Abstract
The legume tree Schizolobium parahyba from the Brazilian Atlantic Forest shows young aerial organs covered with a sticky exudate. Aiming to clarify the functional aspects of the sticky secretions, we performed analyses on the dynamics of secretion through the plant development and characterized the chemical nature of the exudates by histochemical tests. We also studied the secretory tissue using light and electron microscopy. The production of the exudates starts soon after seed germination, being evident in the epicotyl but not in the hypocotyl and cotyledons. The secretory activity extends throughout the juvenile and pre-reproductive phase, in primary stems and leaf portions. After the first flowering, secretion was no longer observed. The lipid exudates are secreted by the epidermis and are composed of mixtures of essential oils and oleoresins. Modified plastids, extensive rough endoplasmic reticulum, proliferated smooth endoplasmic reticulum, enlarged vacuoles containing flocculant materials, membrane debris, and convoluted tubules/lamellae membranes covered with osmiophilic deposits are the main features of the secretory epidermal cells. Secretion exits the protoplast by exocytosis and accumulates in the cuticle, resulting in a sheath of concentric bands of electron-dense deposits, and is released by cuticle peeling. The hydrophobic nature of the secretion, which forms an impermeable layer on the epidermis of young organs, is a relevant attribute of the aerial organs of S. parahyba. In addition to protecting against desiccation, this exudate effectively captures particles and immobilizes insects and other arthropods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of all the major families of vascular plants have species with some combination of sticky, slimy, or oily substances on their surfaces (LoPresti 2016). Sticky secretions on plants have different functions, such as capturing small animals (Adlassnig et al. 2010; Frenzke et al. 2016; Wilder 2019), indirect plant defense (LoPresti et al. 2015), spreading propagules, facilitating germination (Yang et al. 2010; De-Paula et al. 2015), collection of organic particles (Adlassnig et al. 2010), and collection of substrate or inorganic particles (LoPresti and Karban 2016). In most plant taxa with sticky exudates externally accumulated, secretion production and release occur most commonly in glandular trichomes (Fahn 1979; Voigt et al. 2020), causing plant surfaces to become sticky (Adlassnig et al. 2010; Falara and Pichersky 2012).
The adhesive properties of exudates are attributed to chemically diverse substances and include terpenoids, flavonoids, phenylpropanoids, alkaloids, fatty acid derivatives, and acylated sugars that confer mucilaginous or resinous properties to secretions (Werker 2000; Simoneit et al. 2008; Adlassnig et al. 2010; Betz 2010; Frenzke et al. 2016; Voigt et al. 2020). Despite the effectiveness of adhesive secretions in defending plants against herbivory (Levin 1976; Wagner 1991; Peiffer et al. 2009; Tian et al. 2012), specialized arthropods can move across sticky surfaces of plants without getting stuck (Voigt and Gorb 2008, 2010). Many species of invertebrates are known to colonize sticky plants to consume living and dead prey immobilized by adhesive secretions produced by glandular trichomes (Jiménez-Pomárico et al. 2019 and references therein). Biological adhesion has attracted much research interest in ecology, especially in plant–animal interactions (Wheeler and Krimmel 2015; Karban et al. 2019; Voigt et al. 2020). However, the morphological and cellular bases of the sticky exudates and their release to the plant surface are relatively understudied.
Plants collectively produce hundreds of thousands of low molecular but specialized metabolites, restricted to specific taxonomic groups and cell or tissue types (Fan et al. 2019). LoPresti et al. (2015) list about 120 genera distributed in 49 families of taxonomically unrelated angiosperms on which representatives of plants with sticky surfaces can be found, among them Leguminosae. Recent advances in next-generation sequencing and mass spectrometry technologies have unveiled the extraordinary metabolic diversity in all plants, such as acylsugars found in trichomes of Caryophyllaceae, Geraniaceae, Martyniaceae, Rosaceae, and Solanaceae (Liu et al. 2019). Acylsugars from the Solanaceae family—including plants of the Solanum, Physalis, Nicotiana, Petunia, and Salpiglossis genera, are produced in glandular trichome tip cells (Moghe et al. 2017; Fan et al. 2019).
Most of the studies on sticky exudates have been addressed to very specific stages in time and space, such as seed mucilage and leaf bud resins (in juveniles and spring budburst before reproduction), and little is known about the dynamic of secretion through the plant development, which is crucial for understanding their performance in heterogeneous habitats. Understanding the secretory process across the lifespan of the plant acquires particular importance in the ecological context to which the plant is subjected, especially during the seedling and juvenile phases. Seedlings and juvenile plants are usually subjected to environmental stresses, such as high irradiation, evapotranspiration and dehydration, and damage caused by trampling animals, herbivory, and pathogens (Oliveira 1999). All these agents are important potential causes of seedling mortality.
Schizolobium parahyba (Vell.) Blake, commonly known as ‘guapuruvu’ or ‘ficheira’, is a semi-deciduous legume tree reaching up to 20–30 m in height (Lorenzi 1992). Its natural distribution is irregular and discontinuous, widely found in forest gaps and edges in devastated ecosystems, as in the case of the Atlantic Forest of Brazil (Pinto and Brito 2003). Schizolobium parahyba is notable for its growth rate reaching 3 m per year, being of great importance in revegetation and landscaping projects (Lorenzi 1992; Freire et al. 2007).
In the course of a study on seedling and juvenile plants of Leguminosae woody species, Oliveira (1999) repeatedly found a sticky secretion covering the epicotyl and other young aerial portions of stem and leaves of S. parahyba. Motivated by these findings, we started discussing the origin of the superficial exudates and their potential roles during a particular and sensitive plant developmental stage.
In the present study, we performed analyses on the exudates and dynamics of secretion in loco in S. parahyba, investigated the chemical nature of the exudates, and identified the secretory tissues and their ultrastructural organization, aiming to clarify the morpho-functional aspects of the sticky secretions under a developmental point of view.
