Abstract
The reductive acid leaching kinetics of synthetic cadmium-bearing zinc ferrite was investigated, and the influence of reaction temperature, sulfuric acid and hydrazine sulfate were studied. The results illustrated that an increase in the reaction temperature, initial sulfuric acid and hydrazine sulfate significantly enhanced the extraction efficiencies of cadmium, zinc and iron. The leaching kinetics were controlled by a surface chemical reaction based on a shrinking core model. The empirical equation \( 1 - (1 - X)^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 3}}\right.\kern-0pt} \!\lower0.7ex\hbox{$3$}}}} = k_{\text{r}} t \) applied was found to fit well with the kinetics analysis; the leaching processes of cadmium, zinc and iron were similar and the activation energies were 79.9 kJ/mol, 77.9 kJ/mol and 79.7 kJ/mol, respectively. The apparent orders of cadmium-bearing zinc ferrite dissolution with respect to sulfuric acid concentration were 0.83, 0.83 and 0.84 for Cd, Zn and Fe, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc ferrite (franklinite, ZnFe2O4) and its variants, having a stable spinel structure, can be easily formed when completely heated to approximately 950°C because of the inevitable presence of iron in the zinc concentrates.1,2 Zinc ferrite is stable and insoluble in most acidic, alkaline and chelating media under mild conditions. Cadmium is a very toxic metal that causes chronic poisoning through inhalation and digestion; its dispersion into an ecosystem should be strictly controlled.3 However, it has been found that zinc smelting by way of roasting–leaching–purifying–electrowinning is one of the major sources of cadmium contamination in China, because cadmium is most commonly found in association with sphalerite and marmatite in minor amounts and is recovered almost exclusively as a byproduct of zinc smelting.4 Unfortunately, because of the similar chemical properties between cadmium and zinc, under high-temperature conditions, cadmium cations unavoidably enter the crystal lattice of zinc ferrite by substituting zinc to form cadmium-bearing zinc ferrite [(Cd x Zn(1−x))Fe2O4]; this creates problems in zinc and cadmium recovery when hydrometallurgical processes increase the diffusion of cadmium in the zinc smelting process.5
The hydrometallurgical extraction of zinc from zinc ferrite and the acid-leaching kinetics of synthetic zinc ferrite samples have been studied by several researchers,6–8 who revealed that the dissolution of zinc ferrite in an acidic solution is slow and highly variable. Zheng and Muir reported that Fe2O3 and ZnFe2O4 exhibited a reduction of iron(III) to iron(II) in the lattice under cathodic conditions, with Fe2O3 and ZnFe2O4 becoming more reactive to acid.9 Elgersma et al. investigated synthetic and industrial zinc ferrite leaching in H2SO4, HNO3, and HClO4 solutions at temperatures ranging from 75°C to 95°C and estimated the apparent activation energy for the dissolution of synthetic zinc ferrite at 74 ± 2 kJ/mol in H2SO4. 10 Shawabkeh and Montenegro et al. studied the hydrometallurgical treatment of steelmaking electric arc furnace dust (EAFD), which contained primarily zinc ferrite, for the recovery of zinc and other metals such as cadmium.11,12
Many researchers have used hard reaction conditions, such as high acid concentrations, high pressures or high temperatures to intensify the leaching process and thereby achieve higher metal extraction efficiency.13,14 Ultrasonic leaching, microwave heating and reductive acid leaching have recently been examined for enhancing leaching kinetics.15–19 However, there has been no study on the reductive acid-leaching behavior of cadmium in cadmium-bearing zinc ferrite in order to determine whether the cadmium diffusion pollution control is the same as zinc recovery in the zinc smelting process.
Because it is practically impossible to separate and recover pure zinc ferrite from other minerals, synthetic cadmium-bearing zinc ferrite was used. XRD and SEM–EDS analyses were used to compare it with industrial zinc ferrite. Because of its strong reductive ability, hydrazine sulfate was chosen as the reductant. The effects of temperature, sulfuric acid and hydrazine sulfate concentration were studied in detail.
