Abstract
Plants have evolved a variety of defences to reduce losses to herbivores and pathogens. The benefits of these may, however, be modified by resistance evolution in antagonists, changes in antagonist fauna, context-dependent “costs of defence”, and by interactions between antagonists. In Barbarea vulgaris (Brassicaceae), the so-called “G-type” produces triterpenoid saponins that deter important specialist insect herbivores, whereas the “P-type” produces other saponins and are not insect-resistant. In contrast, P-type plants are predominantly resistant to the biotroph pathogen Albugo sp., causing white blister rust, whilst most G-type plants are susceptible. In a field experiment with F3 hybrids between G and P-plants, we tested whether the two resistances are functionally coupled, leads to less disease and herbivory and to better plant performance, and whether insect herbivores and the pathogen interact in their effects on plant performance. The Albugo and insect resistances varied continuously between the F3 plants and mapped to different linkage groups, indicating independent mechanisms and evolution. Plants with high Albugo resistance produced more biomass and survived better than more susceptible plants. Albugo DNA was detected in surface-sterilized green siliques, indicating systemic and sometimes non-symptomatic infection. Plants with high insect resistance were slightly less damaged by herbivores, but did not grow or survive better than more susceptible plants. Interactions between Albugo and insect herbivores did not affect plant performance. In contrast to the Albugo resistance, which clearly benefited the plants, our results show that the saponin-based insect resistance did convey any benefit under the given conditions despite its deterrent effects in controlled experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved a multitude of defences against herbivores and pathogens, based on especially toxic and repellent metabolites and physical structures (Chisholm et al. 2006; Chen 2008; War et al. 2012). Whilst each specific defence type or variety at least initially reduce tissue and resource losses to the plants, antagonists continually evolve resistance and tolerance to these, which may render these defences less beneficial or even useless over time. This may vary between geographical regions within the plant species range, depending on the proportion of the local antagonist fauna that is affected by the specific defence (Thompson 2005). Different antagonists, e.g., insect herbivores and phytopathogens, may in addition interact directly or indirectly with each other and thereby modify the combined fitness impact on the host plant (Thaler et al. 2002, 2012; Koornneef and Pieterse 2008). Thus, the evolutionary benefit of a specific defence type is inherently context-dependent, and expected to vary over time and space.
In contrast, the physiological costs associated with a specific defence type may arguably be less context-dependent, as the production of a defence metabolite and the enzymes and other factors involved in its biosynthesis has intrinsic costs that may be more constant (Gershenzon 1994; Züst and Agrawal 2017). At the organismal level, the overall fitness cost of a defence reaction obviously also depends on the induction level and present resource availability for defence and compensatory reactions during and after attack. A predictable outcome of the coevolution between plants and antagonists is therefore that many specific defences at some point may become too physiologically and ecologically costly compared to their organismal benefits and become replaced or lost over time.
A few species in the Barbarea genus are known to have evolved the ability to produce triterpenoid saponins as the only ones in the large and economically important Cabbage family (Brassicaceae) (Agerbirk et al. 2003a; Nielsen et al. 2010; Badenes-Perez et al. 2014b; Badenes-Pérez et al. 2017). Amongst the saponins produced, especially cellobiosides of hederagenin and oleanolic acid affect several important specialist crucifer herbivores like the yellow-striped flea beetle Phyllotreta nemorum (Phyllotreta for short in the following) and diamondback moth (Plutella xylostella) (Shinoda et al. 2002; Kuzina et al. 2009; Nielsen et al. 2010; Augustin et al. 2012; Badenes-Perez et al. 2014b, 2014a), and seem to affect many other herbivores as well (Badenes-Pérez and López-Pérez 2018; Christensen et al. 2019). Saponins can disrupt biological membranes and otherwise deter and intoxicate a diverse range of organisms (Augustin et al. 2011).
One of the saponin-producing Barbarea species, B. vulgaris s.l., has diverged into two different “plant types” (Agerbirk et al. 2003b; Toneatto et al. 2010, 2012; Hauser et al. 2012; Christensen et al. 2014), of which the so-called “P-type” seems to have lost the oleane-type saponin-based resistance secondarily (Agerbirk et al. 2003b; Toneatto et al. 2012; Lange et al. 2018). This was caused by a shift to produce lupane-type saponins instead, with no known biological function (Augustin et al. 2012; Khakimov et al. 2015; Liu et al. 2019). The B. vulgaris G and P-types also produce different glucosinolates and flavonoids (Agerbirk et al. 2001, 2003a; Dalby-Brown et al. 2011), they differ in hairiness of rosette leaves (G-type: Glabrous rosette leaves; P-type: Pubescent leaves) (Agerbirk et al. 2003b), and have diverged genetically and reproductively (Toneatto et al. 2010, 2012; Christensen et al. 2014, 2016).
Whereas the G-plants are resistant to the insect herbivores, they are readily infected by the oomycete pathogen Albugo candida sp. causing white blister rust (Albugo for short in the following). In contrast, P-plants, which are susceptible to the herbivores, are predominantly resistant to Albugo (van Mölken et al. 2014a, 2014b; Christensen et al. 2014; Heimes et al. 2015). A. candida is a species complex of biotroph oomycetes affecting a wide range of crucifer species (Choi et al. 2011), including Barbarea species. It can grow systemically and asymptomatically within plant tissue and transmit vertically by seeds (Jacobson et al. 1998; Ploch and Thines 2011); white blister rust can severely reduce crucifer crop yields, especially in warmer regions than Denmark.
The association of the insect and pathogen resistances with either of the two closely related plant types raises a number of functional and evolutionary questions. One explanation could be that the two resistances are mechanistically coupled, e.g., determined by the same gene(s) but with opposing allelic effects. For example, if Albugo was negatively affected by lupane-type but not oleane-type saponins, a gene determining the switch between these saponins could have such an effect. In that case the two resistances should be co-localized in the genome to the same QTL.
Alternatively, the insect and pathogen resistances may be determined by different genes and mechanisms but associated with either of the two plant types due to different evolutionary histories and resulting linkage disequilibrium (non-random association of alleles at different loci). Progenitor populations of the present G and P-types may have been isolated from each other and exposed to different communities of herbivores and pathogens, which could have selected for the alternate resistance-states. Later this could have been maintained in regions of secondary sympatry due to the substantial crossing barrier separating them (Toneatto et al. 2010; Christensen et al. 2016). In accordance with this, we previously suggested that the G and P-type have been geographically isolated for long periods in the past based on their substantial population genetic divergence and different overall Eurasian distributions (Hauser et al. 2012; Christensen et al. 2014).
The present coexistence of the G and P-types in Scandinavia and Finland raises the important question, why the saponin-based insect resistance and the Albugo resistance have not spread across all B. vulgaris populations if these resistances indeed reduce losses to important insect herbivores and Albugo-caused white rust. Strongly selected genes are known to be able to cross even substantial hybrid barriers over time (Barton and Hewitt 1985), or one of the two plant types could suppress and replace the other if better fit due to its associated resistance.
Alternatively, the insect and pathogen resistances may not really benefit the plants in this part of their current range, or the resistance benefits may be outweighed by physiological and ecological “costs of defence” (Koricheva 2002; Strauss et al. 2002). Further, interactions between local herbivores, diseases and the plant may modify the fitness benefits and costs of the two resistances, e.g., if a pathogen attack makes the plants more or less vulnerable to simultaneous insect attack (Pieterse et al. 2012; Hauser et al. 2013; Pangesti et al. 2013). Thus, the insect and Albugo resistances may have been beneficial when and where they evolved but not in this part of their present distribution range.
