Abstract
Several secondary metabolites are associated with plant resistance against herbivores. Cassava genotypes present a wide variety of metabolites with insecticidal potential, but little is known about the identity of these compounds. The present work was conducted for the identification of insecticidal molecules present in cassava genotypes. To this purpose, we firstly evaluated the development of the generalist herbivore Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) fed with leaves from six cassava genotypes (IAC-14, IAC-90, IAC-12, IAC-Caapora, Baianinha and MEcu 72). Following bioassays, we measured the levels of two anti-herbivory related groups of compounds (phenolics and steroidal saponins) in all genotypes using colorimetric methods. The metabolic fingerprinting (GC–MS) of three contrasting cassava genotypes selected by their biological interferences over S. frugiperda was performed. The most unsuitable nutritional indices were observed for larvae fed with MEcu 72. Lower reproductive indices were observed for adults in which larval stages were fed with Baianinha or MEcu 72. Steroidal saponin content was similar in all genotypes, but phenolic content was 25% higher in MEcu 72. GC–MS metabolite fingerprinting of MEcu 72, Baianinha and IAC-Caapora resulted in the annotation of 53 metabolites in which 20 presented different relative abundance among the evaluated genotypes. Molecules such as myo-inositol-2-monophosphate, p-coumaric acid, chlorogenic acid, p-hydroxybenzoic acid, octadecadienoic acid and n-hexacosane were more abundant in MEcu 72 or Baianinha than in IAC-Caapora. The possible roles of these molecules in the development of S. frugiperda are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Secondary metabolites produced by plants are not directly involved in growth, development or reproduction of plants but may confer advantages such as protection against competitors or antagonists (Harborne 1999; Bourgaud et al. 2001; Macías et al. 2007; Ibanez et al. 2012). Some well-known plant secondary metabolites such as pyrethrins, nicotine, rotenone, sabadilla, ryanodine and azadirachtin are related to plant defense against herbivores and are commercially exploited as insecticides (Gonzalez-Coloma et al. 2010) or serve as molecular models for the development of synthetic insecticides (Casida 1980; Yamamoto 1999).
Cassava plants (Manihot spp.), even those improved through plant breeding, are well known for their robustness and herbivore tolerance. Molecules with insecticidal potential such as gallic acid, catechin, rutin, saponins and protease inhibitors are present in cassava (Calatayud et al. 1994; Wobeto et al. 2007; Santos et al. 2013; Gazola et al. 2018). Some of these molecules, as well as others still not detected or identified, may be associated with cassava resistance against insects, e.g. with the resistance of the MEcu 72 genotype, a genotype that, after consumed, results in the mortality of 70% of whitefly immatures (Barilli et al. 2019).
Different genotypes of a given plant species may result in different interactions with herbivores, being secondary metabolites some of the main components involved in plant defense against insects (Mithöfer and Boland 2012). For example, steroidal saponins are present in cassava leaves (Wobeto et al. 2007) and the consumption of these molecules may result in increased mortality in herbivores insects (De Geyter et al. 2007). Plant phenolic molecules also present in cassava leaves are frequently associated with negative effects in insect development (Rattan 2010).
Untargeted metabolomics of resistant and susceptible genotypes has been performed as a powerful tool to identify candidate molecules of different chemical classes associated with plant resistance to insects (Fiehn 2002; Leiss et al. 2009b; Jansen et al. 2009; Wang et al. 2017; Undas et al., 2018). Most of insect-resistant cassava genotypes have no commercial value; however, plant breeding using these resistant genotypes can be helpful to associate pest resistance to high yield and root quality in improved genotypes (Bellotti and Arias 2001). In plant breeding programs, instead of performing complex bioassays to detect plant resistance in the entire progeny, the selection of resistant plants can be facilitated by measuring the titers of compounds related to plant resistance against insects in the progeny (Maluf et al. 2010).
For the present work, we selected the polyphagous insect Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae), an important pest of crops such as maize also known as fall armyworm (Blanco et al. 2016; Fatoretto et al. 2017) as model herbivore. Larval S. frugiperda is a generalist herbivore that can feed on many different plant species and, despite of not being a specific pest of cassava, can fully develop using cassava leaves as food (Silva et al. 2017). This ability may be associated to the high phenotypic plasticity of S. frugiperda larvae that results, for instance, in the detoxification of xenobiotic compounds or in the expression of alternative digestive enzymes (Paulillo et al. 2000; Silva-Brandão et al. 2017).
The present work was conducted for the identification of insecticidal molecules present in the leaves of selected cassava genotypes. To this purpose, we evaluated the development of the generalist herbivore S. frugiperda fed with six cassava genotypes, quantified total phenolic and steroidal saponin contents in the six cassava genotypes, and compared the metabolic fingerprinting, using GC–MS untargeted metabolomics, of three contrasting cassava genotypes selected by their biological interferences caused to S. frugiperda.
Materials and methods
Plants
The genotypes IAC-14, IAC-90, IAC-12 and IAC-Caapora were obtained from the breeding program at Instituto Agronômico de Campinas (IAC), the landrace genotype Baianinha from Universidade Estadual do Oeste do Paraná (UNIOESTE), and the wild genotype MEcu 72 from Empresa Brasileira de Pesquisa Agropecuária (Embrapa). The IAC-genotypes and MEcu 72 have been associated with resistance to whitefly, Bemisia tuberculata Bondar, 1923 (Hemiptera: Aleyrodidae), and mealybug, Phenacoccus manihoti Matile-Ferrero, 1977 (Hemiptera: Pseudococcidae) while Baianinha is considered a susceptible genotype for these insects (Rheinheimer 2013; Barilli et al. 2019). Each genotype of cassava was planted in two rows with 20 plants per row spaced by 0.5 m between plants and 1 m between rows at Faculdade de Ciências Agrárias e Veterinárias (FCAV) of the Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP), Jaboticabal-SP.