Experimental
Materials
A synthetic cadmium-bearing zinc ferrite mixture sample, containing 20.3 wt.% Cd, 12.2 wt.% Zn and 44.4 wt.% Fe, was prepared by heating a completely ground stoichiometric mixture of Fe2O3, ZnO and CdO to a temperature of 1000°C for 4 h. The sintered product was ground and calcined at 1000°C for 2 h. The reaction can be described as follows:
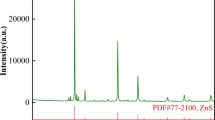
The sample was characterized by x-ray diffraction (XRD, Rigaku, TTR-III) with all peaks matched the standard XRD pattern of octahedral structure ferrite; however, some zinc lattice sites were partially substituted by cadmium and formed the major phases Cd0.25Zn0.75Fe2O4 and Cd0.5Zn0.5Fe2O4, as shown in Fig. 1. SEM analysis revealed that the particles were mostly spherical and porous and a minor unreacted phase of ZnO was also detected. A particle size distribution analysis performed with a lazer particle size analyzer [LS-POP(6)] showed a narrow particle size distribution of cadmium-bearing zinc ferrite mixture primarily in the range of 8–110 μm.
Experimental Set Up and Procedure
A 500-mL four-neck flask equipped with a reflux condenser, mechanical stirrer and a thermometer was used for the leaching experiments and immersed in a thermostatically controlled water bath. Distilled water and concentrated sulfuric acid were employed in preparing the leaching solutions (250 mL), and cadmium-bearing zinc ferrite (5.0 g) was added with stoichiometric hydrazine sulfuric when the temperature reached the required value. Samples of 1 mL were withdrawn at various times. The concentration of cadmium, zinc and iron were analyzed by ICP-AES. The stirring speed was fixed at 400 rpm based on previous studies,7,20 which proved this rate to be sufficient for eliminating the effect of external diffusion throughout the experiment. All chemical reagents employed in this study were of analytical grade.
Kinetic Analysis
Fluid–solid heterogeneous reaction systems have been applied in many chemical and hydrometallurgical processes. In heterogeneous fluid–solid reactions, the soluble reactants diffuse initially across a fluid-film and then through a porous solid layer. Chemical reactions occur thereafter. Thus, the reaction rate may be controlled by reactant diffusion through the fluid-film, or by diffusion through a solid product layer, or by the rate of chemical reaction at the surface of the unreacted core.21,22
To determine the kinetic parameters and rate controlling steps in the reductive acid leaching of cadmium-bearing zinc ferrite, the experimental data were analyzed based on a shrinking core model.23 According to the set of control regimes in the shrinking core model, if the reaction rate is controlled by liquid film diffusion, then the following integrated rate equation will be in effect:
If the reaction rate is controlled by diffusion through the solid product layer, then the integrated rate equation can be described as follows:
If the surface chemical reaction is the slowest step, then Eq. 3 transforms to the following equation:
where X is the fraction achieved, \( k^{\prime}_{\text{d}} \) (min−1) is the kinetic parameter for the liquid film diffusion control, \( k^{\prime\prime}_{\text{d}} \) (min−1) is the kinetic parameter for the product layer diffusion control, k r (min−1) is the kinetic parameter for the surface chemical reaction control and t (min) is the reaction time.
The temperature dependence of the reaction rate constant is defined by the Arrhenius equation.
In this method, there is an implicit assumption that the activation energy does not vary during the course of reaction. In the Arrhenius equation, A is the frequency factor, Ea is the apparent activation energy of the reaction, R is the universal gas constant and T is the absolute temperature, K.
Results and Dissolutions
Chemical Reaction Analysis
In a sulfuric acid solution, cadmium-bearing zinc ferrite reacts with Cd2+, Zn2+ and Fe3+ according to the following equation (Eq. 6).