To evaluate the fitness effects of the Albugo and insect resistances, we needed to study them independent of their genetic background, i.e., break the historical linkage disequilibrium between the resistance genes and other genes associated with either the G or the P-type. Thus, we hybridized G and P-plants in three generations to create F3 hybrids, which were cloned when small and tested for resistances to Phyllotreta and Albugo. The plants were then transplanted to a field experiment, evaluated for white rust disease, insect attack, survival, and biomass production for two years. From this we could correlate and map the Albugo and insect resistances to linkage groups, analyse their effects on losses from white rust and different classes of herbivores and on biomass and survival, in addition to impacts from interactions between Albugo and the different functional classes of herbivores on plant performance. We additionally analysed whether Albugo occurred asymptomatically within plants and spread systemically to developing siliques.
Materials and methods
Crossing design
F3 plants for the field experiment originated from crosses between G and P-plants from three Danish G-populations and three P-populations (G: Herlev, Kvaerkeby, Try-G; P: Tissø, Trundholm, Try-P). The first hybrid generation (F1) was produced by controlled hand pollinations in a half sib design: pollen from six G-plants and six P-plants, two from each population, was transferred to stigmas of 30 maternal plants of the other plant type; each donor pollinated five maternal plants, each maternal plant received pollen from one donor. Only 16 of the maternal plants (9 G, 7 P) produced F1 offspring, the parentage of which included all paternal donors and parental populations, however. At flowering, 36 F1 plants representing this diversity were randomly inter-crossed by bumble bees (Bombus terrestris; Koppert Biological Systems) in an outdoor net tent to produce F2 seeds. From these, 20 F2 parental plants were inter-crossed to produce F3 seeds, this time using blow flies (Lucilia sericata, Koppert Biological Systems) as they do not damage flowers as much. In each generation, seeds were harvested separately for each maternal plant to keep maternal family structure and representation even. Some maternal families were lost during the process, and the final F3 plants for the experiment originated from 8 of the original 30 P0 maternal plants (5G, 3 P), but representing all the six parental populations.
Initial tests of Albugo and Phyllotreta resistances
Ten plants from each of the 20 F3 families were grown in a greenhouse at 16 h daylight, supplemented with metal halide lamps (Philips HPI-T plus 400 W). Each plant (genet) was cloned by leaf cuttings and treated with rooting hormone (CLONEX, Growth Technology, Taunton, UK) to produce ramets for testing Albugo and P. nemorum resistances. At the 5-leaf stage, one leaf was cut from each ramet for P. nemorum assays, whilst the rest of the plant were used for Albugo assays.
Albugo resistance was tested by a method by Dangl et al. (1992) and revised by van Mölken et al. (2014a, b). Briefly, the ramets were placed inside plastic bags at 15 °C for 24 h. Next day, Albugo sporangia were collected from a stock of infected G-plants, added to a slurry of 15 °C deionised water and fresh ground G-type leaves, and vortexed until the suspension was green and foamy. Sporangia were allowed to hydrate approx. 30 min. at 15 °C, and their concentration adjusted to ~ 1.5 × 105/ml. 40 µl of this inoculum was added to each plant, 10 µl drops on each of the four leaves. Plants were again placed in the bags at 15 °C, which were opened seven days after inoculation (dai); 10 dai plants were returned to the greenhouse. At 24 dai, the leaf area covered by pustules were scored visually on all leaves using a categorical scale from 0 to 4: < 11%, 11–25%, 26–50%, 51–75%, 76–100%. A disease index was calculated by multiplying the median of the disease classes (5.5, 18, 38, 63 and 88) by the number of leaves in that category for each plant, summing these for each ramet and dividing by total number of leaves, and averaging for each genet across ramets. The number of ramets tested for Albugo varied from 1 to 4 for each clone. These disease indices were used as a measure of the F3 plants susceptibility in the following analyses.
Resistance to Phyllotreta nemorum larvae (Phyllotreta in the following) was tested using a well-established bioassay (Nielsen 1997a, b). In brief, five larvae were placed on a leaf disc and the number of surviving larvae determined; 4–5 leaves were tested per each genet. The average number of surviving larvae is used in the following analyses as a measurement of F3 plant susceptibility to keep the same polarity as the disease and herbivory measurements from the field.
To ensure that the ramets cloned by leaf cuttings behaved consistently, 6–10 ramets from seven genets were tested for Albugo and Phyllotreta resistance. Cloned offspring behaved sufficiently consistent that this method could be used for the experiment (results not shown).
Mapping resistances to linkage groups
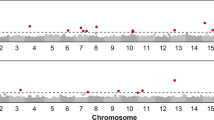
To test whether the insect and pathogen resistance/susceptibility were functionally and genetically linked, the Albugo disease and Phyllotreta survival indices were mapped to genetic linkage groups by association mapping, using twenty-three SSR markers representing preferentially both ends of all the linkage groups known at that time (Kuzina et al. 2011). Leaves from one clone of each of the F3 plants (genets) were freeze dried, DNA extracted, and fragments amplified, separated and analysed as described previously (Kuzina et al. 2011).
Field experiment
Approximately 3 months after inoculation with Albugo, the plantlets were vernalised for at least two months in an outdoor bench, and transplanted to an experimental plot at the university farm of University of Copenhagen in the end of May 2012. The plot was placed in a field that had previously been grazed by horses; in the following years, the plot was surrounded by cereal and oil seed rape crops. Plants were placed 33 cm apart in double rows of 15 m length; double rows were separated by 2 m. Ramets from the 171 F3 genets were planted randomly into three continuous blocks along these double rows, 401 ramets in total. Plants were transferred from their pots into circular holes dug in the ground. Due to dry conditions at planting time, all plants were irrigated initially but not at any other time. During the two growing seasons, the grassy ruderal vegetation surrounding individual plants was cut occasionally by sickle when at the height of the experimental plants, and passages between double rows mowed by a hay cutter. Plants were not treated with any other external input.
During the two growing seasons (2012 and 2013), we evaluated the following disease and herbivory symptoms several times for each plant: (1) White rust, (2) Leaf mines typical for P. nemorum larvae, the weevil Ceutorhynchus minutus and others, (3) Gnaw marks typical for snails and slugs, (4) Bite holes typical for adult P. nemorum and other flea beetles, and (5) Aphid attack, using an ordinal categorical scale: (0) No signs of damage; (1) Single very few occurrences on a single part of the plant; (2) Few occurrences spread across several parts of the plant; (3) Many occurrences and substantial damage; (4) Severe damage affecting all parts of the plants. A qualitative assessment of plants “weakness” was scored at the same time using the same scale, where 0 designated completely healthy-looking plants and 4 plants in a seriously bad constitution.
After the first summer, when inflorescences were dominated by mature but unopened siliques, inflorescences were cut at the lowest pedicel/peduncle on the flowering stems, dried and weighed. This way of harvesting was chosen to allow some above-ground resources for over-wintering. In the second year, all above-ground parts were harvested and weighed. At this stage, plants have very few and withering leaves and most of the above-ground biomass therefore consists of stems and mature siliques. Survival of individual plants was noted both years.
Albugo detection in siliques
To test whether Albugo spread systemically to developing siliques, green siliques were harvested from all plants (ramets) in the first summer and tested with oomycete-specific primers, as described in Ploch et al. (2011).