Larval development, nutritional indices, and fertility life table of Spodoptera frugiperda using leaves from cassava genotypes as food
Larvae of S. frugiperda used in feeding experiments with detached cassava leaves were obtained from a colony reared in controlled conditions (25 ± 2 °C; 12 h photophase) using artificial diet as food (Parra 2001). This colony have been maintained at Applied Ecology Lab (APECOLAB), Unesp, Jaboticabal, SP without insecticide or transgenic plants exposures for at least 15 years. Cassava leaves from non-flowering 5-month-old plants were daily collected in the field and cleaned in a bath in sodium hypochlorite (0.075%) for 1 min followed by two baths in deionized water. Leaf sections were superficially dried at room temperature, transferred to a plastic container (7 cm diameter × 3 cm height) and a single 1-day-old S. frugiperda larva was inserted in each container covered with lid. Larval development feeding on each cassava genotype was observed in five replicates. Each replicate was composed by ten individualized larva. Approximately 0.5 g of cassava leaves from each genotype were daily collected, washed, weighted and offered for each S. frugiperda larva. Larval and pupal periods as well as weight of pupae after 24 h and adult deformation were observed.
Food consumption and utilization were assessed by offering sections of cassava leaves to 30 larvae per genotype kept individualized in plastic containers as described above. Fresh weights of provided and remaining food, feces and larvae were daily weighted until pupation. Ten leaf sections per genotype were kept in the same conditions without larvae to estimate weight reduction by water losses (Parra 1991).
The nutritional indices were calculated for the larval stage of S. frugiperda fed with cassava genotypes following Waldbauer (1968) and Scriber and Slansky Jr. (1981): relative consumption rate (RCR) (mg/mg/day) (RCR = I/Bm × T), relative metabolic rate (RMR) (mg/mg/day) (RMR = M/Bm × T), relative growth rate (RGR) (mg/mg/day) (RGR = B/Bm × T), efficiency of conversion of ingested food (ECI) (%) (ECI = (B/I) × 100), efficiency of conversion of digested food (ECD) (%) (ECD = B/(I − F) × 100), metabolic cost (MC) (%) (MC = 100 − ECD), and approximate digestibility (AD) (%) (AD = (I − F)/I × 100), where T = feeding period (days), I = food consumption (mg) during T, B = larval weight gain (mg) during T, F = feces produced (mg) during T, M = food used in metabolic processes (mg) during T [M = (I − F) − B], and Bm = mean larval weight (mg) during T.
Moths formed from larvae fed with each cassava genotype were kept separated and used for the observation of adult reproductive features. For this, one couple of S. frugiperda moths which larvae were fed in each genotype was placed in a PVC cage (10 cm diameter × 10 cm height) internally covered with paper for oviposition. Aqueous solution of honey (10%) was provided as food to the couples. The mortality of adults was daily observed and egg production was quantified in 48 h intervals until moths’ death. The average of two cages were considered a replicate and five replicates per genotype were made. The second posture of each female was incubated (25 °C; 12 h photophase) until larval hatching to obtain the embryonic development period and egg viability.
Fertility life tables of S. frugiperda moths emerged after larval feeding in each cassava genotype were calculated following Birch (1948), Silvera Neto et al. (1976) and Krebs (1994): net reproductive rate (R0) (♀/♀) (R0 = ∑ lx × mx), mean generation time (T) (days) (T = (∑ lx × mx × x)/(∑ lx × mx)), intrinsic rate of increase (rm) (♀/♀/day) (rm = log R0/T × 0.4343) and doubling time (DT) (days) (DT = ln(2)/rm), where x is the age of the individual in days, lx the specific survival, and mx the specific fertility.
Collection of leaves and metabolite extraction
About 50 leaves of each cassava genotype without pest or diseases symptoms were collected at the same period that leaves were collected for bioassays with S. frugiperda. The leaves used for metabolite extraction were cleaned in water, superficially dried and stored (− 20 °C). Leaf extracts were prepared following Pilon et al. (2016) with minor modifications. For this, leaf samples (3 g of fresh leaves) were ground in mortar with a pestle using liquid nitrogen and transferred to vials containing 30 ml of ethanol (1:10; w:v). After 24 h, leaf extracts were sonicated in ultrasonic bath (3 periods of 15 min), filtered in filter paper and stored (− 20 °C). Filtrates were re-extracted as previously described and combined with the first extract, resulting in approximately 60 ml of extract. Three independent replicates of each genotype were prepared.
Estimation of steroidal saponin and total phenolic contents
The steroidal saponin contents were determined using leaf extracts following the colorimetric method described by Baccou et al. (1977). The saponin contents in cassava leaves were calculated using a standard curve of diosgenin (Sigma Aldrich, Saint Louis, USA) in chloroform (0–20 µg/ml; y = 0.0529x; R2 = 0.9963) and expressed in mg/g of fresh material.
The total phenolic contents in the extracts were estimated by colorimetric method following Ainsworth and Gillespie (2007). The total phenolic contents in the cassava leaves were calculated using a standard curve of gallic acid (Sigma Aldrich, Saint Louis, USA) in ethanol (0–10 µg/ml; y = 0.1008x, R2 = 0.9984) and expressed in mg/g of fresh material.
Metabolic fingerprinting by GC–MS
Leaf extracts from Baianinha (positive reference), IAC-Caapora and MEcu 72 (negative references) were selected to be analyzed by GC–MS. These genotypes were considered contrasting because S. frugiperda larvae fed with Baianinha leaves presented favorable results for larval development when compared with the results observed for larvae fed with IAC-Caapora and MEcu 72 leaves.