Materials and methods
Seeds of S. parahyba were collected in a remnant area of seasonal semi-deciduous forest located in Edgardia farm, in Botucatu municipality (22° 52ʹ S, 48° 26ʹ W, 786 m above sea level), in the central west region of São Paulo State, Brazil. The mean annual temperature is 20.3 °C, and the annual precipitation is 1428 mm. With a strong seasonality, rains occur in the summer (December–March) and drought in the winter (June–September), and with a small hydric deficiency from April to August (Cunha and Martins 2009).
The localization and sequential distribution of the secretory activity were described by observing in loco the seedlings and juvenile plants. For this, a lot of 50 seeds (five repetitions of ten units each) were placed in ger-boxes, between sheets of filter paper moistened with distilled water and kept in a germination chamber (BOD model NT708) at 25 °C ± 1, under continuous white fluorescent lighting (1000 lx, daylight). Subsequently, the seedlings were transplanted into black polyethylene bags with dimensions of 0.18 × 0.30 m and a capacity of 1.3 kg of the substrate, containing a mixture of soil (Red-Yellow Latosol with medium texture) and sand (1: 1 p/p), being kept in a greenhouse with shading nets (50% transmittance), and watered daily. Daily observations were made, considering two phases: seedling phase, from germination (determined by the protrusion of the primary root) to the expansion of the first photosynthetic leaf (eophyll), and the juvenile phase, from the seedling to the appearance of the first leaf with typical features of the species (metaphyll) (Oliveira 1999). At the same time, seedlings, juvenile, pre-reproductive (from the seedling to the first flowering) and adult (after the first flowering) specimens were observed at the field. Reference vouchers were deposited in the BOTU Herbarium, Department of Botany, Institute of Biosciences-São Paulo State University, UNESP.
The secretory tissues and cells in the seedlings, juvenile, and pre-reproductive plants were observed in samples from the epicotyl, other internodes of the primary stem, and leaves in the different developmental stages using stereoscopic microscopy (macroscopical analyses), scanning electron microscopy (SEM, surface examination), light microscopy (LM, anatomical and histochemical examination), and transmission electron microscopy (TEM, ultrastructural examination).
For anatomical analysis under LM, samples were fixed in formaldehyde, acetic acid, and 50% ethanol (FAA 50—Johansen 1940), dehydrated in a graded ethanol series, and embedded in methacrylate resin (Leica Microsystems Inc., Heidelberger, Germany). Cross and longitudinal sections (4–6 µm thick) were prepared with a rotary microtome (Leica RM2255) and stained with toluidine blue pH 4.7 (O’Brien et al. 1964). The sections were mounted on glass slides using a synthetic resin (Entellan New, Merck, Darmstadt, Germany). Observations and photographs were taken with a digital camera (Leica DC 300F) coupled with a light microscope (Leica DM5500B, Leica Microsystems, Wetzlar, Germany). Alternatively, hand-cut fresh sections were double-stained using astra blue and safranin (9:1 v/v; Bukatsch 1972); cell wall polysaccharides such as cellulose and pectins stain with astra blue, and safranin shows an affinity for lipids (Berlyn and Miksche 1976).
We registered the compounds present in the epidermal cells and in the superficial exudate using histochemical assays on sections of fresh materials obtained from the epicotyl and first internode above of juvenile plants (two months old). Sudan IV (Johansen 1940) detected total lipids and Nile blue (Cain 1947) for acidic (blue color) or neutral (pink color) lipids. Nadi’s reagent (a-naphthol and N, N-dimethyl-p-phenylenediamine) detected essential oils and oleoresin (David and Carde 1964). A 10% aqueous solution of ferric chloride highlighted phenolic compounds (Johansen 1940). A 0.02% aqueous solution of ruthenium red detected pectins (Jensen 1962). Lugol’s reagent highlighted starch grains (Johansen 1940). Standard control materials were prepared simultaneously. We examined and documented all specimens using a light microscope (Olympus BX41) equipped with a digital camera (Olympus C7070).
For the surface examination under SEM, samples were fixed in 2.5% glutaraldehyde in 0.1 mol L−1 phosphate buffer (pH 7.2), dehydrated in an ethanol series, and subjected to the critical drying point using liquid CO2. Samples were mounted on aluminum stubs, coated with gold (10 nm), and examined under a Quanta 200 scanning electron microscope (Fei Company, FEI, Gräfelfing, Germany) at 20 kV.
For ultrastructural characterization of the secretory cells under TEM, samples of epicotyl and first internode above were fixed in 2.5% glutaraldehyde in 0.1 mol L−1 phosphate buffer (pH 7.2) and left overnight at 4 °C. The material was then post-fixed in 1% osmium tetroxide (OsO4) solution in the same buffer for 2 h at room temperature, dehydrated in an acetone series, and embedded in epoxy resin (Araldite 502, Electron Microscopy Sciences, Hatfield, USA). Ultrathin sections were stained with uranyl acetate and lead citrate (Reynolds 1963). The sections were examined with a Tecnai Spirit TEM (FEI) at 80 kV.
Results
Location and temporal distribution of secretory activity
Schizolobium parahyba presented epigeal germination, and its cotyledons are leaf-like and photosynthetic (Fig. 1a). On the surface of the cotyledons and the hypocotyl, the secretory activity is absent. The surface of the epicotyl (Fig. 1a, b), subsequent internodes of the primary stem, pulvinus, and petiole (Fig. 1c) of the eophylls of S. parahyba seedlings was covered with a hyaline and sticky fluid, which can also be seen covering the epidermis of the young stem of juvenile and pre-reproductive plants (Figs. 1c, d, 2a–c). Soon after seed germination, the expanding epicotyl began to produce sticky secretion (Fig. 1a–c). The production of this secretion only occurred during organ expansion, both stem and leaves. Under SEM, the secretion was seen as a homogeneous material accumulation (asterisk) on the cuticle with a lattice aspect (Fig. 2d, e). In both the eophylls and metaphylls during pre-reproductive phase, secretory activity occurred in all the pulvinus and petiole, not having been observed in the leaflets blade. In the rachis just the abaxial surface presents secretory activity (Fig. 1d). The sticky exudate remained adhered to the surface of the immature portions of the organs, being gradually lost as they reached maturity, even in cases where the epidermis was persistent, as in the leaves. After the first flowering, sticky secretion was no longer observed. Therefore, in adult plants epidermis shows no secretory activity and a black indument (Fig. 1e) with simple non-glandular trichomes can be seen.