Statistical analysis
Resistance correlations and association mapping
The correlation between Phyllotreta survival and disease index of the F3 seedlings before transplant to the field was estimated with Spearman’s rho in JMP, Version 10.0.0, SAS Institute Inc., Cary, NC.
For the genetic mapping of the resistances with SSR markers, we used association mapping with a mixed linear model, implemented in Tassel 2.1 (Bradbury et al. 2007). For the relatedness of the F3 plants (inferred ancestry of individuals: Q matrix) we used two estimates of the number of subpopulations: (1) Equal to the number of maternal F2 families (k = 20) and (2) Determined by the software Structure (Pritchard et al. 2000), using the change in log-probability of the data for increasing number of subpopulations (k = 1–20; Evanno et al. (2005)). Structure parameters were set to allow admixture and did not include prior information about population membership; models were run with 10,000 burn-ins and 100,000 Markov chain Monte Carlo replications.
Herbivore damage, disease and biomass in field experiment
Individual scorings of white rust, herbivore damage, weakness, survival and biomass in the field were averaged across ramets for each genet, as were the initial scorings of Albugo and Phyllotreta susceptibility. Effects of (1) Albugo and Phyllotreta susceptibility (as determined at the seedling stage) on disease and herbivore damage, and (2) Effects of disease and herbivore damage on plant biomass and survival were analysed by univariate analyses of covariance and by structural equation modelling (Grace 2006).
In the analyses of covariance, the disease and herbivory damage was analysed with a model including (1) Initial disease indices and Phyllotreta survival as continuous explanatory variables and (2) F2 family of the genet as a categorical random variable. These models, including all interactions, were (1) Manually reduced based on Chi-square tests of likelihood differences (Crawley 2007), omitting in sequence three-way and two-way interactions, and variables with no significant effects on the model likelihood, and by (2) Automated stepwise regression based on Akaike statistics (R: step function). Data were Box-Cox transformed to improve normality and variance uniformity (R: MASS library). Scorings of disease, herbivore damage and plant weakness were in these analyses averaged across scoring times, after inspection of the single time measurements. All analyses were done in R version 2.15.0 (R Development Core Team 2020).
To test more complex interactions between the two resistances, between white rust and the different classes of herbivory, in addition to direct effects of the resistances on plant fitness not accounted for by disease and herbivore damage (e.g., costs of resistance), we used Structural equation modelling (SEM), as implemented in the “sem” R package. Here, we included latent variables for disease and the different classes of herbivory (mining, gnawing, holing, aphids; see Fig. 1), estimated from the observations at different census dates.
Initial model (Model 0 in Online Resource 2) used for structural equation analyses of complex relationships between resistances, symptoms in the field, and plant biomass. The two left boxes indicate susceptibility to Albugo sp. and Phyllotreta nemorum, as tested before plants were transplanted to the field, small boxes and connected circles indicate the field scorings of disease and herbivore damage at different time points (t1–t3) and the associated latent variable for each damage class, and the right box indicates total biomass produced in the first and second year. Black arrows indicate assumed simple relationships between the resistances and potential damage based on existing knowledge (identified by lower case letters), red arrows additional complex relationships tested (upper case letters). For details, see Methods and Online Resource 2
Our approach was to start from the most simple but biologically meaningful model (M0) including all direct effects of (a) Albugo susceptibility on disease in the field, (b–e) Phyllotreta susceptibility on the different herbivory classes (mining, gnawing, holing, aphids), and (f–j) Disease and herbivore damage on plant biomass (Fig. 1: black unbroken lines). After fitting model M0 to the data, we initially tested whether inclusion of covariances between the different classes of herbivory improved the basic model, which it did. Effects of Albugo susceptibility on white rust in the field, and of Phyllotreta susceptibility on the different classes of herbivory were tested by sequential exclusion of each regression from the model; insignificant regressions were omitted from the working model (Online Resource 2). After this, effects of disease and herbivory on total plant biomass (sum of biomass for the two harvests; f–j in Fig. 1) were tested likewise. We then tested whether more complex interactions between variables improved model fit (hatched lines in Fig. 1): effects of (A) Phyllotreta susceptibility on disease severity in the field, (B) Albugo susceptibility on the different classes of herbivory, (G–(C–F)) disease on herbivory and vice versa ((C–F)–G), and (H and I) direct effects of the two resistances/susceptibilities on plant weight independent on their effects via disease and herbivory. Only one of these connections (A) improved and was included in the working model; whether this affected previous exclusion of variables was subsequently checked. Inclusion/exclusion of regression/covariance-variables was guided by differences in Akaike, Bayesian and log-Likelihood between the reduced/expanded model and the present working model.
Results
Correlations and genetic mapping of Albugo and Phyllotreta resistances
Albugo symptoms and Phyllotreta survival were not correlated in the initial tests on plantlets before transplant to the field (rho = − 0.01, P = 0.91); this was the case both for descendants from original G or P-type maternal P0 plants (Fig. 2).
Correlation between Albugo and Phyllotreta susceptibility of young plantlets before transplantation to the field, shown for descendants of original G and P maternal plants. Susceptibility to white rust was determined by leaf area covered by pustules, and susceptibility to Phyllotreta by the number of Phyllotreta larvae surviving out of five in bioassays
Initial analyses estimated the most likely number of subpopulations amongst the samples (k) to be three. Using this value and also k = 20 (number of maternal F2 families), significant associations were found between the Albugo susceptibility and markers Bv128 on linkage group 3 of Khakimov et al. (2015), Bv161 on linkage group 16 (for k = 3), and Bv65 on linkage group 1 (k = 20). Phyllotreta susceptibility did not associate with any of those linkage groups, but with one marker on linkage group 5 (Bv60) (Online Resource 1).
Effects of the Albugo resistance
Plants from genets with high susceptibility (low resistance) to Albugo, as determined before transplant, developed much more white blister rust in the field than the less susceptible (more resistant) plants (averaged across first and second year scorings); this was found by both univariate and SEM modelling (Table 1; Online Resource 2; Figs. 3, 4). Disease in the field was also affected by flea beetle susceptibility of the genets; however, this interaction was complex, with more disease on plants that were intermediately susceptible to Phyllotreta than on weakly or strongly susceptible plants (Figs. 3, 4). This effect explained about 2% of the variation in the univariate analyses. Genets with high susceptibility to Albugo also were less vigorous in the field (subjective scoring of plant vigour in the field; Table 1; Fig. 3).
Effect of Albugo susceptibility, as tested on plantlets before transplant, on severity of white rust, plant weakness (subjective scoring of plant health), and plant survival in the field experiment (averaged across multiple scorings). Simple regression lines are shown for illustration; for disease, for the lowest, middle and highest third of Phyllotreta susceptibility
Final model from structural equation analyses of complex relationships between resistances, symptoms in the field, and plant biomass combined over two years. The two left boxes indicate susceptibility to Albugo sp. and Phyllotreta nemorum, as tested before plants were transplanted to the field, small boxes and connected circles indicate field scorings of disease and herbivore damage at different time points (t1–t3) and the associated latent variable for each damage class, and the right box indicates total biomass produced in the first and second year. Black unidirectional arrows indicate significant regressions, with unstandardized and standardized regression coefficients; bold values indicate coefficients that are also significant by Wald’s test (Z). Bi-directional arrows indicate significant covariance’s, with associated unstandardized and standardized values. Proportion of variance explained by the model (R2) are given for each estimated variable (in circle and square). Detailed analysis results in Online Resource 2
Genets with high susceptibility to Albugo produced less biomass in year 2 and in total (year 1 + year 2; Table 1; Online Resource 2; Fig. 5); likewise, genets that were strongly affected by white rust disease in the field developed less total biomass than less affected genets (years 1 + 2; Online Resource 2; Figs. 4, 6). The Albugo resistance/susceptibility explained appr. 28% of the variation in total biomass (product of a–f relative coefficients, Fig. 4). Albugo susceptibility did not have any direct effects on biomass apart from effects through disease in the field (path H; Online Resource 2; Figs. 1, 4).