The extracts were evaporated in rotary evaporator Laborota 4000 (Heidolph, Schwabach, Germany) at 45 °C and 175 mbar followed by 24 h of drying at 35 °C and 1.0 Torr in a vacuum concentrator SpeedVac (R) (Thermo, Milford, USA). Derivatization was made by adding 80 µl of methoxyamine hydrochloride (20.0 mg/ml prepared in pyridine, Sigma-Aldrich, St. Louis, USA) to 5 mg of the dried samples, followed by incubation at 30 °C for 90 min. Silylation was made by adding 200 µl of N-methyl-N-trimethylsilyl-trifluoroacetamide solution (Sigma-Aldrich, St. Louis, USA) to the samples, which were homogenized and incubated at 37 °C for 30 min, with a final incubation step at 5 °C for 24 h (Carnevale Neto et al. 2016). Afterwards, samples were filtered using BioFil® nylon syringe filter (13.0 mm diameter × 0.22 µm pore size).
Metabolic fingerprinting of the leaves of cassava genotypes were analyzed using gas chromatography coupled to mass spectrometer (Shimadzu QP-2020, Tokyo, Japan) (GC–MS). Samples were injected with an automatic sampler AOC-20Si (injector temperature at 270 °C) to a fused-silica capillary column Restek Rtx-5MS (5% phenyl-methylpolysiloxane, 30 m × 0.25 mm × 0.25 μm). The flow of the carrier gas (Helium) was adjusted at 1 ml/min and injections of 1 µl occurred in split mode (1:10). The oven temperature was kept at 140 °C for 3 min and then programmed to increase at 3 °C/min until 320 °C. The mass spectrometer was operated in electron ionization (EI) mode at 70-eV and the acquisition mass range was m/z 35–700. A C8–C40 alkanes calibration standard (Supelco, Bellefonte USA) was used as reference to calculate the retention index (RI) of detected substances (Carnevale Neto et al. 2016).
Metabolite data acquisition was performed using the GC–MS Solutions software version 1.02 (Tokyo, Japan). Peak detection, deconvolution, retention time alignment and library matching were performed using the TargetSearch package (Cuadros-Inostroza et al. 2009) in R studio. Metabolites were annotated by comparing their RI (± 2 s) and spectra (similarity > 600) against the data available in the Golm Metabolome Database (Kopka et al. 2005). Metabolite intensities were normalized using total ion chromatogram (TIC).
Statistical analyses
The data of S. frugiperda development, consumption and utilization of food and the estimation of steroidal saponin and total phenolics contents, in cassava genotypes were tested for normality and for homogeneity of the variance of errors. Variables were subjected to ANOVA followed by the Scott–Knott test (α = 0.05) using the SISVAR software (Ferreira 2011).
Parameters of fertility life table were estimated by Jackknife technique, and the means were compared using PROC GLM (SAS Institute, 2002) (Maia et al. 2000).
Annotated metabolites and their relative abundances obtained from cassava genotypes after GC–MS were statistically analyzed by combining multi and univariate analysis using the MetaboAnalyst (Chong et al. 2019). For this, data were filtered by interquantile range (IQR), log transformed and scaled by Pareto scaling. Partial least squares discriminant analysis (PLS-DA) were performed to reduce the dimensionality of the data, and to visualize samples grouping and possible outliers. Then, we performed one-way ANOVA (FDR adjusted, α = 0.05) followed by the test of Tukey (α = 0.05) to define differences in the peak area means of metabolites observed in the cassava genotypes.
Results
Nutritional indices of Spodoptera frugiperda larvae fed with leaves of cassava genotypes
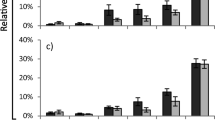
The consumption and utilization of food by S. frugiperda larvae were influenced by the cassava genotypes. Among the cassava genotypes analyzed, Baianinha was the most suitable to be used as food to S. frugiperda larvae. Consumption of Baianinha resulted in lowest values for relative consumption rate (RCR), relative metabolic rate (RMR) and metabolic cost (MC). Besides, larvae fed with Baianinha showed high efficiency of conversion of digested food (ECD), when compared with other cassava genotypes. After consumption of leaves of MEcu 72, IAC-Caapora and IAC-12, S. frugiperda larvae had lower values for efficiency of conversion of ingested food (ECI) and higher values for MC (Fig. 1a, b).
Nutritional indices of Spodoptera frugiperda larvae fed with leaves of cassava genotypes (T: 25 ± 2 °C; photophase: 12 h) (mean ± standard error). RCR relative consumption rate (F5.12 = 7.12, p = 0.003); RGR relative growth rate (F5.12 = 9.46, p < 0.001); RMR relative metabolic rate (F5.12 = 13.24, p < 0.001) (a); AD approximate digestibility (F5.12 = 5.06, p = 0.010); ECI efficiency of conversion of ingested food (F5.12 = 16.21, p < 0.001); ECD efficiency of conversion of digested food (F5.12 = 24.07, p < 0.001); MC metabolic cost (F5.12 = 24.07, p < 0.001) (b). Means followed by the same letter do not differ by Scott–Knott test (p > 0.05). Number of insects: IAC-14 = 30; IAC-90 = 30; IAC-12 = 28; Baianinha = 28; MEcu 72 = 29; IAC-Caapora = 27
Development of Spodoptera frugiperda fed with leaves of cassava genotypes
Larval period (F5.24 = 8.10, p < 0.001) and larval survival (F5.24 = 2.92, p = 0.033) of S. frugiperda changed according to the cassava genotype consumed by the insect. The developmental time of larvae fed with leaves of IAC-12 and IAC-Caapora was approximately 2 days shorter when compared to the larval period of larvae fed with leaves of the other genotypes. Larval survival of S. frugiperda fed with leaves of Baianinha, IAC-Caapora and MEcu 72 was approximately 10% reduced when compared with larval survival observed for larvae fed with leaves of IAC-14, IAC-90, and IAC-12 (Table 1).