Sticky exudates on aerial organs of Schizolobium parahyba (Vell.) Blake. a Seedling showing leaf-like cotyledons elevated by the hypocotyl and expanded epicotyl with a pair of eophylls. The detail of the cotyledon node shows shine in the epicotyl, contrasting with the opacity of the hypocotyl, as the result of presence and absence of secretion, respectively. The same shiny appearance can be seen in the detail of the eophyll node, also conferred by the secretion. b–d Juvenile plants. b Hypocotyl-epicotyl zone after cotyledons abscission showing the shiny appearance of the secretion in the epicotyl, in contrast to the suberized hypocotyl. c Apical bud, in which petioles and stem are covered with sticky exudate. Note that the young and unexpanded leaf has a glabrous and shiny surface. d Detail of an metaphyll in apical view; the distal portion of the petiole presents the shine adhesive exudate, while this face is not secretory in the rachis. e Apical portion of an adult plant during flowering. Note, in the unexpanded portion, that the stem and leaves are covered with a dense indument formed by black non-glandular trichomes, also present in flower buds (arrows). The absence of secretion is easily noticed, especially when compared to figure (c). Symbols: co cotyledon, e1 first eophyll, ep epicotyl, hp hypocotyl, pe petiole, ra rachis
Adhesive exudates on the first internode above the epicotyl of Schizolobium parahyba (Vell.) Blake juvenile plants. a–c Abundant hyaline fluid. Note particles, arthropods, and remains of dead animals adhered on the superficial exudate. d, e Scanning electron microscopy showing secretion (*) on the cuticle. Note the lattice appearance of the cuticle
The presence of this secretion can trap particles, and immobilize small insects and mites, among other organisms which die attached to the plant (Fig. 2a–c) or manage to escape leaving parts of the body, such as wings and segments of the locomotor limbs (Fig. 2c). It was possible to observe visits by Brazilian stingless bees Tetragonista angustula Latreille (Apidae: Meliponina) in the field. These bees avoided the younger portions of the stem axis, in which the secretion is more abundant than the older, but they were observed collecting the secretion, especially in the rachis.
Structure, histochemistry, and secretion dynamics
In all sampled organs, secretory features of the epidermis can be recognized under the LM and TEM. Therefore, we chose to describe the epidermal tissue in the epicotyl samples and first internode above.
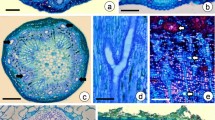
The epidermis is single-layered and constituted by juxtaposed, papilla-shaped columnar cells in a palisade-like arrangement (Figs. 3a–h, 4a). Scarce glandular trichomes consisting of a multicellular body with a cushion-like base were observed (Fig. 3a). The epidermal cells have dense content (Fig. 3a, b), thin walls, and a thickened cuticle (Fig. 3b). The cell content was stained purple with toluidine blue (Fig. 3a), pink with safranin (Fig. 3c), and reacted positively to the reagents to detect total and acid lipids (Fig. 3d, e), phenolic substances (Fig. 3g) and mucilage (Fig. 3h). The exudate deposited on the epicotyl surface reacted positively to the reagents to detect total and neutral lipids (Fig. 3d, e) and mixtures of essential oils and oleoresins (Fig. 3f). The results of histochemical tests carried out on the epidermis of the first internode above the epicotyl are summarized in Table 1.
Anatomical and histochemical characterization of the secretory epidermis in the young stem of Schizolobium parahyba (Vell.) Blake. a Epidermis formed by papilla to columnar cells with thin walls and dense content (cell walls stained in pink and protoplast stained in purple). Note sparse glandular trichomes. Coloration: toluidine blue. b Epidermal cells with dense content and lipid bodies, thin walls, and thickened cuticle. Note the heterogeneous aspect of the cuticle. c Epidermal cells double-stained with astra blue (cell wall polysaccharides stained in blue color) and basic safranin (protoplast and cutinized walls stained in vivid purplish red color). d Positive reaction to Sudan IV inside the epidermal cells and superficial exudate. e Positive reaction to Nile blue indicating acid lipids inside the epidermal cells and neutral lipids in the superficial exudate. f Positive reaction to Nadi’s reagent indicating a mixture of essential oils and oleoresins in the superficial exudates. g Phenolic substances in the epidermal cells were detected with ferric chloride. h Positive reaction to ruthenium red indicating mucilage inside the epidermal cells
TEM micrographs of the secretory epidermis in the epicotyl of Schizolobium parahyba (Vell.) Blake. a Columnar epidermal cells exhibit prominent nucleus, dense cytoplasm, and vacuoles of different sizes. b Numerous plasmodesmata in the transverse walls connect the epidermal and parenchyma cells. Note the irregularly contoured plasma membrane and periplasmic spaces adjacent to the transverse walls, a voluminous nucleus with heterochromatin lumps, and plastids with inner, poorly developed membranes. c Nucleus with evident nucleolus, plastids with oil inclusions, and periplasmic space along the anticlinal walls. Note vacuole fused with the plasma membrane. d Plasmodesmata in the anticlinal cell walls, peripheral RER, plastids with translucent globules, mitochondria with well-developed cristae, and small vacuoles with flocculant materials. e Golgi bodies with attached vesicles and polyribosomes in the dense and abundant cytoplasm. f Cortical microtubules oriented longitudinally to the plasma membrane. g Tubules and vesicles of SER in the cell periphery underlying the anticlinal walls. Observe dark material in the plasmodesmata. Symbols: cw cell wall, Gb Golgi body, mt microtubules, mi mitochondria, ps periplasmic space, nu nucleus, pl plastid, RER rough endoplasmic reticulum, SER smooth endoplasmic reticulum, va vacuole
Under TEM, epidermal cells of the epicotyl exhibited prominent nuclei, dense cytoplasm, and vacuoles of different sizes (Fig. 4a). The nucleus was spherical with heterochromatin lumps (Fig. 4a, b) and nucleolus (Fig. 4c). Numerous plasmodesmata (Fig. 4b) connect the epidermal and parenchyma cells. The plasma membrane had an irregular outline and, at some points, was detached from the cell wall forming small periplasmic spaces (Fig. 4b, c). Plastids with poorly developed inner membranes and filled with large translucent globules (Fig. 4b–d), extensive rough endoplasmic reticulum (RER) arranged in parallel rows along the anticlinal walls, mitochondria with prominent cristae (Fig. 4d), well-developed Golgi bodies with attached vesicles and polyribosomes (Fig. 4e) characterized the cytoplasm of the epidermal cells. Vesicles containing dense inclusion in close juxtaposition or fused with the plasma membrane were visible (Fig. 4f, g). At the same time, a network of cortical microtubules was seen longitudinally oriented along the anticlinal walls (Fig. 4f). Tubules and vesicles of smooth endoplasmic reticulum (SER) occurred in the cell periphery underlying the anticlinal walls, forming a network of reticular appearance (Fig. 4g). In these cells, plasmodesmata exhibited prominent pores filled with dark material.