Effect of white blister rust (white rust) and different classes of herbivory, on total plant above-ground biomass (g dry weight) for the two years combined. Leaf mines, gnawing, and bite holes may be symptoms of feeding by P. nemorum larvae, snails and slugs, and P. nemorum adults, respectively. Significant regressions are indicated
Genets with high susceptibility to Albugo survived less frequently to the end of the experiment than plant with low susceptibility (more resistant) (Table 1; Fig. 3); the 25% most susceptible plants had 8% lower survival than the 25% least susceptible.
Albugo susceptibility had little or no effects on herbivore damage. Using automatic model reduction in the univariate analyses, herbivore gnawing was significantly affected by both Albugo susceptibility and an interaction between the two resistances (Table1); however, this was not the case in the user-guided reduction or from the SEM modelling (Online Resource 2: 4.BD).
Albugo DNA was found inside green siliques of 45 plants from 35 genets in the field; 10 of these genets did not show any symptoms of white rust at the initial tests before transplant to the field, eight genets did not show any symptoms during the field experiment.
Effects of the Phyllotreta resistance
Plants from genets with high susceptibility (low resistance) to Phyllotreta in initial bioassays were more affected by leaf mines and gnawing than plants with low susceptibility; in contrast, they were less affected by aphids (averaged across first and second year scorings; Table 1; Online Resource 2; Figs. 4 and 7). In the univariate analyses, plants with high Phyllotreta susceptibility were found to be more affected by biting holes (Table 1); this was, however, not found in the SEM analyses. The effects of Phyllotreta resistance/susceptibility on herbivore damage were relatively weak, however; the regression models only explained about 3% of the variation (relative path coefficients in Fig. 4). Similarly, Phyllotreta susceptibility had no effect on the scorings of “plant weakness” in the field (Table 1).
Effect of Phyllotreta susceptibility, as tested on plantlets before transplant, on damage by leaf mines, gnawing, bite holes, and aphids in the field experiment. Damage scorings were averaged over censuses. Significant regressions are indicated; see Table 1 and Online Resource 2
The severity of gnaw and aphids in the field had a negative effect on total biomass over the two years in the univariate analyses (Fig. 6), as did mines and aphids in the structural equation modelling (Online Resource 2; Fig. 4); surprisingly, biting holes were positively correlated with total biomass.
Overall, Phyllotreta resistance had no effect on plant biomass when analysed by univariate models (Table 1; Fig. 5), and only a weak negative effect in the SEM analyses (Online Resource 2; Fig. 4) through herbivore damage (b–e, g–j). Thus, only approximate 2% of the variation in total biomass was explained by the paths through mine damage and aphids (sum of the products of the b → g and e → j relative coefficients, Fig. 4), the only paths that contributed significantly to the SEM model. There was no direct effect of the resistance on biomass, apart from through herbivory (path I in Fig. 1; Online Resource 2). Phyllotreta resistance did not affect the plant survival.
The plants’ resistance to Phyllotreta affected white rust severity in the field, but as mentioned above the combined effect with the Albugo resistance was not easily interpreted.
Correlations between disease and herbivory in the field
White rust severity in the field was not correlated with any of the herbivory classes in the overall SEM analysis across years (paths G–(C–F) in Fig. 1; Online Resource 2). Disease severity at the first census was positively pairwise correlated with gnaw at the second census (r = 0.23, p < 0.01) and with biting holes at the same census (r = 0.16, p < 0.05). Disease was also positively correlated to aphid infestation at the same and different censuses in the first summer (r = 0.14–0.18, p = 0.06–0.03).
The severity of leaf mines was negatively correlated with gnaw and biting holes in the SEM analysis (Fig. 4); in contrast, leaf mines were positively pairwise correlated with biting holes at one census in the second summer (r = 0.16, p < 0.05). Gnaw was negatively pairwise correlated with aphids at two of the censuses in the first year (r = − 0.17 and − 0.33; p = 0.03 and < 0.001, respectively), but not at other times; gnaw was in contrast positively pairwise correlated with biting holes at three of the censuses both years (r = 0.22–0.29; P < 0.01) and between censuses within years.
Family variation
Genets from different maternal F2 families had somewhat different degrees of leaf mines, gnawing, vigour and survival, as well as interactions with Phyllotreta and Albugo (Table 1); however, these effects were only detected by automatic model reduction, and only a three-way interaction with the two resistances was marginally significant in likelihood-based tests. Additional preliminary analyses based on other models also found no strong family variation (results not shown).
Discussion
Association between Albugo and Phyllotreta resistances
The incentive for our study was the discovery that G-populations of B. vulgaris are resistant to herbivory by Phyllotreta nemorum flea beetles, but frequently and strongly affected by white blister rust, caused by Albugo ssp.; in contrast, P-populations are predominantly resistant to Albugo but susceptible to the herbivores (Nielsen 1997a; Nielsen et al. 2010; van Mölken et al. 2014a, b; Christensen et al. 2014, 2019). We here show that the negative association between the two resistances breaks down in F3 hybrids and that the two resistances map to different genetic linkage groups. This implies that the negative association between the Albugo and Phyllotreta resistances in natural G and P-populations in Europe (Hauser et al. 2012; Christensen et al. 2014) is due to linkage disequilibrium, i.e., associations amongst allelic states of unlinked genes, evolved during independent evolution of the two plant types. This has previously been suggested for the associations of hairiness, glucosinolate and saponin profiles of the two plant types (Kuzina et al. 2011; Khakimov et al. 2015; Byrne et al. 2017). The alternative explanation, that the two resistances are functionally, but antagonistically acting, is thereby ruled out.
Phyllotreta resistance mapped to a marker, Bv60 on linkage group 5, which has previously been shown to be close to, or within, a strong QTL for Phyllotreta resistance (Kuzina et al. 2011; Byrne et al. 2017). The same region on linkage group 5 harbours a QTL for the saponins conferring the resistance to Phyllotreta (Kuzina et al. 2009, 2011; Khakimov et al. 2015), which has been used for finding genes and enzymes involved in the saponin biosynthesis (Augustin et al. 2012; Khakimov et al. 2015; Erthmann et al. 2018). Albugo resistance mapped to three different linkage groups, 1, 3, and 16 of Khakimov et al. (2015), of which linkage groups 1 and 3 are syntenic to Arabidopsis thaliana chromosome 1 and linkage group 6 to A. thaliana chromosome 5 (Khakimov et al. 2015), both of which contain genes and regions involved in Albugo resistance (Borhan et al. 2004, 2008; Panjabi-Massand et al. 2010). The goal of our study was not to map precisely the Albugo resistance in B. vulgaris, but to verify independence from the Phyllotreta resistance. Association mapping of our material is in any case challenging as recombination between G and P-type chromosomes is most likely selected against due to hybrid inviability and dysfunction (Toneatto et al. 2010; Christensen et al. 2016)).
