steel with martenstic transformation after quenching, the present steel with a completely bainitic microstructure in the as-quenched condition was subject to tempering heat treatments from 100°C to 700°C. The mechanical properties evaluated after tempering is shown in Fig. 6, from which it can be seen that tempering the steel ranging from 100°C to 550°C does not show any variation in the mechanical properties (yield strength, ultimate tensile strength, ductility and toughness), unlike tempered alloy steels. In alloy steels, where martenite forms on quenching, tempering transforms the bct marteniste to equilibrium bcc ferrite and carbide. The investigated steel which showed a microstructure of upper bainite with carbide on quenching (Fig. 3a–c) has equilibrium ferrite and carbide in it and does not undergo phase transformation until 550°C, resulting in no change in the microstructure as shown in Fig. 7a and b. Unlike martensitic alloy steels which show tempered martensite embrittlement (~350°C) or temper embrittlement (~550°C), the present steel does not show such embrittlements.
Tempering in the range from 550°C to 650°C, shows a secondary hardening phenomenon, as evident in Fig. 6. At this temperature, microstructural changes take place even in steel with a bainitic ferrite-carbide microstructure. Tempering above 550°C leads to diffusion of the residual carbon and solutes in the ferrite to precipitate alloy carbides that coarsen the existing bainitic carbides and create ferrite free of solid solution.7 , 8 Above 600°C, diffusion of carbon and alloying elements becomes possible and the thermodynamic driving force enables precipitation of alloy carbides that lead to secondary hardening in this steel similar to that of martenistic alloy steels.
The secondary hardening peak for the present steel occurs at about 650°C, at which the mechanical properties showed an increase in yield strength and ultimate tensile strength but with severe deterioration of the impact toughness at the peak hardening temperature. The tensile strength improved by 100 MPa, while there was a decrease in CVN impact toughness from 24 J to 9 J at the peak hardened condition. The optical micrography of the samples at the peak hardness at 650°C, given in Fig. 7c, shows no significant change in optical microstructure. The SEM micrographs were then examined for the samples tempered at 500°C, 650°C and 700°C. The structure at 500°C showed thickening of the carbides in the ferrite matrix (Fig. 8a), while at 650°C peak hardened condition, the structure showed fine carbides in the ferrite matrix (Fig. 8b). The loss in toughness in peak hardening is observed in many steels and is attributed to the fine carbides that have unfavourable orientation effects with the precipitating phases and coherency strains, which decrease the impact toughness.9 When the present steel is tempered above 650°C, the strength starts to decrease, while there is a significant improvement in impact toughness (Fig. 6). The decrease in strength and the enhancement of toughness occurs between a very narrow temperature range of 650°C–720°C. At 700°C tempering, the steel shows significant improvement in impact toughness to a value as high as 220 J at the ultra high strength level (UTS = 938 MPa; YS = 865 MPa) as shown in Fig. 6. Thus, the steel responds to a narrow tempering temperature regime, where technically superior toughness and impact strength can be achieved. A precise control of temperature is needed in this range where even a 5°C change can give a very large variation in the impact toughness. The higher toughness and loss in strength at a tempering temperature above 680°C may be attributed to the coarsening of the alloy carbides along with bainitic cementite and recrystallization of ferrite grains. The tempering temperature is high enough for enhanced mobility of the elements, which form coarse carbides.
The ferrite which is free of alloying elements due to carbide precipitation is soft and exhibits improved toughness. The optical microstructures of the steel tempered at 700°C, as in Fig. 7d, show coarsening of the ferrite. The SEM micrographs show coarse recrystallised ferrite as in Fig. 8c. An effort was made to assess the carbides in TEM, where disk-shaped carbides were found, as shown in Fig. 9. Probably, these carbides are V4C3.10 A meaningful EDAX pattern and SAD pattern could not be obtained to directly decipher the disc-shaped phase.