At this time, in the peripheral cytoplasm facing the outer periclinal walls, there was an increase in the population of the Golgi bodies, SER tubules, and vesicles (Fig. 5a). Vacuoles containing membrane debris and osmiophilic deposits attached to the inner surface of the vacuolar membrane occurred in the peripheral cytoplasm (Fig. 5a). These vacuoles became enlarged and exhibited flocculant materials, membrane debris, and clusters of dense convoluted tubules/lamellae membranes covered with osmiophilic deposits (Fig. 5b). The convoluted membranes were attached to the inner surface of the vacuolar membrane (Fig. 5c) and seemed to have been originated from the intrusion and proliferation of the SER tubules into the vacuole.
TEM micrographs of the secretory epidermis in the young stem of Schizolobium parahyba (Vell.) Blake. a Golgi body and vesicles near the vacuoles featured by dark content and membrane debris. Note the loose appearance of the outer cell wall. b Enlarged vacuole containing flocculant materials, membrane debris, and clusters of convoluted tubules/lamellae membranes covered with osmiophilic deposits. c Detail of the previous figure showing the convoluted tubules/lamellae membranes. d Small vacuoles containing flocculant materials adjacent to the plasma membrane or vacuolar membrane and osmiophilic deposits in the cuticle. e Golgi bodies, SER tubules, and vesicles in the peripheral cytoplasm facing the outer periclinal wall. Observe plasma membrane to be irregular in outline and flocculant materials in the periplasmic space. f Large vesicles containing flocculant materials and lipid inclusions merged with the plasma membrane. Note osmiophilic deposits on the outer surface of the cell wall and concentric bands of electron-dense materials in the cuticular layer. Note the frayed appearance of the cuticle. g Outer epidermal cell wall showing a precise gradation from a cellulose layer followed by the cuticle exhibiting a developed cuticular layer. h Cuticle showing a clear distinction between the inner and outermost zones. Note a cellulose microfibrils framework encrusted with large deposits of fatty substances (cuticular layer) to an outermost region (proper cuticle). i Cellulose layer exhibiting a loose appearance and irregular thickness. j Cuticle showing signs of degradation. k Degraded cuticle showing holes interspersed with remnants of the cuticular layer, while the outermost layer (cuticle proper) is no longer observed. l General view showing the loose appearance of the cuticle. Symbols: cw cell wall, cl cuticular layer, ct cuticle proper, Gb Golgi body, ps periplasmic space, va vacuole
Vacuoles containing flocculant inclusions were seen near the vacuole or in the peripheral cytoplasm near the outer epidermal cell wall (Fig. 5d). Flocculant materials and lipid accumulation, globose and translucent in appearance, occurred between the plasma membrane and the outer periclinal cell walls (Fig. 5d, e). Images suggesting the fusion of SER elements and vesicles with the plasma membrane were regularly observed (Fig. 5f). Deposits of dense materials occurred on the outer surface of the wall and scattered in the cuticle, often layered, resulting in a sheath of concentric bands in the cuticle (Fig. 5f) that had a frayed appearance (Fig. 5a, d, f).
During the secretion, we identified changes in the outer epidermal cell wall linked with the exudate release. The outer epidermal wall exhibited a complex structure and showed a clear gradation from a cellulose layer (facing the protoplast) followed by an intermediate zone and the cuticle proper at the outermost region. The intermediate zone presented osmiophilic material that protrudes through a cellulose microfibrils framework encrusted with large deposits of fatty substances to an outermost region (Fig. 5g). The outermost region of the cuticle (cuticle proper) is featured by a loose appearance and was free of the dense ramifications, exhibiting dense deposits with a granular appearance (Fig. 5h). During the secretory process, the cellulose layer exhibited a loose appearance and irregular thickness (Fig. 5i), and the cuticle showed signs of degradation (Fig. 5j). Lastly, the cuticle exhibited holes interspersed with remnants of the cuticular layer, while the outermost layer (cuticle proper) was no longer observed (Fig. 5k). As the secretion progressed, the cuticle appeared loose and porous (Fig. 5l), consistent with the lattice aspect seen in SEM (Fig. 2d, e).