Pupal period of females (F5.24 = 3.19, p = 0.024), male (F5.24 = 4.98, p = 0.003) and female pupal weight (F5.24 = 3.75, p = 0.012) were different according to the genotypes consumed during larval stage. Pupae formed after consumption of Baianinha and MEcu-72 leaves had lowest pupal weight (Table 2).
The cassava genotype consumed by the larvae did not influenced the adults parameters such as preoviposition period (F5.24 = 2.06, p = 0.106), male (F5.36 = 0.64, p = 0.673) and female longevity (F5.36 = 0.70, p = 0.628) and fecundity (F5.24 = 1.20, p = 0.340). Preoviposition period of S. frugiperda ranged from 3.6 to 4.9 days, male longevity from 8.8 to 10.7 days, female longevity from 14.9 to 19.4 days and the fecundity from 2122.1 to 2645.5 eggs. However, a larger number of deformed adults were observed when insects were fed during larval stage with MEcu 72 leaves (25%), followed by IAC-14, IAC-12 and IAC-Caapora leaves (ca. 10%), IAC-90 and Baianinha leaves (2.5%).
Fertility life table parameters, i.e. the reproductive performance, changed according to the cassava genotype used as food during larval stage of S. frugiperda. In general, we observed that consumption of IAC-12 or IAC-Caapora leaves resulted in a net reproduction rate (R0) 25% superior than of that observed after consumption of Baianinha leaves. Feeding with IAC-12 or IAC-Caapora leaves also resulted in lower mean generation time (T), higher intrinsic rate of increase (rm) and lower doubling time (DT) than those parameters observed after consumption of the other genotypes leaves (Table 3).
Estimation of saponin and total phenolic contents in cassava leaves
Steroidal saponin contents in IAC-14 and MEcu 72 leaves were lower than in the other cassava genotypes (Fig. 2a). The total phenolic contents in the MEcu 72 leaves were approximately 25% (3.0 mg/g) higher than in the other cassava genotypes (Fig. 2b).
Metabolic fingerprinting of selected cassava leaves using GC–MS
In total, we detected 53 putative metabolites (Supplementary Table 1; Supplementary Fig. 1) and, from these, 29 were unambiguously annotated. The others 24 metabolites were annotated as “unknown” and were kept in our analysis to observe differences in relative abundances of these metabolites among cassava genotypes. An unknown metabolite means a compound that although unidentified or unclassified can still be differentiated and quantified based upon spectral data (Sumner et al. 2007).
PLS-DA score plots demonstrated a clear distinction among the genotypes Baianinha, MEcu 72 (major negative effects on adults) and IAC-Caapora (best reproductive performance). The two first principal components of PLS-DA explained 61.4% of the total variance observed among the genotypes (R2 = 0.9821; Q2 = 0.7929, values were obtained by cross-validation using 2 components). Based on the first principal component (33.3%), we observed the separation of Baianinha from the IAC-Caapora and MEcu 72 and the second principal component (27.8%), separated IAC-Caapora from the Baianinha and MEcu 72 (Fig. 3).
Twenty metabolites were differentially abundant (FDR adjusted p ≤ 0.05) among at least two cassava genotypes tested (Supplementary Table 1), and, from these, 9 metabolites have known structure. According to ANOVA and Tukey’s analysis, the distribution of the differentially abundant metabolites among the genotypes was grouped into nine clusters (Table 4). These clusters were formed according to the pattern of relative abundance of metabolites in the different genotypes. For example, Cluster 1 was composed by two metabolites, 1 myo-inositol-2-monophosphate (I2P) and trehalose, with higher relative abundance in MEcu 72, in comparison to Baianinha and IAC-Caapora. In Cluster 2, p-coumaric acid was detected in a relative abundance higher in MEcu 72, intermediary in Baianinha and lower in IAC-Caapora. Cluster 3 shows higher abundance of a sugar like ribulose and three unknown metabolites (ID 1442, ID 600, ID 906) in MEcu 72 and IAC-Caapora and lower or absent relative abundance in Baianinha. Cluster 4 indicates higher relative abundance of the metabolites methyl isoheptadecanoate, chlorogenic acid, p-hydroxybenzoic acid (PHBA) and one unknown metabolite (ID 1662) in Baianinha and MEcu 72 and lower relative abundance in IAC-Caapora. Cluster 5 showed higher relative abundance of laminaribiose in IAC-Caapora, intermediary in MEcu 72 and lower in Baianinha. Cluster 6 indicates higher relative abundance of lactose and n-hexacosane in Baianinha, intermediary in MEcu 72, and lower in IAC-Caapora. Cluster 7 indicates higher relative abundance of one unknown metabolite (ID 1653) in Baianinha and IAC-Caapora and lower relative abundance in MEcu 72. Cluster 8 indicates higher relative abundance of two unknown metabolites (ID 461, ID 2027) in IAC-Caapora when compared with Baianinha and MEcu72. Cluster 9 indicates higher relative abundance of octadecadienoic acid and two unknown metabolites (ID 1717, ID 1766) in Baianinha when compared with IAC-Caapora and MEcu 72.