Effect of resistances on damage, biomass and survival
Plants determined to be Albugo-resistant before transplant to the field developed much less white rust in the following growing seasons, grew larger, appeared healthier, and survived better than susceptible plants, as may be expected. All plantlets (genets) were inoculated during the initial resistance tests, and Albugo probably established systemically in some plants, from which the disease could develop in the field and spread secondarily to other plants. Albugo spp. may occur asymptomatically within the tissue, spread into fruits and seeds, and thereby become vertically transmitted (Jacobson et al. 1998; Ploch and Thines 2011). In accordance with this, plants that did develop symptoms did not necessarily do that at all censuses and Albugo DNA was detected within surface-sterilised green siliques of experimental plants that had not shown any white rust symptoms at any of the several field scorings. Thus, the disease pressure was probably higher than found in most natural B. vulgaris populations (van Mölken et al. 2014a). White rust can have devastating effects on both crucifer crops (Chattopadhyay et al. 2015) and wild species (Alexander and Burdon 1984), and our results show that it can certainly also have serious negative effects on wild B. vulgaris.
In contrast to the Albugo-resistant plants, Phyllotreta-resistant plants were only slightly less affected by herbivory and did not differ in biomass and survival from susceptible plants. Typical crucifer herbivores, including the yellow-striped flea beetle P. nemorum, were present and at times abundant in the field experiment during both years (TP Hauser, personal observations) and caused substantial damage (Fig. 7). The low effect of the Phyllotreta resistance on herbivory and no effect on plant performance was therefore unexpected. Resistance to Phyllotreta in B. vulgaris is caused by triterpenoid saponins of the oleane type, especially cellobiosides of hederagenin and oleanolic acid (Kuzina et al. 2009; Augustin et al. 2012; Khakimov et al. 2015; Liu et al. 2019), which also confer resistance to the devastating agricultural pest diamondback Plutella xylostella (Shinoda et al. 2002; Agerbirk et al. 2003a). G-type plants, producing these saponins, are less affected by powdery mildew, molluscs, a nematode, and several other specialist and generalist arthropod herbivores (Renwick 2002; Badenes-Pérez and López-Pérez 2018; Christensen et al. 2019); this may, however, also be caused by other traits differing between the two plant types.
The lack of effect of the saponin-based Phyllotreta resistance on plant performance could have several non-exclusive explanations. The resistance-conferring saponins may have affected only a subset of the important herbivores in the field experiment, with neglectable effects on plant performance, or costs associated with the saponin-based defence may have outweighed benefits of reduced herbivory; this was however not evident from our analyses (non-significant path I, Figs. 1 and 4). Alternatively, the saponin-conferred resistances of vernalised adult plants may not be precisely estimated by our bioassays on non-vernalised plantlets before transplantation. Vernalisation and other ontogenetic changes may modify the relative content of the saponins amongst genotypes, and saponin content may decrease in older, vernalised plants. The saponin-based insect resistance is determined by (at least) three major QTLs (Kuzina et al. 2011; Wei et al. 2013; Zhang et al. 2015; Khakimov et al. 2015; Byrne et al. 2017), which should produce a range from full susceptibility to full Phyllotreta resistance in F3 plants, as is found in F2 (Kuzina et al. 2009). Whether and how vernalisation and age interacts with these QTLs has never been studied, however. The resistance-conferring saponins decrease in older B. vulgaris leaves, and in plants older than 8 weeks, under greenhouse conditions (Badenes-Perez et al. 2014a), but still remain relatively high at 12 weeks. G-type plants in natural Danish populations are resistant to Phyllotreta in summer until September, but not in October (Agerbirk et al. 2001); most of these plants, including cauline rosettes formed from the root neck of plants that flowered earlier in the same year, must have been naturally vernalised in early spring, suggesting that vernalisation does not drastically alter resistance. As the majority of herbivory in our experiment clearly happened during the summer months, the resistance-conferring saponins were most likely still present. Thus, until more is known on ontogenetic and environmental influences on saponin biosynthesis, we assume that our pre-transplantation estimates of Phyllotreta resistance are also valid for adult plants in the field.
The strongest effect of the Phyllotreta resistance on herbivory was found for leaf gnawing in the structural equation modelling. Gnawing is a sign of mollusc and lepidopteran herbivory, and consistent with this, previous studies show that molluscs prefer to consume P-type leaves and are caught more often in plots with P-plants (Heimes et al. 2016; Christensen et al. 2019). Hederagenin-based saponins have been reported to have molluscicidal effects in other studies as well (Hostettmann 1980; Marston et al. 1988; Ekabo et al. 1996). Likewise, the lepidopteran pest diamondback moth, P. xylostella, is severely affected by the resistance-conferring saponins (Shinoda et al. 2002; Badenes-Perez et al. 2014b, 2014a) and G-plants hardly support development of P. rapi (Christensen et al. 2019). Effects of the Phyllotreta resistance on mines and bite holes was smaller; this kinds of damage can be produced P. nemorum larvae and adults that are clearly affected by the saponins. However, other herbivores and flea beetles produce similar symptoms but may not be affected by the saponins. Phyllotreta-resistant plants seemed to be (slightly) more attacked by aphids (Figs. 4 and 7). This seems, however, to be an artefact of a close to significant three-way interaction between the two resistances and F2 family. Adjusting for this, an overall negative effect of the Phyllotreta resistance on aphid attack was suggested. Consistent with this, Myzus persicae aphids prefer to settle on P-plants in controlled choice experiments, and fewer aphids are caught by suction sampling on G than on P-plants in the field (Christensen et al. 2019). Aphids have been found to be negatively affected by saponins also in other studies (Sylwia et al. 2006; Goławska 2007; De Geyter et al. 2012).
The strongest negative and consistent effect of herbivore damage on plant biomass and survival was found for aphids; however, this effect was also influenced by an interaction with F2 family. Some experimental plant families were strongly attacked by cabbage aphids, Brevicoryne brassicae, at one of the censuses; however, we do not know what causes this (TP Hauser, personal observations).
Interactions between disease and different classes of herbivory
White rust in the field seemed to be affected also by the degree of Phyllotreta resistance, with more severe disease on plants that were more resistant to Phyllotreta (path A in Fig. 4), even though the two resistances were uncorrelated before transplant (see above). Plants are likely to produce more saponins under field conditions, as indicated by increased saponin concentrations upon herbivore feeding (van Mölken et al. 2014b), and the degree of Albugo sporulation and blister formation could be affected by concentrations and composition of these.
Surprisingly, we found no correlations between white rust and any of the four classes of herbivore damage (paths (G– (C–F) in Fig. 1), except a few pairwise correlations. Interactions between disease and herbivory can otherwise be expected both from several direct and indirect processes between the two types of antagonists (Hatcher 1995; Pieterse and Dicke 2007; Hauser et al. 2013; Pangesti et al. 2013). Positive interactions between Albugo and insect herbivores could be caused by, e.g., tissue modification by Albugo to become more attractive or nutritious for herbivores and by manipulation of the plant’s immune system to become less efficient (Chou et al. 2000; Belhaj et al. 2017; Prince et al. 2017). Disease severity was indeed positively pairwise correlated with biting holes and aphids at two censuses, and with gnaw, holes and aphids at different time points, and in a previous study, Phyllotreta larvae consumed more leaf material when also infected by Albugo (van Mölken et al. 2014b). However, Heimes et al. (2015) found Phyllotreta exposure to diminish white rust in B. vulgaris, and Albugo-affected Lepidium plants host less Pieris rapae eggs and larvae (Hasenbank et al. 2011). Negative interactions between disease and herbivory could, instead, be expected from cross-talk between the plants’ defence signals. Albugo infection is signalled by Salicylic acid (SA)-based cascades (Prince et al. 2017), which may have priority to the Jasmonic acid (JA)-based signals from chewing, biting and mining (Thaler et al. 2002, 2012; Pieterse et al. 2012; Vos et al. 2015). However, only one pairwise correlation between disease and biting holes was negative and two others were positive.