Discussion
Structure–function relationships of the adhesive secretion in developing aerial organs
The occurrence of the epidermis specialized in the production of sticky fluid in the young aerial organs is of particular significance and constitutes a relevant attribute of plants of S. parahyba. The epidermal tissue entirely formed by secretory cells explains the long-term release of large amounts of this fluid in the developing aerial organs. Although many plants (20–30% of all vascular plants) have glandular trichomes, which often produce adhesive exudates (Duke 1994), this study demonstrated that glandular trichomes are very sparse in S. parahyba, and the ordinary epidermal cells were the main site of sticky fluid production. A continuous layer of sticky secretion covering an extensive area of the plant body, as we observed in S. parahyba, seems to be effective in catching and immobilizing arthropods. Although apparently less common, this strategy appears to be as efficient as catching insects by glandular trichomes as reported in different plant species (Voigt and Gorb 2010; Krimmel and Pearse 2013; LoPresti et al. 2015, 2018; Voigt et al. 2020). It is also important to consider that trichomes can be very sparse and have weak peduncles in some plant species, making prey capture less efficient.
The presence of adhesive fluid on the surface of the entire epicotyl in seedlings of S. parahyba can play a dual role in establishing new plants of this species. First, these superficial impermeable exudates constitute an effective barrier protecting the plumule and young stem against environmental stresses (e.g., Voigt et al. 2020), essential for seedling establishment when the most mortality occurs. The young phase is critical in the plant growth cycle since they depend greatly on the prevailing environmental conditions and determine the plant survival in natural habitats (Hadas 2005). Therefore, the occurrence of sticky exudates in the epicotyl surface could be significant to the immobilization of small insects and mites, among other organisms, which died attached to the epicotyl surface. The role of sticky secretions in indirect plant defense by providing predatory insects with entrapped insects has been reported for different plant species (Krimmel and Pearse 2013; LoPresti et al. 2015, 2018), contributing to reducing herbivory and increasing plant fitness. In general, the viscous secretion has no repellent properties but has immobilizing and toxicant effects on trapped animals (Sutherst et al. 1982).
The adhesive properties of the fluid covering the surface of the aerial organs of S. parahyba may be associated with the presence of lipids and terpenes (mixtures of essential oils and oleoresins) in this exudate, besides mucilage and phenolic substances detected in the epidermal cells. Natural adhesives consist of mixtures of different chemicals, frequently including terpenes (Betz 2010) and polyphenolics (Rischka et al. 2010). These compounds are bioactive and physiologically relevant in those plants bearing adhesive exudates (Jiménez-Pomárico et al. 2019).
Cytological events linked to the secretory process
The ultrastructural organization of the secretory epidermal cells in S. parahyba is similar to that described in previous reports for cytological events associated with the synthesis, transport, and release of mixed secretions composed by hydrophilic (mucilage) and lipidic substances (lipids and terpenes mixtures of essential oils and oleoresins) (Sadala-Castilho et al. 2016; Tresmondi et al. 2017). The abundance in ribosomal components is suggested to be connected with increased metabolic activity associated with the production of enzymes involved in the synthesis of secretion components and the cell wall modifications (Hall et al. 1981). Accumulation of Golgi bodies and vesicles in the vicinity of the cell walls is evidence of the role of this organelle in the synthesis and delivery of polysaccharides (Fahn 1979, 2000). The abundance of RER and SER elements in the peripheral cytoplasm and indications of Golgi body vesicles, or of SER tubules in juxtaposition to, or merging with, the plasma membrane, is compatible with subcellular localization studies, which show that the core reactions of the lipids and terpenes synthetic pathway occur at the endoplasmic reticulum (Nawrath et al. 2013). The juxtaposition of ER and plasma membrane observed here is evidence of the direct transfer of lipids from the ER to the plasma membrane. Modified plastids are involved in the fatty acid synthesis, giving rise to acyl chains that can be exported and modified by the key lipid synthesis enzymes located in the RER (Nawrath et al. 2013). Golgi and trans-Golgi network-mediated vesicle trafficking are involved in exporting secreted substances (polysaccharides and lipids) to the apoplast by exocytosis (McFarlane et al. 2014). In our study, the increase in the plasma membrane area originating periplasmic spaces indicates of merocrine secretion via exocytosis. The relationship between increased plasma membrane area and this pathway of cellular secretion has been highlighted in different types of glands and has important physiological significance in the secretory process (e.g., Fahn 1979, 2000; Evert 2006).
During secretion, the abundance of microtubules in the periphery of the cytoplasm is noticeable. The involvement of microtubules in organizing the organelle positioning, trafficking of cargos (particularly between the endoplasmic reticulum and the Golgi apparatus), the traffic through the Golgi apparatus itself, and the transport via exocytosis to the cell surface has been highlighted in the recent literature (Fourriere et al. 2020). In addition, the abundance of mitochondria with well-developed cristae can likely be associated with secretions transported through the plasma membrane and into the cell wall. This process involves ATP-binding cassette transporters and, possibly also, lipid transfer proteins (Nawrath et al. 2013).
We found that plasmodesmata connections were not interrupted by sticky secretions. In addition, plasmodesmata usually exhibited a large central cavity filled with dark content, consistent with active involvement in the symplastic pathway of the secretions. Studies using fluorescence redistribution after photobleaching (FRAP) have provided evidence that the ER membranes of plasmodesmata can serve as dynamic diffusion pathways for the movement of lipids and lipid signaling molecules between bordering cells (Epel 1994).
In S. parahyba, the secretion accumulates in the cuticular layer of the outer epidermal cell walls and is released by peeling the cuticle, as reported in colleters of Rubiaceae (Machado et al. 2012). This mechanism of secretion is distinct of those reported for glandular trichomes producing sticky secretion (Werker 2000; Jiménez-Pomárico et al. 2019), in which the exudate accumulates within a subcuticular space in the secretory head and then is released by cuticle rupture (Gregory et al. 1986; Pichersky and Gershenzon 2002; Peiffer et al. 2009) or through the cuticular pores (Wagner 1991; Wagner et al. 2004; Paiva 2016; Jiménez-Pomárico et al. 2019). Thus, we believe that the mechanism of elimination of the secretion by the epidermal cells could explain the abundance of exudate over the entire surface of developing organs in S. parahyba.