Discussion
Similarly to the present results obtained for S. frugiperda larvae, negative effects of the consumption of MEcu 72 leaves was already reported for three species of whiteflies (Hemiptera: Aleyrodidae) and one species of mealybug (Hemiptera: Pseudococcidae) (Bellotti and Arias 2001; Carabalí et al. 2010; Omongo et al. 2012; Rheinheimer 2013; Barilli et al. 2019). Baianinha genotype, previously classified as susceptible to sap sucking insects (Rheinheimer 2013; Barilli et al. 2019), was also suitable for larval development of S. frugiperda.
However, negative effects in post larval development, such as reduced pupal weight and reduced net reproduction rate, were observed after S. frugiperda larvae were fed with leaves of Baianinha genotype. Discrepant results obtained for the fitness of larval and adult stages highlight how different genotypes may affect herbivores. In Lepidoptera, nutrition of the larval stage is very important for reproductive performance, and the effects of genotypes on insect development may be apparent only after the larval stage (Awmack and Leather 2002). Lepidopteran egg production is based on energetic reserves captured at the larval stage (Honek 1993) and Baianinha leaves, despite of being suitable for larval development, were apparently not suitable to confer energetic reserves for reproduction in the adult stage.
In this study, steroidal saponins levels were similar in all cassava genotypes. Apparently, the levels of steroidal saponins in the leaves of the selected cassava genotypes are not enough to result in changes in the development of S. frugiperda or the steroidal saponins present in these leaves have low or no insecticidal activity (Adel et al. 2000). Therefore, part of the negative effects observed in larvae fed with MEcu 72 leaves may be correlated with higher total phenolics contents observed in this genotype (Rattan 2010).
GC–MS analysis revealed higher abundance of the phenolic acids p-coumaric, chlorogenic and p-hydroxybenzoic (PHBA), in MEcu 72 and Baianinha leaves. Spodoptera frugiperda showed lower pupal weight and reproductive performance when fed with both genotypes. These compounds are phenylpropanoids, derived from the shikimate-phenylpropanoid pathway, usually classified into four categories: hydroxycinnamates and their derivatives, salicylate-derived phenolic glycosides, flavonoids and condensed tannins (Chen et al. 2009).
The p-coumaric acid is a hydroxycinnamate that can be oxidized to reactive molecular species, such as quinones and quinones methides. Both metabolites bind to dietary proteins of the herbivores, decrease the nutritive value of the food and may result in negative effects on insect development (Lattanzio 2013). The chlorogenic acid is also a hydroxycinnamate that can be oxidized to chlorogenoquinone and binds to free amino acids and proteins, which reduce the availability of amino acids and decrease digestibility of dietary proteins (Felton et al. 1989, 1991). Both compounds play roles at chemical defense of plants against herbivores and may result in negative effects in development, survival and fecundity of chewing and sap sucking insects (Leiss et al. 2009b; Chrzanowski et al. 2012; Torp et al. 2013). The PHBA is a salicylate-derived phenolic glycoside, also known as 4-hydroxybenzoic acid or p-salicylate acid. Many benzenoid metabolites derived from salicylic acid, such as benzoic acid, are related to the constitutive resistance of plants against pests (Wang et al. 2017).
Myo-inositol-2-monophosphate (I2P) and trehalose were detected in higher abundance in MEcu 72 genotype. The ingestion of myo-inositol did not result in negative effects on the larval stage of Hyphantria cunea (Drury, 1773) (Lepidoptera: Arctiidae) (Wang et al. 2017) and this compound was found in higher intensity in a Mediterranean fly susceptible peach genotype when compared to the resistant genotype (Capitani et al. 2013). However, the ingestion of I2P by Coptotermes formosanus Shiraki, 1909 (Isoptera: Rhinotermitidae) resulted in deterioration of antennae segments, turning into brown and falling off (Grimball et al. 2017). Therefore, we suggest that I2P may be involved with the largest number of S. frugiperda adults deformed after feeding with MEcu 72 leaves during larval stage.
The sugar trehalose, formed by two units of glucose, is hydrolyzed by digestive trehalases present in S. frugiperda midgut (Silva et al. 2009), and is not a strong candidate molecule to explain the observed negative effects. Trehalose is correlated with plant response to biotic and abiotic stresses (Lunn et al. 2014). Higher levels of trehalose in MEcu 72 leaves suggest that this sugar could be a precursor for other molecules associated to negative effects on insect development, such as phenolic molecules (Ali et al. 2012).
The octadecadienoic acid was more abundant in Baianinha leaves. Spodoptera frugiperda larvae fed with this genotype showed nutritional indices results that suggest better utilization of Baianinha leaves as food during larval stage than results obtained using leaves of IAC-Caapora or MEcu 72 as food. However, the consumption of this genotype resulted in reduced pupal weight and net reproduction rate. This compound is classified as a conjugated linoleic acid (CLA), i.e. a group of fatty acids that contain two conjugated double bonds, which differ from each other by double bond position along the carbon chain (Yurawecz et al. 1995). CLAs have been reported to have antioxidant properties (Park et al. 1997). In this study, octadecadienoic acids may have reduced the oxidation of phenolic acids p-coumaric, chlorogenic and PHBA in the midgut of S. frugiperda larvae, resulting in improved consumption and utilization of food and reduced metabolic costs when larvae were fed with Baianinha leaves. On the other hand, octadecadienoic acids may also reduce fat deposition and increase lipolysis in adipocytes of rabbits (Park et al. 1997). This way, despite the favorable nutritional indices results observed for larvae fed with Baianinha leaves, a probable reduction in lipid storage during larval stage caused by octadecadienoic acid may have resulted in the reduced net reproduction rate observed in S. frugiperda adults (Honek 1993).