Hydrazine sulfate dissolves easily in hot water and its solution is acidic because of hydrolysis. The Fe3+ was quickly reduced to Fe2+ by hydrazine sulfate when the temperature was above 80°C; this can be presented as follows:
The potential-pH diagrams of the Fe-Cd-Zn-H2O system at different temperatures are shown in Fig. 2a and b. The potential of Fe3+/Fe2+ increases with an increase in temperature from 25°C to 95°C, denoting that the feasibility that Fe3+ is reduced by hydrazine sulfate when the temperature is increased. In addition, the coexisting potential of Fe2+, Cd2+ and Zn2+ cations increases with the increasing of temperature, but narrows the pH range of Fe2+, Cd2+ and Zn2+ in the solution. Zinc ferrite has a large stability field, but it is less stable under reducing acidic conditions. The stability field of zinc ferrite has a pH range of approximately 4.6–14 and a range of potential from 1.6 V to below −0.8 V at 25°C. When the temperature was increased from 25°C to 95°C, the decomposition pH of zinc ferrite decreases from approximately 4.6 to below 4, i.e., it becomes more acid, which illustrates that an increase in temperature disadvantages the decomposition of zinc ferrite from the thermodynamics perspective. Therefore, to dissolve zinc ferrite under reducing conditions, the temperature and pH of solution should be maintained appropriately.
Effect of Temperature
From a thermodynamics point of view, temperature has significant influence on the reductive acid leaching of cadmium-bearing zinc ferrite. Therefore, a range of temperatures (55–95°C) below the water boiling point of water was chosen in this study to investigate the effect of temperature on the reductive-leaching of cadmium-bearing zinc ferrite and the changes of redox potential during the leaching process.
As shown in Fig. 3a–c, the reaction temperatures have a noticeable influence on the leaching of cadmium, zinc and iron from cadmium-bearing zinc ferrite. At 55°C, 62.2% of the cadmium, 63.7% of the zinc and 63.0% of the iron were extracted after 360 min, and these values increased to 95.6%, 95.6% and 95.8%, respectively, at 95°C after 60 min.
The cadmium, zinc and iron extraction data at different temperatures were plotted according to the shrinking core model. The results illustrate that the dissolution rate of cadmium-bearing zinc ferrite is controlled by the surface chemical reactions and not by diffusion control. Figure 4a–c show the plots of the shrinking core model for chemical reaction control and have the linear relationship between \( 1 - (1 - X)^{1/3} \) and the leaching time, with the values of the correlation coefficient (R 2) close to 1. The apparent rate constants (k r) were calculated as slopes of the straight lines and were significantly affected by changes in the reaction temperature. Using the apparent rate constants obtained in Fig. 4a–c, the Arrhenius plot (lnk r versus 1000/T) was obtained (Fig. 4d), and the apparent activation energy was calculated to be 79.9 kJ/mol, 77.9 kJ/mol and 79.7 kJ/mol for cadmium, zinc and iron, respectively, in the temperature range of 55°C to 95°C. There were no serious discrepancies noted among the activation energies corresponding to cadmium, zinc and iron, which indicates a similar leaching behavior, although the apparent activation energy calculated for zinc is slightly lower than that for cadmium and iron because of the coexistence of ZnO. These values clearly confirm that this process is most likely controlled by the surface chemical reaction, which is similar to other reports.1,10,15,24
The variation of 1 − (1 − X)1/3 with time at various temperatures: (a) Cd, (b) Zn, (c) Fe, (d) Arrhenius plot of reaction rate for Cd, Zn and Fe against reciprocal temperature. (H2SO4 80 g/L, \( {\text{N}}_{{{\text{N}}_{ 2} {\text{H}}_{ 4} {\text{H}}_{ 2} {\text{SO}}_{ 4} }} \):NFe = 1:1, L/S = 50 mL/g, 400 rpm)
To describe the variation of redox potential in the solution during the leaching process at various temperatures, the potential was measured with a platinum electrode similar to a calomel electrode; the values were recalculated relative to a normal hydrogen electrode. The results are shown in Fig. 5, where the redox potential of the solution sharply increases within the first 30 min of reaction, due to the dissolution of Fe3+ into the solution and its simultaneous reduction to Fe2+ via hydrazine sulfate increasing with a higher speed at 95°C than that at 65°C. The redox potential of the solution decreases with the increase in reaction temperature, which obviously implies that a higher temperature contributes to the redox reaction between Fe3+ and hydrazine sulfate which may accelerate the dissolution of cadmium-bearing zinc ferrite. The pH of the reaction was also measured and is shown in Fig. 5. During the leaching process, the pH changed slightly, maintaining a range from −1.33 to −1.13 due to the excess of employed sulfuric acid. The measured values of redox potential and pH are in the region of the coexisting stability field of Cd2+, Zn2+ and Fe2+ in solution compared with the analysis in Fig. 2, which is in accordance with the theoretical analysis.