In contrast to the weak or missing interactions between white rust and herbivory, the different classes of herbivory were inter-correlated at several censuses and overall. Pairwise correlations between leaf mining and leaf gnawing or biting holes could be caused by manipulation of plant defence and physiology by the mining larvae, as has been described in other plant species (Giron et al. 2016; Zhang et al. 2016, 2017). The negative correlations between gnaw and aphid infestation could be due to direct interactions, changes in tissue quality by gnawing, and to interactions during plant defence signalling, as discussed above. The positive correlations between gnawing and biting, found at several censuses, is likely caused by genetic variation in defence ability against tissue-damaging herbivores, which differed significantly amongst F2 plant families.
Conclusions and implications
Our study of advanced F3 hybrids between G and P-type B. vulgaris clearly shows that the Albugo and Phyllotreta resistances of the two plant types are not physically or functionally linked. Thus, the association of resistance to Phyllotreta and other insects but susceptibility to Albugo in G-populations, and the opposite in P-populations, is best explained by independent and contrasting defence evolution in the two plant types, probably to different antagonist faunas, as suggested previously (Hauser et al. 2012; Christensen et al. 2014). In their present region of sympatry, the two resistance genes probably cannot spread freely from one plant type to the other, due to a partial reproductive barrier (Toneatto et al. 2010; Christensen et al. 2016), maintaining the historical associations. However, over time genes with a positive fitness effect may still transcend incomplete crossing barriers (Barton and Hewitt 1985; Martin and Jiggins 2017), suggesting that this could happen for the Albugo resistance that clearly benefitted the plants. However, the frequency of exposure to Albugo in natural populations of B. vulgaris is unknown, even if white rust can be frequently found here (van Mölken et al. 2014a). In contrast, the saponin-based insect resistance seems to be more or less neutral, possibly because costs and benefits outweigh each other, or may be context-dependent and more beneficial in other regions of B. vulgaris geographical distribution, as suggested by the geographic mosaic theory of coevolution (Thompson 2005). Despite assumed benefits at the time when biosynthesis of the resistance-conferring saponins evolved, a changed antagonist fauna, climate, and other environmental conditions may have reduced the benefits of this resistance in parts of the geographical range today and thus the selection forces driving its spread amongst plant types. Evolution of the P-type of B. vulgaris, with its secondary loss of the Phyllotreta resistance, supports that the benefits of the resistance-conferring saponins may be marginal or absent.
References
Agerbirk N, Olsen CE, Nielsen JK (2001) Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 58:91–100. https://doi.org/10.1016/S0031-9422(01)00151-0
Agerbirk N, Olsen CE, Bibby BM, Frandsen HO, Brown LD, Nielsen JK, Renwick JAA (2003a) A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J Chem Ecol 29:1417–1433
Agerbirk N, Ørgaard M, Nielsen JK (2003b) Glucosinolates, flea beetle resistance, and leaf pubescence as taxonomic characters in the genus Barbarea (Brassicaceae). Phytochemistry 63:69–80. https://doi.org/10.1016/S0031-9422(02)00750-1
Alexander HM, Burdon JJ (1984) The effect of disease induced by Albugo candida (white rust) and Peronospora parasitica (downy mildew) on the survival and reproduction of Capsella burna-pastoris (shepherd’s purse). Oecologia 64:314–318
Augustin JM, Kuzina V, Andersen SB, Bak S (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72:435–457. https://doi.org/10.1016/j.phytochem.2011.01.015
Augustin JM, Drok S, Shinoda T, Sanmiya K, Nielsen JK, Khakimov B, Olsen CE, Hansen EH, Kuzina V, Ekstrøm CT, Hauser T, Bak S (2012) UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance. Plant Physiol 160:1881–1895. https://doi.org/10.1104/pp.112.202747
Badenes-Pérez FR, López-Pérez JA (2018) Resistance and susceptibility to powdery mildew, root-knot nematode, and western flower thrips in two types of winter cress (Brassicaceae). Crop Prot 110:41–47
Badenes-Perez FR, Gershenzon J, Heckel DG (2014a) Insect attraction versus plant defense: young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 9:e95766. https://doi.org/10.1371/journal.pone.0095766
Badenes-Perez FR, Reichelt M, Gershenzon J, Heckel DG (2014b) Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 98:137–144. https://doi.org/10.1016/j.phytochem.2013.11.009
Badenes-Pérez FR, Márquez BP, Petitpierre E (2017) Can flowering Barbarea spp. (Brassicaceae) be used simultaneously as a trap crop and in conservation biological control? J Pest Sci 90:623–633. https://doi.org/10.1007/s10340-016-0815-y
Barton NH, Hewitt GM (1985) Analysis of hybrid zones. Annu Rev Ecol Syst 16:113–148. https://doi.org/10.1146/annurev.es.16.110185.000553
Belhaj K, Cano LM, Prince DC, Kemen A, Yoshida K, Dagdas YF, Etherington GJ, Schoonbeek H-J, van Esse HP, Jones JDG, Kamoun S, Schornack S (2017) Arabidopsis late blight: infection of a nonhost plant by Albugo laibachii enables full colonization by Phytophthora infestans. Cell Microbiol. https://doi.org/10.1111/cmi.12628
Borhan MH, Holub EB, Beynon JL, Rozwadowski K, Rimmer SR (2004) The arabidopsis TIR-NB-LRR gene RAC1 confers resistance to Albugo candida (white rust) and is dependent on EDS1 but not PAD4. Mol Plant Microbe Interact 17:711–719. https://doi.org/10.1094/MPMI.2004.17.7.711
Borhan MH, Gunn N, Cooper A, Gulden S, Tör M, Rimmer SR, Holub EB (2008) WRR4 encodes a TIR-NB-LRR protein that confers broad-spectrum white rust resistance in Arabidopsis thaliana to four physiological races of Albugo candida. Mol Plant Microbe Interact 21:757–768. https://doi.org/10.1094/MPMI-21-6-0757
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635. https://doi.org/10.1093/bioinformatics/btm308
Byrne SL, Erthmann PØ, Agerbirk N, Bak S, Hauser TP, Nagy I, Paina C, Asp T (2017) The genome sequence of Barbarea vulgaris facilitates the study of ecological biochemistry. Sci Rep 7:40728. https://doi.org/10.1038/srep40728
Chattopadhyay C, Kolte SJ, Waliyar F (2015) Diseases of edible oilseed crops. CRC Press, Taylor and Francis Group, Boca Raton
Chen M-S (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15:101–114. https://doi.org/10.1111/j.1744-7917.2008.00190.x
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814. https://doi.org/10.1016/j.cell.2006.02.008