The n-hexacosane metabolite was detected in higher relative abundance in Baianinha leaves, intermediary in MEcu 72 and lower in IAC-Caapora may also be associated with the reduced reproductive performance observed for S. frugiperda adults which larval stage was fed with Baianinha and MEcu 72 leaves. Larval feeding with sublethal concentrations of a fractionated extract of Couroupita guianensis L. flowers containing n-hexacosane resulted in reduced pupal weight and reproductive performance of Spodoptera litura (Fabricius, 1775) (Lepidoptera: Noctuidae) (Ponsankar et al. 2016).
Results obtained in here provide useful information for specialists in plant–insect interactions to investigate the effects of the suggested metabolites with greater constitutive relative abundance in resistant genotypes over the development of target insects (Mouden et al. 2017). These bioassays may be conducted using artificial diets or susceptible genotypes as basis for the incorporation of the suggested metabolites in realistic concentrations, i.e. those observed in plants (Maag et al. 2015).
Moreover, plant breeders may also use the suggested analytical tools and metabolites as molecular markers to search for insect-resistant genotypes among progenies obtained from crosses of insect-resistant genotypes with high quality and productive genotypes, which are frequently insect-susceptible (Leiss et al. 2009a).
References
Adel MM, Sehnal F, Jurzysta M (2000) Effects of alfalfa saponins on the moth Spodoptera littoralis. J Chem Ecol 26(1065–1078):2. https://doi.org/10.1023/A:1005445217004
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Ali Q, Ashraf M, Anwar F, Al-Qurainy F (2012) Trehalose-induced changes in seed oil composition and antioxidant potential of maize grown under drought stress. J Am Oil Chem Soc 89:1485–1493. https://doi.org/10.1007/s11746-012-2032-z
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844. https://doi.org/10.1146/annurev.ento.47.091201.145300
Baccou JC, Lambert F, Sauvaire Y (1977) Spectrometric method for the determination of total steroidal sapogenin. Analyst 102:458–465. https://doi.org/10.1039/an9770200458
Barilli DR, Wengrat APGS, Guimarães ATB, Pietrowski V, Ringenberg R, Garcia MS (2019) Resistance of cassava genotypes to Bemisia tuberculata. Arthropod-Plant Interact 13:663–669. https://doi.org/10.1007/s11829-019-09694-z
Bellotti AC, Arias B (2001) Host plant resistance to whiteflies with emphasis on cassava as a case study. Crop Prot 20:813–823. https://doi.org/10.1016/S0261-2194(01)00113-2
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26. https://doi.org/10.2307/1605
Blanco CA, Chiaravalle W, Dalla-Rizza M, Farias JR, García-Degano MF, Gastaminza G, Mota-Sánchez D, Murúa MG, Omoto C, Pieralisi BK, Rodríguez J, Rodríguez-Maciel JC, Terán-Santofimio H, Terán-Vargas AP, Valencia SJ, Willink E (2016) Current situation of pests targeted by Bt crops in Latin America. Curr Opin Insect Sci 15:131–138. https://doi.org/10.1016/j.cois.2016.04.012
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851. https://doi.org/10.1016/S0168-9452(01)00490-3
Calatayud PA, Rahbé Y, Delobel B, Khuong-Huu F, Tertuliano M, Le Rü B (1994) Influence of secondary compounds in the phloem sap of cassava on expression of antibiosis towards the mealybug Phenacoccus manihoti. Entomol Exp Appl 72:47–57. https://doi.org/10.1007/BF02382414
Capitani D, Sobolev AP, Tomassini A, Sciubba F, De Salvador FR, Mannina L, Delfini M (2013) Peach fruit: metabolic comparative analysis of two varieties with different resistances to insect attacks by NMR spectroscopy. J Agric Food Chem 61:1718–1726. https://doi.org/10.1021/jf303248z
Carabalí A, Bellotti AC, Montoya-Lerma J, Fregene M (2010) Resistance to the whitefly, Aleurotrachelus socialis, in wild populations of cassava, Manihot tristis. J Insect Sci 10:1–10. https://doi.org/10.1673/031.010.14130
Carnevale Neto F, Pilon AC, Selegato DM, Freire RT, Gu H, Raftery D, Lopes NP, Castro-Gamboa I (2016) Dereplication of natural products using GC-TOF mass spectrometry: improved metabolite identification by spectral deconvolution ratio analysis. Front Mol Biosci 3:59. https://doi.org/10.3389/fmolb.2016.00059
Casida JE (1980) Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect 34:189–202. https://doi.org/10.1289/ehp.8034189
Chen F, Liu CJ, Tschaplinski TJ, Zhao N (2009) Genomics of secondary metabolism in Populus: interactions with biotic and abiotic environments. Crit Rev Plant Sci 28:375–392. https://doi.org/10.1080/07352680903241279
Chong J, Yamamoto M, Xia J (2019) MetaboAnalystR 2.0: from raw spectra to biological insights. Metabolites 9:57. https://doi.org/10.3390/metabo9030057
Chrzanowski G, Leszczynski B, Czerniewicz P, Sytykiewicz H, Matok H, Krzyzanowski R (2012) Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop Prot 35:71–77. https://doi.org/10.1016/j.cropro.2012.01.005
Cuadros-Inostroza A, Caldana C, Redestig H, Kusano M, Lisec J, Peña-Cortés H, Willmitzer L, Hannah MA (2009) TargetSearch—a bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinform 10:428. https://doi.org/10.1186/1471-2105-10-428
De Geyter E, Gellen D, Smagghe G (2007) First result on the insecticidal action of saponins. Commun Appl Biol Sci 72:645–648