Effect of Sulfuric Acid Concentration
To investigate the influence of sulfuric acid concentration on the decomposition of cadmium-bearing zinc ferrite, experiments were conducted in solutions containing different initial H2SO4 concentrations (35–80 g/L) at 85°C. The molar ratio between the hydrazine sulfate and the iron content in the cadmium-bearing zinc ferrite mixture was 1:1; the liquid–solid ratio was fixed at 50 mL/g and the stirring speed was 400 rpm.
As shown in Fig. 6a, the increase in sulfuric acid concentration increased the dissolution rate of the cadmium. For concentrations below 50 g/L, there was virtually an acid deficiency effect. Nevertheless, there was no beneficial influence from increasing the acid concentration by more than 80 g/L. Similar results were achieved for the extraction of zinc and iron from cadmium-bearing zinc ferrite, as shown in Fig. 6b and c, respectively. The stoichiometric sulfuric acid concentration for a total dissolution of cadmium-bearing zinc ferrite was calculated to be approximately 23 g/L. However, the dissolution of cadmium-bearing zinc ferrite actually occurred in more acidic conditions because of its stable spinel structure. The extraction efficiencies after 60 min leaching at 80 g/L H2SO4 were found to be 87.8%, 89.1% and 84.8% for Cd, Zn and Fe, respectively.
The effect of leaching time on the dissolution of the cadmium-bearing zinc ferrite is also evident in Fig. 6. The leaching efficiency of Cd, Zn and Fe increased with the increase of leaching time with the initial dissolution of cadmium-bearing zinc ferrite being very rapid. As shown in Fig. 6, 120 min of leaching was found to be efficient in this study.
The extraction data of Fig. 6 were used in the surface chemical reaction control model (Eq. 4) and to determine the k r values for different sulfuric acid concentrations, as shown in Fig. 7a–d. According to the apparent rate constant k r and the sulfuric acid concentration values, the lnk r versus ln[H2SO4] was plotted and is shown in Fig. 7d. The order of reaction for the dissolution of cadmium-bearing zinc ferrite with respect to sulfuric acid was proportional to the 0.83, 0.83 and 0.84 for Cd, Zn and Fe, respectively, with a correlation coefficient of more than 0.99. Furthermore, the values of the reaction order for Cd, Zn and Fe are parallel to each other, which indicates that the effect of sulfuric acid on the extraction of cadmium, zinc and iron from cadmium-bearing zinc ferrite is similar. Ramachandra Sarma et al. reported that the order of the zinc ferrite–sulfuric acid reaction with respect to [H+] was 0.6 in 0.5–2.5 mol/L H2SO4 at 70–99°C.25
The variation of 1 − (1 − X)1/3 with time at various sulfuric acid concentration: (a) Cd, (b) Zn, (c) Fe, (d) Plot for determination of reaction order with respect to ln[H2SO4]. (85°C, \( {\text{N}}_{{{\text{N}}_{ 2} {\text{H}}_{ 4} {\text{H}}_{ 2} {\text{SO}}_{ 4} }} \):NFe = 1:1, L/S = 50 mL/g, 400 rpm)
Effect of Hydrazine Sulfate
The relationship between the concentration of hydrazine sulfate and the leaching efficiency of the metals was investigated. The added amount of hydrazine sulfate was calculated using the molar ratios between the hydrazine sulfate and the total iron content in the material as 0.6, 0.8, 1.0, 1.2 and 1.4, respectively, namely the range of hydrazine sulfate concentrations from 12.4 g/L to 28.8 g/L. Other fixed parameters included a sulfuric acid concentration of 50 g/L, a leaching temperature of 85°C, a liquid–solid ratio of 50 mL/g and a stirring speed of 400 rpm.