Choi Y-J, Shin H-D, Ploch S, Thines M (2011) Three new phylogenetic lineages are the closest relatives of the widespread species Albugo candida. Fungal Biol 115:598–607. https://doi.org/10.1016/j.funbio.2011.02.006
Chou HM, Bundock N, Rolfe SA, Scholes JD (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1:99–113. https://doi.org/10.1046/j.1364-3703.2000.00013.x
Christensen S, Heimes C, Agerbirk N, Kuzina V, Olsen CE, Hauser TP (2014) Different geographical distributions of two chemotypes of Barbarea vulgaris that differ in resistance to insects and a pathogen. J Chem Ecol 40:491–501. https://doi.org/10.1007/s10886-014-0430-4
Christensen S, Sørensen H, Munk KR, Hauser TP (2016) A hybridisation barrier between two evolutionary lineages of Barbarea vulgaris (Brassicaceae) that differ in biotic resistances. Evol Ecol 30:887–904. https://doi.org/10.1007/s10682-016-9858-z
Christensen S, Enge S, Jensen KR, Müller C, Kiær LP, Agerbirk N, Heimes C, Hauser TP (2019) Different herbivore responses to two co-occurring chemotypes of the wild crucifer Barbarea vulgaris. Arthropod Plant Interact 13:19–30. https://doi.org/10.1007/s11829-018-9633-x
Crawley MJ (2007) The R Book. John Wiley and Sons, Ltd, Chichester
Dalby-Brown L, Olsen CE, Nielsen JK, Agerbirk N (2011) Polymorphism for novel tetraglycosylated flavonols in an eco-model crucifer, Barbarea vulgaris. J Agric Food Chem 59:6947–6956. https://doi.org/10.1021/jf200412c
Dangl JL, Holub EB, Debener T, Lehnackers H, Ritter C, Crute IR, Koncz C, Chua NH, Schell J (1992) Genetic definition of loci involved in Arabidopsis-pathogen interactions. In: Koncz C, Chua NH, Schell J (eds) Methods in Arabidopsis research. World Scientific Publishing Co, Singapore, pp 393–418
De Geyter E, Smagghe G, Rahbé Y, Geelen D (2012) Triterpene saponins of Quillaja saponaria show strong aphicidal and deterrent activity against the pea aphid Acyrthosiphon pisum. Pest Manag Sci 68:164–169. https://doi.org/10.1002/ps.2235
Ekabo OA, Farnsworth NR, Henderson TO, Mao G, Mukherjee R (1996) Antifungal and molluscicidal saponins from Serjania salzmanniana. J Nat Prod 59:431–435. https://doi.org/10.1021/np960208r
Erthmann PØ, Agerbirk N, Bak S (2018) A tandem array of UDP-glycosyltransferases from the UGT73C subfamily glycosylate sapogenins, forming a spectrum of mono- and bisdesmosidic saponins. Plant Mol Biol 97:37–55. https://doi.org/10.1007/s11103-018-0723-z
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20:1281–1328. https://doi.org/10.1007/BF02059810
Giron D, Huguet E, Stone GN, Body M (2016) Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J Insect Physiol 84:70–89. https://doi.org/10.1016/j.jinsphys.2015.12.009
Goławska S (2007) Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J Chem Ecol 33:1598–1606. https://doi.org/10.1007/s10886-007-9333-y
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Hasenbank M, Brandon A, Hartley S (2011) White butterfly (Pieris rapae) an the white rust Albugo candida on Cook’s scurvy grass (Lepidium oleraceum). N Z J Ecol 35:69–75
Hatcher PE (1995) Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol Rev 70:639–694. https://doi.org/10.1111/j.1469-185X.1995.tb01655.x
Hauser TP, Toneatto F, Nielsen JK (2012) Genetic and geographic structure of an insect resistant and a susceptible type of Barbarea vulgaris in western Europe. Evol Ecol 26:611–624. https://doi.org/10.1007/s10682-011-9515-5
Hauser TP, Christensen S, Heimes C, Kiaer LP (2013) Combined effects of arthropod herbivores and phytopathogens on plant performance. Funct Ecol 27:623–632. https://doi.org/10.1111/1365-2435.12053
Heimes C, Thiele J, van Mölken T, Hauser TP (2015) Interactive impacts of a herbivore and a pathogen on two resistance types of Barbarea vulgaris (Brassicaceae). Oecologia 177:441–452. https://doi.org/10.1007/s00442-014-3113-5
Heimes C, Agerbirk N, Sørensen H, van Mölken T, Hauser TP (2016) Ecotypic differentiation of two sympatric chemotypes of Barbarea vulgaris (Brassicaceae) with different biotic resistances. Plant Ecol 217:1055–1068. https://doi.org/10.1007/s11258-016-0631-8
Hostettmann K (1980) Saponins with molluscicidal activity from Hedera helix L. Helv Chim Acta 63:606–609. https://doi.org/10.1002/hlca.19800630307
Jacobson DJ, LeFebvre SM, Ojerio RS, Berwald N, Heikkinen E (1998) Persistent, systemic, asymptomatic infections of Albugo candida, an oomycete parasite, detected in three wild crucifer species. Can J Bot 76:739–750. https://doi.org/10.1139/b98-039
Khakimov B, Kuzina V, Erthmann PØ, Fukushima EO, Augustin JM, Olsen CE, Scholtalbers J, Volpin H, Andersen SB, Hauser TP, Muranaka T, Bak S (2015) Identification and genome organization of saponin pathway genes from a wild crucifer, and their use for transient production of saponins in Nicotiana benthamiana. Plant J 84:478–490. https://doi.org/10.1111/tpj.13012
Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146:839–844. https://doi.org/10.1104/pp.107.112029
Koricheva J (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176. https://doi.org/10.2307/2680130
Kuzina V, Ekstrøm CT, Andersen SB, Nielsen JK, Olsen CE, Bak S (2009) Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol 151:1977–1990. https://doi.org/10.1104/pp.109.136952
Kuzina V, Nielsen JK, Augustin JM, Torp AM, Bak S, Andersen SB (2011) Barbarea vulgaris linkage map and quantitative trait loci for saponins, glucosinolates, hairiness and resistance to the herbivore Phyllotreta nemorum. Phytochemistry 72:188–198. https://doi.org/10.1016/j.phytochem.2010.11.007
Lange CBA, Hauser TP, Deichmann V, Ørgaard M (2021) Hybridization and complex evolution of Barbarea vulgaris and related species (Brassicaceae). In review
Liu Q, Khakimov B, Cárdenas PD, Cozzi F, Olsen CE, Jensen KR, Hauser TP, Bak S (2019) The cytochrome P450 CYP72A552 is key to production of hederagenin-based saponins that mediate plant defense against herbivores. New Phytol 222:1599–1609. https://doi.org/10.1111/nph.15689
Marston A, Gafner F, Dossaji SF, Hostettmann K (1988) Fungicidal and molluscicidal saponins from Dolichos kilimandscharicus. Phytochemistry 27:1325–1326. https://doi.org/10.1016/0031-9422(88)80186-9
Martin SH, Jiggins CD (2017) Interpreting the genomic landscape of introgression. Curr Opin Genet Dev 47:69–74. https://doi.org/10.1016/j.gde.2017.08.007
Nielsen JK (1997a) Variation in defences of the plant Barbarea vulgaris and in counteradaptations by the flea beetle Phyllotreta nemorum. Entomol Exp Appl 82:25–35. https://doi.org/10.1046/j.1570-7458.1997.00110.x
Nielsen JK (1997b) Genetics of the ability of Phyllotreta nemorum larvae to survive in an atypical host plant, Barbarea vulgaris ssp. arcuata. Entomol Exp Appl 82:37–44. https://doi.org/10.1046/j.1570-7458.1997.00111.x