The relationship between secretory epidermis histochemistry, subcellular structures, and the mechanisms of sticky secretion is considered and helps us better understand the probable functions of the secretions. Despite the great diversity of secretory structures in Leguminosae, in vegetative and reproductive organs, including glandular trichomes, secretory epidermal cells, nectaries, idioblasts, canals, and cavities (e.g., Solereder 1908; Metcalfe and Chalk 1950; Uphof 1962; Lackey 1978; Leelavathi and Ramayya 1983; Marquiafável et al. 2009; Rodrigues et al. 2011; Matos and Paiva 2012; Marinho et al. 2015; Vargas et al. 2015, 2018), detailed studies regarding the lipid secretion processes and histochemistry of the secretory epidermis in legumes are scarce. Lipid secretions (neutral lipids and mixtures of essential oils and oleoresins) are common to other secretory structures in legume species (Vargas et al. 2018) and have been considered an important adaptive trait linked with defense function (Wink 2003). The specialized metabolites detected on the epicotyl and young stem surface of S. parahyba can function as a defense against microbial attacks and herbivores and help prevent damage by UV radiation (Harborne 1993). The external lipid secretion affects the survival capacity in environments with high light intensity and high temperatures (Tresmondi et al. 2017), as in the case of seedlings of S. parahyba. Due to their hydrophilic properties, the polysaccharides inside the epidermal cells can act to maintain the high water potential and protect the organs against desiccation damage (Sawidis 1998). This species is epigeous-phanerocotyledonar (Oliveira 1999) and is thus able to explore the multiple microhabitats of the environment (forest gaps and edges in devastated ecosystems), mainly those related to light conditions.
Conclusions and perspectives
As far as we know, sticky external secretions have been widely reported in various non-carnivorous plant genera and species (LoPresti et al. 2015, 2018), among which less than 10% appear to be shrubs or trees. Our results showing animal remnants accumulated on the sticky surface of seedlings and young plants from S. parahyba are consistent with the growing evidence that the stickiness provides an important and widespread indirect defense against herbivory (Karban et al. 2019). Therefore, the hypothesis that such plants benefit from the carrion of trapped insects makes much sense. Some sticky plants can absorb nutrients from the feces of predators, which are attracted by carrion trapped in sticky secretion (Anderson 2005), or as common in carnivorous plants, they can absorb nutrients directly from trapped insects (LoPresti et al. 2015). Regarding large tree species as S. parahyba, the relevance of this kind of nutrient acquirement seems unlikely, but this remains to be tested. For protocarnivorous plants inhabiting eutrophic or mesotrophic soils, Adlassnig et al. (2010) doubt if prey-derived nutrients were significant in meeting the nutritional demand of these plants, as opposed to carnivorous plants, in which significant amounts of nitrogen come from prey. To allow the absorption of nutrients from the carcasses of glued animals, the epidermis of the plant must be permeable; such permeability is characteristic in carnivorous plants (see Adlassnig et al. 2010 and references therein). In the case of S. parahyba, we did not observe any evidence of permeability, making the acquisition of nutrients unlikely. On the contrary, the hydrophobic nature of the secretion forms an impermeable layer that covers the epidermis. It is interesting to emphasize that seedlings and pre-reproductive plants are notably vulnerable phases and can survive in the undergrowth of a tropical forest for several years and wait for favorable light conditions to start growing (Halle et al. 1978; Whitmore 1990).
We want to emphasize that S. parahyba is a native pioneer tree of the Atlantic rainforest with a strong preference for clearings (Lorenzi 1992). According to Freire et al. (2007), the expansion of its populations may have occurred with deforestation since the eighteenth century. Thus, this species is classified as a seasonal semi-deciduous forest invader adapted to rapid colonization of forest gaps and edges or disturbed environments (Magalhães Filho 2013). In this context, the role of adhesive exudates on the young aerial organs has evident physiological and ecological implications. Although we did not develop a bioassay, our results suggest that secretion production on the entire surface of the developing organs from the seedling stage contributes to its pioneer and invasive character favoring local adaptability to numerous environmental impacts, mainly the light conditions and behavior of animal populations (Halle et al. 1978; Whitmore 1990).
Our study adds information to the understanding of the diversity of adhesive plant secretions, shedding light on particular structure–function relationships. In addition, they are essential for understanding the biological and ecological characteristics of forest species. Data presented here may provide useful information for future commercial development of biomimetic adhesives.
Data availability
Not applicable.
Code availability
The analysis code can be requested by emailing the corresponding author (silvia.machado@unesp.br).