The present steel has 1% Cr, 1% Mo and 0.75% V with 0.2% C content. Based on thermodynamic tendencies, this steel is reported to precipitate V4C3 as the secondary hardening phase.9–13 At 700°C, the Fe3C phase in the microstructure is replaced with the formation of more stable V4C3. According to Baker and Nutting,13 V4C3-type carbide has secondary hardening behaviour and the carbide occurs in plate morphology with a typical aspect ratio of 5:6. It has also been reported that the precipitate grows without a change in morphology with increasing tempering temperatures. Bhadeshia et al.14 have investigated tempering of similar steel, and they reported that the alloying elements diffuse into cementite and ultimately precipitate V4C3 and Mo2C. In the initial stages, the coherency between the precipitate and the ferrite lattice is reported to have a 3% mismatch, and the replacement of the bainitic carbide with its dissolution and replacement with fine V4C3 is reported to enhance the strength at the expense of impact toughness.15,16 Tempering at 700°C is reported to ensure the complete precipitation of alloy carbides from the ferrite and the coarsening of the precipitates by Ostwald ripening, in addition to some recrystallization of the ferrite grains which gives a softer ferrite and enhanced toughness.
Phase Analysis by XRD
XRD analysis was carried out with two typical samples in the as-quenched and 700°C tempered condition. The as-quenched sample with the upper bainitic microstructure shows broad ferrite peaks and some minor cementite peaks (Fig. 10a). The tempered steel shows sharp and distinct high-intensity ferrite peaks (200)α and (211)α compared with the as-quenched steel (Fig. 10b). In addition, the tempered steel shows a high degree of noise compared with the quenched steel, making indexing of minor peaks difficult (Fig. 10b). The sharpness of the ferrite peak may be attributed to the depletion of carbon from the ferrite solid solution to form more stable carbides such as V4C3. The noise level is an indication that there are microstructural changes taking place. It is difficult to distinctly decipher the presence of the hardening alloy carbides such as V4C3 due to the low volume percentage. However, the location of the V4C3 and Fe3C was marked in the XRD chart to match with minor peaks. The lattice parameter of the ferrite before and after tempering was calculated and the change in the lattice volume was estimated to be 1.5%, indicating the residual carbon precipitating from the ferrite forming alloy carbides.
Effect of Carbide Fraction on Mechanical Properties of Bainitic Steels
The volume percentages of the carbides (bainitic and alloy carbides) observed in the SEM images were quantified at various tempering temperatures using ImageJ software, as shown in Fig. 11a and b. It was observed that the carbide percentages in the as-quenched condition was highest at 24%. This was attributed to the fact that the upper bainitic microstructure has Fe3C-type plates between the sheaves (Fig. 3c). The carbide content formed for the steel at 0.2%C using the Lever rule, matches with the value obtained in the as-quenched sample. The carbide volume percentage was found to decrease with tempering temperature. It was 9% at 500°C tempering and 3.2% at the peak hardening temperature of 650°C.
The change in carbide volume fractions is an indication that the bainitic ferrite content was increasingly being consumed by the secondary hardening alloying element to form alloy carbides (probably V4C3). At 650°C, the alloy carbides have replaced the Fe3C which leads to increased hardening, while the impact toughness observed was poor. Increasing the tempering temperature to 700°C showed a drastic lowering of strength and significant improvement in toughness, similar to that observed in alloy steels. At 700°C, the alloy carbide volume increased to 5.5%, indicating coarsening of the carbide by precipitation of the carbon from the ferrite solid solution. This precipitation is the reason for the ferrite peaks in XRD becoming sharper after tempering (Fig. 10b). According to Bhadesia,7 the Fe3C dissolves up to the peak hardening stage, where the precipitation of V4C3 starts taking place. Thus, in a fully bainitic steel, the initial Fe3C carbide in the upper bainitic microstructure is replaced with alloy carbides at high-temperature tempering beyond 550–700°C similar to that observed in martensitic low alloy steels.
The overall carbide content of the as-quenched steel as observed in SEM is shown in Fig. 11a. A correlation between the carbide fraction in the steel and the mechanical properties can be established by studying Figs. 6 and 11b. It can be seen that in the as-quenched condition where the bainitic carbide Fe3C is 24%, the strength (UTS = 1079 MPa; YS = 935 MPa) and impact toughness (CVN = 24 J) are lower. As tempering increased to 500°C, the carbide content is lowered (carbide fraction = 9%), where there is an enhancement of strength due to fine carbides with their enhanced strength in the matrix (UTS = 1138 MPa; YS = 996 MPa) with the same impact toughness (CVN = 24 J). At the peak hardening tempering temperature of 650°C, the carbide content is the lowest (carbide fraction = 3.5%), which has led to further enhancement of the strength (UTS = 1205 MPa; YS = 1140 MPa) but with severe loss in impact toughness (CVN = 9 J). Tempering above the peak hardening range at 700°C shows an increase in alloy carbide content (carbide fraction = 5.5%), where there is a drastic fall-off in strength (UTS = 938 MPa; YS = 865 MPa) and very high impact toughness (CVN = 230 J). In summary, the as-quenched fully bainitic steel responds to tempering heat-treatment beyond 550°C, similar to that of martensitic alloy steel.