The results, which are shown in Fig. 8a–c, reveal that the increase in hydrazine sulfate concentration contributes to an increase of leaching efficiency in cadmium-bearing zinc ferrite. The extraction efficiency of cadmium from the cadmium-bearing zinc ferrite is 67% after leaching 60 min at 12.4 g/L of hydrazine sulfate, and increases to 80.0% when the hydrazine sulfate concentration increases to 28.8 g/L (Fig. 8a). Similar results were found in the extraction of zinc and iron from cadmium-bearing zinc ferrite, as shown in Fig. 8b and c. The leaching efficiency of zinc from the cadmium-bearing zinc ferrite increases from 70.1% to 81.7% and that of iron increases from 67.8% to 76.3% after 60 min of leaching when the concentration of hydrazine sulfate increases from 12.4 g/L to 28.8 g/L. However, the results show that the effect of hydrazine sulfate concentration on the extraction efficiency of metals from cadmium-bearing zinc ferrite is smaller than the effects of temperature and sulfuric acid, which may be attributed to reducing the Fe3+ to Fe2+ in the solution; however, there is no direct effect of hydrazine sulfate on the iron in the lattice of cadmium-bearing zinc ferrite.
The variation of redox potential in the solution was also measured to describe the role of hydrazine sulfate as a reductant. The values of potential were obtained with a platinum electrode relative to a calomel electrode and recalculated relative to a normal hydrogen electrode. The results are shown in Fig. 9. The sulfuric acid was oxidized, and the redox potential was measured at 0.736 V for 80 g/L sulfuric acid solution at 65°C. The redox potential of the solution increased slowly at the beginning of leaching and remained constant at the end. The leaching efficiency of metals was still low at 95°C, although the redox potential of the solution was no more than 0.3 V during the leaching process, when hydrazine sulfate was the only lixiviant used for leaching the cadmium-bearing zinc ferrite. This could be attributed to a deficiency of H+ in the solution, as the solution of hydrazine sulfate was acidic because of hydrolysis. The redox potential of the sulfuric acid and hydrazine sulfate mixed solution was sharply increased within the first 30 min of reaction at 95°C. The potential values were less than 0.607 V throughout the leaching process, which was within the region of the coexisting stability field of Cd2+, Zn2+ and Fe2+ in the solution and in accordance with the analysis in Fig. 2d. These findings indicate that the addition of hydrazine sulfate as a reductant was beneficial in accelerating the extraction rate of metals from cadmium-bearing zinc ferrite.
Conclusion
The reductive acid leaching residue of synthetic cadmium-bearing zinc ferrite keeps the same spinel structure as the raw sample. The dissolution kinetics of synthetic cadmium-bearing zinc ferrite in sulfuric acid hydrazine sulfate reductant solutions are dramatically dependent on temperature and sulfuric acid. A shrinking core model was used to analyze the kinetics of cadmium, zinc and iron leaching, and it showed that the rate was controlled by the surface chemical reaction, with apparent activation energies calculated to be 79.9 kJ/mol, 77.9 kJ/mol and 79.7 kJ/mol for cadmium, zinc and iron, respectively, in the range of temperature from 55°C to 95°C. The apparent order of cadmium-bearing zinc ferrite dissolution with respect to sulfuric acid concentrations were 0.83, 0.83 and 0.84 for Cd, Zn and Fe, respectively. The similarity of cadmium, zinc and iron leaching illustrates that cadmium had substituted into the crystal lattice of zinc ferrite.