References
Adlassnig W, Lendl T, Peroutka M, Lang I (2010) Deadly glue-adhesive traps of carnivorous plants. In: von Byern J, Grunwald I (eds) Biological adhesive systems. Springer, Vienna
Anderson B (2005) Adaptations to foliar absorption of feces: a pathway in plant carnivory. Ann Bot 95:757–761
Berlyn GP, Miksche JP (1976) Botanical microtechnique and cytochemistry. Ames, Iowa
Betz O (2010) Adhesive exocrine glands in insects: morphology, ultrastructure, and adhesive secretion. In: Byern J, Grunwald I (eds) Biological adhesive systems—from nature to technical and medical application. Springer, New York, pp 111–152
Bukatsch F (1972) Bemerkungen zur Doppelfärbung: Astrablau-Safranin. Mikrokosmos 61:255
Cain AJ (1947) The use of Nile blue in the examination of lipoids. J Cell Sci 88:383–392
Cunha AR, Martins D (2009) Classificação climática para os municípios de Botucatu e São Manuel-SP. Irriga 14:1–11
David R, Carde JP (1964) Coloration différentielle des inclusions lipidiques et terpeniques des pseudophylles du pin maritime au moyen du reactif Nadi. C R Hebd Séances Acad Sci 258:1338–1340
De-Paula OC, Marzinek J, Oliveira DMT, Paiva EAS (2015) Roles of mucilage in Emilia fosbergii, a myxocarpic Asteraceae: efficient seed imbibition and diaspore adhesion. Am J Bot 102(9):1413–1421
Duke SO (1994) Glandular trichomes—a focal point of chemical and structural interactions. Int J Plant Sci 155:617–620
Epel BL (1994) Plasmodesmata: composition, structure and trafficking. Plant Mol Biol 26:1343–1356
Evert RF (2006) Esau’s plant anatomy. Wiley, New Jersey
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Fahn A (2000) Structure and function of secretory cells. Adv Bot Res 31:37–75. https://doi.org/10.1016/S0065-2296(00)31006-0
Falara V, Pichersky E (2012) Plant volatiles and other specialized metabolites: synthesis, storage, emission, and function. In: Vivanco JM, Baluska F (eds) Secretions and exudates in biological systems, signaling and communication in plants. Springer, Berlin, pp 109–123
Fan P, Leong BJ, Last RL (2019) Tip of the trichome: evolution of acylsugar metabolic diversity in Solanaceae. Curr Opin Plant Biol 49:8–16
Fourriere L, Jimenez AJ, Perez F, Boncompain G (2020) The role of microtubules in secretory protein transport; review. J Cell Sci 133:237016. https://doi.org/10.1242/jcs.237016
Freire JM, Piña-Rodrigues FCM, Lima ER, Sodré SRC, Corrêa RX (2007) Genetic structure of Schizolobium parahyba (Vell.) Blake (guapuruvu) populations by RAPD markers. Sci for 74:27–35
Frenzke L, Lederer A, Malanin M, Eichhorn KL, Neinhuis C, Voigt D (2016) Plant pressure sensitive adhesives: similar chemical properties in distantly related plant lineages. Planta 244:145–154. https://doi.org/10.1007/s00425-016-2496-4
Gregory P, Ave DA, Bouthyette PY, Tingey WM (1986) Insect-defensive chemistry of potato GT. In: Juniper BE, Southwood TRE (eds) Insects and the plant surface. E. Arnold, London, pp 173–183
Hadas A (2005) Encyclopedia of Soils in the Environment. The Volcani Center, Bet Dagan, pp 130–137
Hall LJ, Flowers TJ, Roberts RM (1981) Plant cell structure and metabolism, 2nd edn. Longman Group Limited, New York
Hallé F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests: an architectural analysis. Springer, Berlin
Harborne JB (1993) Introduction to ecological biochemistry, 4th edn. Academic Press, London
Jensen WA (1962) Botanical histochemistry: principles and practice. W.H. Freeman, San Francisco
Jiménez-Pomárico A, Avila-Núñez JL et al (2019) Chemical and morpho-functional aspects of the interaction between a Neotropical resin bug and a sticky plant. Rev Biol Trop 67:454–465. https://doi.org/10.15517/rbt.v67i3.33525
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Karban R, Lopresti E, Pepi A, Grof-Tisza P (2019) Induction of the sticky plant defense syndrome in wild tobacco. Ecology 100(8):e02746
Krimmel BA, Pearse IS (2013) Sticky plant traps insects to enhance indirect defence. Ecol Lett 16:219–224
Lackey JA (1978) Leaflet anatomy of Phaseoleae (Fabaceae, Papilionoideae) and its relation to taxonomy. Bot Gaz 139:346–446
Leelavathi PM, Ramayya N (1983) Structure, distribution and classification of plant trichomes in relation to taxonomy III. Papilionoideae. Indian J for 92:421–441
Levin DA (1976) The chemical defenses of plants to pathogens and herbivores. Annu Rev Ecol Syst 7:121–159
Liu Y, Jing SX, Luo SH, Li SH (2019) Non-volatile natural products in plant glandular trichomes: chemistry, biological activities and biosynthesis. Nat Prod Rep 36(4):626–665
LoPresti EF (2016) Chemicals on plant surfaces as a heretofore unrecognized, but ecologically informative, class for investigations into plant defence. Biol Rev 91(4):1102–1117. https://doi.org/10.1111/brv.12212
LoPresti EF, Karban R (2016) Chewing sandpaper: grit, plant apparency, and plant defense in sand-entrapping plants. Ecology 97:826–833. https://doi.org/10.1890/15-1696.1
LoPresti EF, Pearse IS, Charles GK (2015) The siren song of a sticky plant: Columbines provision mutualist arthropods by attracting and killing passerby insects. Ecology 96:2862–2869
LoPresti EF, Krimmel B, Pearse IS (2018) Entrapped carrion increases indirect plant resistance and intra-guild predation on a sticky tarweed. Oikos 127(7):1033–1044
Lorenzi H (1992) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Plantarum, Nova Odessa
Machado SR, Barreiro DP, Rocha JF, Rodrigues TM (2012) Dendroid colleters on vegetative and reproductive apices in Alibertia sessilis (Rubiaceae) differ in ultrastructure and secretion. Flora 207:868–877. https://doi.org/10.1016/j.flora.2012.09.013
Magalhães Filho G (2013) Caracterização dos padrões genéticos de populações invasoras e naturalizadas de Schizolobium parahyba (Caesalpinioideae–Fabaceae) por restriction-site associated DNA-sequencing. Dissertation, Universidade Estadual Paulista Júlio de Mesquita Filho
Marinho CR, Oliveira RB, Teixeira SP (2015) The uncommon cavitated secretory trichomes in Bauhinia s.s. (Fabaceae): the same roles in different organs. Bot J Linn Soc 180:104–122