Conclusions
The steel 20CrMoVTiB410 shows a completely upper bainitic structure in the as-quenched condition with good strength, ductility and impact toughness. The presence of Ti in the steel shows a fine grain size of ASTM No. 9–9.5. The hardenability of the steel showed a through hardening capability, which is favourable for the development of a uniform bainitic microstructure with a large thickness. Tempering the steel up to 550°C showed stable strength, toughness and ductility levels. At further increased tempering temperatures, there is a secondary hardening effect observed in a bainitic matrix, where the strength enhances with a deterioration of toughness at the peak hardening condition of 650°C. The secondary hardening response of the steel is attributed to the formation of fine carbides, observed in TEM, and which was deduced to be V4C3-type precipitation based on thermodynamic assessment. There is a significant improvement in toughness with reasonable strength, when the tempering of the steel is carried out at 700°C. The microstructure and the carbide distribution and content were quantified and correlated to the mechanical properties with the SEM microstructure. The formation of the ferrite and carbides in the as-quenched condition was established by the XRD investigation, and the changes in peak intensity and minor peaks of alloy carbide beyond peak hardening confirmed the response to tempering treatment.
References
K. Singh, BHEL J 27, 15 (2006).
D.T. Llewellyn, Steels Metallurgy and Applications, 2nd ed. (Oxford: Butterworth Heinemann, 1992), p. 190.
Y. Koutski, R. Pokornyi, and V. Vanecheck, Met. Sci. Heat Treat. 21, 896 (1979).
J.R. Davis, ASM Speciality Handbook on Heat Resistant Materials (OH: ASM International, 1997), pp. 89.
H. Everson, J. Orr, and D. Dulieu, Technical paper Prod/EP6, Corus engineering steels, 1 (2001).
T. Gladman, Physical Metallurgy of Microalloyed Steels, 1st ed. (London, UK: Institute of Metals, 1997), pp. 363.
H.K.D.H Bhadeshia, Bainite in Steels, 2nd ed. (London, UK: Institute of Materials Communication Ltd., 2001), pp. 454.
H.K.D.H. Bhadeshia, Steels: Microstructures and Properties, 2nd ed. (Oxford: Butterworth and Heinemann Ltd, 2006), p. 145.
O.G. Kasatkin and B.B. Vinokur, Met. Sci. Heat Treat. 26, 27 (1984).
K.A. Taylor, Scripta Metall. 32, 7 (1995).
M.F. Ashby and K.E. Easterling, Acta Metall. 30, 1969 (1982).
Q. Shaw, ISIJ Int. 10, 85 (1957).
J. Nutting, ISIJ Int. 207, 872 (1969).
S. Yamasaki and H.K.D.H. Bhadeshia, Mater. Sci. Technol. 10, 1335 (2003).
S. Yamasaki and H.K.D.H. Bhadeshia, Mater. Sci. Technol. 10, 723 (2003).
J.D. Robson and H.K.D.H. Bhadeshia, Mater. Sci. Technol. 13, 631 (1997).
Acknowledgements
The authors wish to thank the constant encouragement and support provided by Dr. Baba Kalyani, Chairman and Managing Director and Mr. R. K. Goyal, Managing Director, Kalyani Carpenter Special Steels Ltd., Pune. A special thanks to the Kalyani Centre for Technology and innovation (KCTI) for their constant support. The authors would like to thank the Department of Scientific and Industrial Research for the recognition and support of our R&D activities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivatsa, K., Srinivas, P., Balachandran, G. et al. Room Temperature Microstructure and Property Evaluation of a Heat Treated Fully Bainitic 20CrMoVTiB410 Steel. JOM 68, 2704–2712 (2016). https://doi.org/10.1007/s11837-016-2063-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2063-2