References
Y. Zhang, X. Li, L. Pan, X. Liang, and X. Li, Hydrometallurgy 100, 172 (2010).
J.C. Balarini, L.D.O. Polli, T.L.S. Miranda, R.M.Z.D. Castro, and A. Salum, Miner. Eng. 21, 100 (2008).
E. Bidari, M. Irannejad, and M. Gharabaghi, J. Environ. Chem. Eng. 1, 1269 (2013).
M. Sadegh Safarzadeh, M.S. Bafghi, D. Moradkhani, and M. Ojaghi Ilkhchi, Miner. Eng. 20, 211 (2007).
J.E. Dutrizac, D.J. Hardy, and T.T. Chen, Hydrometallurgy 41, 269 (1996).
C. Núñez and J. Viñals, J. Metall. Trans. B. 15, 221 (1984).
N. Leclerc, E. Meux, and J.-M. Lecuire, Hydrometallurgy 70, 175 (2003).
Š. Langová, J. Leško, and D. Matýsek, Hydrometallurgy 95, 179 (2009).
Z.-Y. Lu and D.M. Muir, Hydrometallurgy 21, 9 (1988).
F. Elgersma, G.F. Kamst, G.J. Witkamp, and G.M. van Rosmalen, Hydrometallurgy 29, 173 (1992).
R.A. Shawabkeh, Hydrometallurgy 104, 61 (2010).
V. Montenegro, P. Oustadakis, P.E. Tsakiridis, and S. Agatzini-Leonardou, Metall. Mater. Trans. B 44, 1058 (2013).
H. Xie, J. Wang, H. Lu, and C. Bao, Hydrometallurgy 134, 96 (2013).
M.A.R. Önal and Y.A. Topkaya, Hydrometallurgy 142, 98 (2014).
L. Zhang, J. Mo, X. Li, L. Pan, X. Liang, and G. Wei, Metall. Mater. Trans. B 44, 1329 (2013).
X.-H. Li, Y.-J. Zhang, L.-P. Pan, and Y.-S. Wei, Trans. Met. Soc. China. 23, 1512 (2013).
X. Wu, S. Wu, W. Qin, X. Ma, Y. Niu, S. Lai, C. Yang, F. Jiao, and L. Ren, Hydrometallurgy 113, 195 (2012).
M.S. Safarzadeh, N. Dhawan, M. Birinci, and D. Moradkhani, Hydrometallurgy 106, 51 (2011).
W. Xin, C. Srinivasakannan, D. Xin-hui, P. Jin-hui, Y. Da-jin, and J. Shao-hua, Sep. Purif. Technol. 115, 66 (2013).
D. Moradkhani, M. Rasouli, D. Behnian, H. Arjmandfar, and P. Ashtari, Hydrometallurgy 115, 84 (2012).
V. Safari, G. Arzpeyma, F. Rashchi, and N. Mostoufi, Int. J. Miner. Process. 93, 79 (2009).
A. Velardo, M. Giona, A. Adrover, F. Pagnanelli, and L. Toro, Chem. Eng. J. 90, 231 (2002).
K.C. Liddell, Hydrometallurgy 79, 62 (2005).
Y. Zhang, X. Li, L. Pan, Y. Wei, and X. Liang, Hydrometallurgy 102, 95 (2010).
V.N. Ramachandra Sarma, K. Deo, and A.K. Biswas, Hydrometallurgy 2, 171 (1976).
Acknowledgements
The authors gratefully acknowledge Project (2012FJ1010) supported by the Key Project of Science and Technology of Hunan province; Project (51474247) supported National Natural Science Foundation of China; Project (201509050) supported by the Special Program on Environmental Protection for Public Welfare. The project was supported Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, J., Min, X. et al. Kinetics of Reductive Acid Leaching of Cadmium-Bearing Zinc Ferrite Mixture Using Hydrazine Sulfate. JOM 67, 2028–2037 (2015). https://doi.org/10.1007/s11837-015-1553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1553-y