Marquiafável FS, Ferreira MDS, Teixeira SP (2009) Novel reports of glands in Neotropical species of Indigofera L. (Leguminosae, Papilionoideae). Flora 200:189–197
Matos EC, Paiva EAS (2012) Structure, function and secretory products of the peltate glands of Centrolobium tomentosum (Fabaceae, Faboideae). Aust J Bot 60:301–309
McFarlane HE, Watanabe Y, Yang W, Huang Y, Ohlrogge J, Samuels AL (2014) Golgi- and trans-Golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol 164:1250–1260
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons: leaves, stem and wood in relation to taxonomy with notes on economic uses, vol I. Clarendon Press, Oxford
Moghe GD, Leong BJ, Hurney S, Jones AD (2017) Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway. Elife 6:e28468
Nawrath C, Schreiber L, Franke RB, Geldner N, Pinto JJR, Kunst L (2013) Apoplastic diffusion barriers in Arabidopsis. The Arabidopsis Book. https://doi.org/10.1199/tab.0167
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oliveira DMT (1999) Morfologia de plântulas e plantas jovens de 30 espécies arbóreas de Leguminosae. Acta Bot Bras 13(3):263–269
Paiva EAS (2016) How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann Bot 117:533–540
Peiffer M, Tooker JF, Luthe DS, Felton GW (2009) Plants on early alert: GT as sensors for insect herbivores. New Phytol 184:644–656
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Pinto LP, Brito CW (2003) Dynamics of biodiversity loss in the Brazilian Atlantic Forest: an introduction. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America; biodiversity status, threats and outlook. Island Press, London, pp 405–434
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Rischka K, Richter K, Hartwig A, Kozielec M, Slenzka K, Sader R, Grunwald I (2010) Bio-inspired polyphenolic adhesives for medical and technical applications. In: von Byern J, Grunwald I (eds) Biological adhesive systems from nature to technical and medical application. Springer, Vienna, pp 201–211
Rodrigues TM, Teixeira SP, Machado SR (2011) The oleoresin secretory system in seedlings and adult plants of copaíba (Copaifera langsdorffii Desf., Leguminosae–Caesalpinioideae). Flora 206:585–594
Sadala-Castilho R, Sá-Haiad B, Machado SR, Lima HA (2016) Oil-resin glands in Velloziaceae flowers: structure, ontogenesis and secretion. Plant Syst Evol 302(5):585–599
Sawidis TH (1998) The subglandular tissue of Hibiscus rosa-sinensis nectaries. Flora 193:327–335
Simoneit BRT, Medeiros PM, Wollenweber E (2008) Triterpenoids as major components of the insect-trapping glue of Roridula species. Z Für Nat 63:625–630
Solereder H (1908) Systematic anatomy of the dicotyledons. A handbook for laboratories of pure and applied botany, vol 2. Clarendon Press, Oxford
Sutherst RW, Jones RJ, Schnitzerling HJ (1982) Tropical legumes of the genus Stylosanthes immobilize and kill cattle ticks. Nature 295:320–321
Tian D, Tooker J, Peiffer M, Chung SH, Felton GW (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236:1053–1066
Tresmondi F, Canaveze Y, Guimarães E, Machado SR (2017) Colleters in Rubiaceae from forest and savanna: the link between secretion and environment. Sci Nat 104:17. https://doi.org/10.1007/s00114-017-1444-x
Uphof JCT (1962) Plant hairs. Encyclopedia of plant anatomy band IV/5. Gebr. Borntraeger, Berlin
Vargas W, Sartori ALB, Dias ES (2015) Novelties in secretory structures and anatomy of Rhynchosia (Fabaceae). Anais Acad Bras Cienc 87:83–87
Vargas W, Machado SR, Lewis GP, Candido ES, Vatamparast M, Fortuna-Perez AP (2018) Revisiting the leaflet secretory structures in subtribe Cajaninae Benth. (Leguminosae, Phaseoleae). Int J Plant Sci 179(9):697. https://doi.org/10.1086/699288
Voigt D, Gorb S (2008) An insect trap as habitat: cohesion-failure mechanism prevents adhesion of Pameridea roridulae bugs to the sticky surface of the plant Roridula gorgonias. J Exp Biol 211:2647–2657. https://doi.org/10.1242/jeb.019273
Voigt D, Gorb S (2010) Locomotion in a sticky terrain. Arthropod-Plant Interact 4:69–79
Voigt D, Kim J, Jantschke A, Varenberg M (2020) Robust, universal, and persistent bud secretion adhesion in horse-chestnut trees. Sci Rep 10:16925. https://doi.org/10.1038/s41598-020-74029-5
Wagner GJ (1991) Secreting GT: more than just hairs. Plant Physiol 96:675–679
Wagner G, Wang E, Shepherd R (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot 93:3–11
Werker E (2000) Trichome diversity and development. Advances Bot Res 31:1–35
Wheeler AG, Krimmel BA (2015) Mirid (Hemiptera: Heteroptera) specialists of sticky plants: adaptations, interactions, and ecological implications. Ann Rev Entomol 60:393–414
Whitmore TC (1990) An introduction to Tropical Rain Forests. Clarendon Press, Oxford
Wilder JA (2019) A true “migrant trap”: Boerhavia (Nyctaginaceae) entanglement as a recurring cause of avian entrapment and mortality. Wilson J Ornithol 131:658–663
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Yang X, Dong M, Huang Z (2010) Role of mucilage in the germination of Artemisia sphaerocephala (Asteraceae) achenes exposed to osmotic stress and salinity. Plant Physiol Biochem 48:131–135. https://doi.org/10.1016/j.plaphy.2009.12.006
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/ Edital Universal Proc. 401053/2016-4) and also by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES), Finance Code 001. EASP, DMTO, and SRM received research grants from CNPq (Proc. 305638/2018-1, 305686/2018-6, and 308982/2020-7, respectively).
Author information
Authors and Affiliations
Contributions
SRM, DMTO, and EASP conceived and designed the research. SRM, EASP and YC carried out the work. All the authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor Dagmar Voigt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paiva, E.A.S., Oliveira, D.M.T., Canaveze, Y. et al. Adhesive secretion in Schizolobium parahyba (Vell.) Blake (Leguminosae: Caesalpinioideae): histochemical and morpho-functional characterization of this unusual feature in woody plants. Arthropod-Plant Interactions 16, 249–261 (2022). https://doi.org/10.1007/s11829-022-09888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-022-09888-y