Fatoretto JC, Michel AP, Silva Filho MC, Silva N (2017) Adaptive potential of fall armyworm (Lepidoptera: Noctuidae) limits Bt trait durability in Brazil. J Integr Pest Manag 8:1–10. https://doi.org/10.1093/jipm/pmx011
Felton GW, Donato K, Del Vecchio RJ, Duffey SS (1989) Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol 15:2667–2694. https://doi.org/10.1007/BF01014725
Felton GW, Donato KK, Broadway RM, Duffey SS (1991) Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38:277–285. https://doi.org/10.1016/0022-1910(92)90128-Z
Ferreira D (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Fiehn O (2002) Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol 48:155–171. https://doi.org/10.1023/A:1013713905833
Gazola D, Zucareli C, Ringenberg R, Oliveira MCN, Graça JP, Nunes EO, Hoffmann-Campo CB (2018) Secondary metabolite contents in different parts of cassava plants infested by Phenacoccus manihoti Matile-Ferrero (Hemiptera: Pseudococcidae). Arthropod-Plant Interact 13:359–366. https://doi.org/10.1007/s11829-018-9649-2
Gonzalez-Coloma A, Reina M, Diaz CE, Fraga BM (2010) Natural product-based biopesticides for insect control. In: Liu H, Mander L (eds) Comprehensive natural products II. Elsevier, Oxford, pp 237–268
Grimball B, Veillon L, Calhoun T, Fronczek FR, Arceneaux E, Laine RA (2017) Cyclohexylamine inexplicably induces antennae loss in formosan subterranean termites (Coptotermes formosanus Shiraki): cyclohexylamine hydrogen phosphate salts are novel termiticides. Pest Manag Sci 73:2039–2047. https://doi.org/10.1002/ps.4610
Harborne JB (1999) Classes and functions of secondary products from plants. In: Walton NJ, Brown DE (eds) Chemicals from plants. Imperial College Press and World Scientific Publishing, London, pp 1–25. https://doi.org/10.1142/9789812817273_0001
Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492. https://doi.org/10.2307/3544943
Ibanez S, Gallet C, Despres L (2012) Plant insecticidal toxins in ecological networks. Toxins 4:228–243. https://doi.org/10.3390/toxins4040228
Jansen JJ, Allwood W, Marsden-Edwards E, van der Putten WH, Goodacre R, van Dam NM (2009) Metabolomic analysis of the interaction between plants and herbivores. Metabolomics 5:150–161. https://doi.org/10.1007/s11306-008-0124-4
Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmüller E, Dörmann P, Weckwerth W, Gibon Y, Stitt M, Willmitzer L, Fernie AR, Steinhauser D (2005) GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21:1635–1638. https://doi.org/10.1093/bioinformatics/bti236
Krebs CJ (1994) Ecology: the experimental analysis of distribution and abundance. Harper & Row, New York
Lattanzio V (2013) Phenolic compounds: introduction. In: Lattanzio V (ed) Natural products. Springer Press, Berlin, pp 1543–1580. https://doi.org/10.1007/978-3-642-22144-6_57
Leiss KA, Choi YH, Abdel-Farid IB, Verpoorte R, Klinkhamer PG (2009a) NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J Chem Ecol 35:219–229. https://doi.org/10.1007/s10886-008-9586-0
Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PG (2009b) Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol 150:1567–1575. https://doi.org/10.1104/pp.109.138131
Lunn JE, Delorge I, Figueroa CM, Dijck PV, Stitt M (2014) Trehalose metabolism in plants. Plant J 79:544–567. https://doi.org/10.1111/tpj.12509
Maag D, Erb M, Glauser G (2015) Metabolomics in plant–herbivore interactions: challenges and applications. Entomol Exp Appl 157:18–29. https://doi.org/10.1111/eea.12336
Macías FA, Galindo JLG, Galindo JCG (2007) Evolution and current status of ecological phytochemistry. Phytochemistry 68:2917–2936. https://doi.org/10.1016/j.phytochem.2007.10.010
Maia AHN, Luiz AJB, Campanhola C (2000) Statistical inference on associated fertility life table parameters using Jackknife technique: computational aspects. J Econ Entomol 93:511–518. https://doi.org/10.1603/0022-0493-93.2.511
Maluf WR, Maciel GM, Gomes LAA, Cardoso MG, Gonçalves LD, Silva EC, Knapp M (2010) Broad-spectrum arthropod resistance in hybrids between high- and low-acylsugar tomate lines. Crop Sci 50:439–450. https://doi.org/10.2135/cropsci2009.01.0045
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450. https://doi.org/10.1146/annurev-arplant-042110-103854
Mouden S, Klinkhamer PG, Choi YH, Leiss KA (2017) Towards eco-friendly crop protection: natural deep eutectic solvents and defensive secondary metabolites. Phytochem Rev 16:935–951. https://doi.org/10.1007/s11101-017-9502-8
Omongo CA, Kawuki R, Bellotti AC, Alicai T, Baguma Y, Maruthim MN, Bua A, Colvin J (2012) African cassava whitefly, Bemisia tabaci, resistance in African and South American cassava genotypes. J Integr Agric 11:327–336. https://doi.org/10.1016/S2095-3119(12)60017-3
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32:853–858. https://doi.org/10.1007/s11745-997-0109-x
Parra JRP (1991) Consumo e utilização de alimento por insetos. In: Panizzi AR, Parra JRP (eds) Ecologia nutricional de insetos e suas implicações no manejo de pragas. Manole, São Paulo, pp 9–65
Parra JRP (2001) Técnicas de criação de insetos para programas de Controle Biológico. FEALQ, Piracicaba