Nielsen JK, Nagao T, Okabe H, Shinoda T (2010) Resistance in the plant, Barbarea vulgaris, and counter-adaptations in flea beetles mediated by saponins. J Chem Ecol 36:277–285. https://doi.org/10.1007/s10886-010-9758-6
Pangesti N, Pineda A, Pieterse CMJ, Dicke M, van Loon JJA (2013) Two-way plant mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front Plant Sci 4:414. https://doi.org/10.3389/fpls.2013.00414
Panjabi-Massand P, Yadava SK, Sharma P, Kaur A, Kumar A, Arumugam N, Sodhi YS, Mukhopadhyay A, Gupta V, Pradhan AK, Pental D (2010) Molecular mapping reveals two independent loci conferring resistance to Albugo candida in the east European germplasm of oilseed mustard Brassica juncea. Theor Appl Genet 121:137–145. https://doi.org/10.1007/s00122-010-1297-6
Pieterse CMJ, Dicke M (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci 12:564–569. https://doi.org/10.1016/j.tplants.2007.09.004
Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055
Ploch S, Thines M (2011) Obligate biotrophic pathogens of the genus Albugo are widespread as asymptomatic endophytes in natural populations of Brassicaceae. Mol Ecol 20:3692–3699. https://doi.org/10.1111/j.1365-294X.2011.05188.x
Prince DC, Rallapalli G, Xu D, Schoonbeek H-J, Çevik V, Asai S, Kemen E, Cruz-Mireles N, Kemen A, Belhaj K, Schornack S, Kamoun S, Holub EB, Halkier BA, Jones JDG (2017) Albugo-imposed changes to tryptophan-derived antimicrobial metabolite biosynthesis may contribute to suppression of non-host resistance to Phytophthora infestans in Arabidopsis thaliana. BMC Biol 15:20. https://doi.org/10.1186/s12915-017-0360-z
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959. https://doi.org/10.1093/genetics/155.2.945
R Development Core Team R (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Renwick JAA (2002) The chemical world of crucivores: lures, treats and traps. Entomol Exp Appl 104:35–42. https://doi.org/10.1046/j.1570-7458.2002.00988.x
Shinoda T, Nagao T, Nakayama M, Serizawa H, Koshioka M, Okabe H, Kawai A (2002) Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J Chem Ecol 28:587–599
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol (amst) 17:278–285. https://doi.org/10.1016/S0169-5347(02)02483-7
Sylwia G, Leszczynski B, Wieslaw O (2006) Effect of low and high-saponin lines of alfalfa on pea aphid. J Insect Physiol 52:737–743. https://doi.org/10.1016/j.jinsphys.2006.04.001
Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131:227–235. https://doi.org/10.1007/s00442-002-0885-9
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270. https://doi.org/10.1016/j.tplants.2012.02.010
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Toneatto F, Nielsen JK, Ørgaard M, Hauser TP (2010) Genetic and sexual separation between insect resistant and susceptible Barbarea vulgaris plants in Denmark. Mol Ecol 19:3456–3465. https://doi.org/10.1111/j.1365-294X.2010.04760.x
Toneatto F, Hauser TP, Nielsen JK, Ørgaard M (2012) Genetic diversity and similarity in the Barbarea vulgaris complex (Brassicaceae). Nord J Bot 30:506–512. https://doi.org/10.1111/j.1756-1051.2012.01546.x
van Mölken T, Heimes C, Hauser TP, Sundelin T (2014a) Phylogeny of an Albugo sp. infecting Barbarea vulgaris in Denmark and its frequency of symptom development in natural populations of two evolutionary divergent plant types. Fungal Biol 118:340–347. https://doi.org/10.1016/j.funbio.2014.01.008
van Mölken T, Kuzina V, Munk KR, Olsen CE, Sundelin T, van Dam NM, Hauser TP (2014b) Consequences of combined herbivore feeding and pathogen infection for fitness of Barbarea vulgaris plants. Oecologia 175:589–600. https://doi.org/10.1007/s00442-014-2928-4
Vos IA, Moritz L, Pieterse CMJ, Van Wees SCM (2015) Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front Plant Sci 6:639. https://doi.org/10.3389/fpls.2015.00639
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. https://doi.org/10.4161/psb.21663
Wei X, Zhang X, Shen D, Wang H, Wu Q, Lu P, Qiu Y, Song J, Zhang Y, Li X (2013) Transcriptome analysis of Barbarea vulgaris infested with diamondback moth (Plutella xylostella) larvae. PLoS ONE 8:e64481. https://doi.org/10.1371/journal.pone.0064481
Zhang X, Liu T, Wei X, Qiu Y, Song J, Wang H, Shen D, Agerbirk N, Li X (2015) Expression patterns, molecular markers and genetic diversity of insect-susceptible and resistant Barbarea genotypes by comparative transcriptome analysis. BMC Genomics 16:486. https://doi.org/10.1186/s12864-015-1609-y
Zhang H, Dugé de Bernonville T, Body M, Glevarec G, Reichelt M, Unsicker S, Bruneau M, Renou J-P, Huguet E, Dubreuil G, Giron D (2016) Leaf-mining by Phyllonorycter blancardella reprograms the host-leaf transcriptome to modulate phytohormones associated with nutrient mobilization and plant defense. J Insect Physiol 84:114–127. https://doi.org/10.1016/j.jinsphys.2015.06.003
Zhang H, Guiguet A, Dubreuil G, Kisiala A, Andreas P, Emery RJN, Huguet E, Body M, Giron D (2017) Dynamics and origin of cytokinins involved in plant manipulation by a leaf-mining insect. Insect Sci 24:1065–1078. https://doi.org/10.1111/1744-7917.12500
Züst T, Agrawal AA (2017) Trade-offs between plant growth and defense against insect Herbivory: an emerging mechanistic synthesis. Annu Rev Plant Biol 68:513–534. https://doi.org/10.1146/annurev-arplant-042916-040856
Acknowledgements
Many thanks to Professor Sven Bode Andersen, who unfortunately passed away before publishing, for guiding the genetic analyses. Also, thanks to Morten Læssøe Stephensen, who took care of the plants in the greenhouse, Mai-Britt Sauer and Mads Nielsen, who transplanted into the field, Vinnie Deichmann, who extracted DNA, and Karen R Munk, who weighed the plants. This work was carried out as part of Stina Christensen’s PhD projects, funded by the Faculty of Science, University of Copenhagen, and by a grant from the Danish Council for Independent Research, Technology and Production Sciences to Thure Hauser (No. 09-065899).
Funding
This work was carried out as part of Stina Christensen’s PhD projects, funded by the Faculty of Science, University of Copenhagen, and by a grant from The Danish Council for Independent Research, Technology and Production Sciences to Thure Hauser (No. 09-065899).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Yes.
Additional information
Communicated by Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hauser, T.P., Christensen, S., Kuzina, V. et al. Effects of a saponin-based insect resistance and a systemic pathogen resistance on field performance of the wild crucifer Barbarea vulgaris. Arthropod-Plant Interactions 15, 683–698 (2021). https://doi.org/10.1007/s11829-021-09858-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09858-w