Paulillo LCMS, Lopes AR, Cristofoletti PT, Parra JRP, Terra WR, Silva Filho MC (2000) Changes in midgut endopeptidase activity of Spodoptera frugiperda (Lepidoptera: Noctuidae) are responsible for adaptation to soybean proteinase inhibitors. J Econ Entomol 93:892–896. https://doi.org/10.1603/0022-0493-93.3.892
Pilon AC, Carnevale Neto F, Freire RT, Cardoso P, Carneiro RL, Bolzani VS, Castro-Gamboa I (2016) Partial least squares model and design of experiments toward the analysis of the metabolome of Jatropha gossypifolia leaves: extraction and chromatographic fingerprint optimization. J Sep Sci 39:1023–1030. https://doi.org/10.1002/jssc.201500892
Ponsankar A, Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, SamEdwin E, Selin-Rani S, Kalaivani K, Hunter WB, Alessandro RT, Abdel-Megeed A, Paik C, Duraipandiyan V, Al-Dhabig NA (2016) Target and non-target toxicity of botanical insecticide derived from Couroupita guianensis L. flower against generalist herbivore, Spodoptera litura Fab. and an earthworm, Eisenia foetida Savigny. Ecotoxicol Environ Saf 133:260–270. https://doi.org/10.1016/j.ecoenv.2016.06.043
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920. https://doi.org/10.1016/j.cropro.2010.05.008
Rheinheimer AR (2013) Resistência de variedades de mandioca à cochonilha Phenacoccus manihoti (Matile-Ferrero) e sua influência sobre o parasitoide Anagyrus lopezi (De Santis). Thesis, Universidade Estadual do Oeste do Paraná
Santos MAI, Corrêa AD, Alves APC, Simão AA, Alves DS, Oliveira RL, Saczk AA, Carvalho GA (2013) Extrato metanólico de folhas de mandioca como alternativa ao controle da lagarta-do-cartucho e de formigas cortadeiras. Semin Ciênc Agrár 34:3501–3512. https://doi.org/10.5433/1679-0359.2013v34n6Suppl1p3501
Scriber JM, Slansky F Jr (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211. https://doi.org/10.1146/annurev.en.26.010181.001151
Silva MC, Ribeiro AF, Terra WR, Ferreira C (2009) Sequencing of Spodoptera frugiperda midgut trehalases and demonstration of secretion of soluble trehalase by midgut columnar cells. Insect Mol Biol 18:769–784. https://doi.org/10.1111/j.1365-2583.2009.00920.x
Silva DM, Bueno AF, Andrade K, Stecca CS, Neves PMOJ, Oliveira MCN (2017) Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci Agric 74:18–31. https://doi.org/10.1590/1678-992x-2015-0160
Silva-Brandão KL, Horikoshi RJ, Bernardi D, Omoto C, Figueira A, Brandão MM (2017) Transcript expression plasticity as a response to alternative larval host plants in the speciation process of corn and rice strains of Spodoptera frugiperda. BMC Genomics 18:792. https://doi.org/10.1186/s12864-017-4170-z
Silvera Neto S, Nakano O, Barbin D, Villa Nova NA (1976) Manual de ecologia dos insetos. Agronômica Ceres, São Paulo
Sumner LW, Amberg A, Barrett D et al (2007) Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3:211–221. https://doi.org/10.1007/s11306-007-0082-2
Torp M, Lehrman A, Stenberg JA, Julkunen-Tiitto R, Björkman C (2013) Performance of an herbivorous leaf beetle (Phratora vulgatissima) on Salix F2 hybrids: the importance of phenolics. J Chem Ecol 39:516–524. https://doi.org/10.1007/s10886-013-0266-3
Undas AK, Weihrauch F, Lutz A, van Tol R, Delatte T, Verstappen F, Bouwmeester H (2018) The use of metabolomics to elucidate resistance markers against Damson-hop aphid. J Chem Ecol 44:711–726. https://doi.org/10.1007/s10886-018-0980-y
Waldbauer GP (1968) Consumption and utilization of food. In: Beament JWL, Treherne JE, Wigglesworth VB (eds) Advances in insect physiology. Academic Press, New York, pp 229–288
Wobeto C, Corrêa AD, Abreu CMP, Santos CD, Pereira HV (2007) Antinutrients in the cassava (Manihot esculenta Crantz) leaf powder at three ages of the plant. Ciênc Tecnol Aliment 27:108–112. https://doi.org/10.1590/S0101-20612007000100019
Wang L, Qu L, Hu J, Zhang L, Tang F, Lu M (2017) Metabolomics reveals constitutive metabolites that contribute resistance to fall webworm (Hyphantria cunea) in Populus deltoides. Environ Exp Bot 136:31–40. https://doi.org/10.1016/j.envexpbot.2017.01.002
Yamamoto I (1999) Nicotine to nicotinoids: 1962 to 1997. In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo, pp 3–27
Yurawecz MP, Hood JK, Mossoba MM, Roach JAG, Ku Y (1995) Furan fatty acids determined as oxidation products of conjugated octadecadienoic acid. Lipids 30:595–598. https://doi.org/10.1007/BF02536995
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
DRB performed all bioassays and participated in all data collection. IGFB and DRB analyzed GC–MS data. JLBJ and DRB performed GC–MS analysis. VSB supervised and secured funds for GC–MS analysis. ALBJ, DRB and GDR conceived and designed the research. DRB and GDR interpreted data and wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research was registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under protocol ACF6CB7.
Additional information
Handling Editor: Anna-Karin Borg-Karlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barilli, D.R., Budzinski, I.G.F., Bronzel Junior, J.L. et al. GC–MS analyses reveal chemical differences in the leaves of Manihot esculenta Crantz genotypes with different anti-herbivore effects. Arthropod-Plant Interactions 15, 387–398 (2021). https://doi.org/10.1007/s11829-021-09822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09822-8