Abstract
Despite potential negative interactions between biological control agents, the release of multiple agents against invasive alien weeds is often justified. The leaf-feeding beetle Mada polluta Mulsant (Coleoptera: Coccinellidae), released against Tecoma stans (L.) Juss ex Kunth var. stans in South Africa, has so far been unable to contain the weed. Consequently, the root-feeding flea beetle Heikertingerella sp. (Coleoptera: Galerucinae) was introduced to complement M. polluta. The effects of the interaction between the two beetles on their performance and on the target weed were studied on potted T. stans plants in a quarantine glasshouse to assess whether they were additive, synergistic or negative. There was no significant difference in the percentage survival of the P1 adults of either beetle when tested alone or in combination. Mada polluta produced significantly more F1 adult progeny than Heikertingerella sp. when tested alone, while both beetles produced significantly fewer offspring when tested in combination. Leaf damage by M. polluta alone was higher than that caused by Heikertingerella sp. alone, but in combination was not significantly higher than damage by M. polluta alone. Although both beetles on their own caused a significant reduction in leaf density relative to the control, leaf density was significantly lower when in combination. Despite significant reductions in plant height relative to the control, the differences between the three beetle treatments were not significant. Although competitive interactions caused a trade-off between agent proliferation and their impact on the growth of T. stans, these data need to be confirmed in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The release of multiple agents to control invasive plant species has been applied in many biological control programmes, with success often increasing with the number of agents released (Denoth et al. 2002). While there has been support for the release of multiple agents in both concept and practice (e.g. Hoffmann and Moran 1998; Jimenez and Balandra 2007), there has also been criticism of this approach (e.g. Myers 1985; Myers et al. 1989; McEvoy and Coombs 2000; Denoth et al. 2002; Crowe 2003). Critics have described this as a “lottery approach” (McEvoy and Coombs 2000), arguing that control arising from the release of multiple agents is due to the increased probability of releasing an effective agent, rather than the combined impact of the suite of agents. Although competition among phytophagous insects appears rare in their native ranges (Rathcke 1976; Strong et al. 1984), introduced herbivores typically experience a decrease in regulation by their natural enemies and thus higher population densities (Harley and Forno 1992; Keane and Crawley 2002), which could have profound negative consequences for established biocontrol agents. In particular, greater competition for shared food resources or the same niches can cause antagonistic effects on agent populations (Denno et al. 1995; Paynter and Hennecke 2001; April et al. 2011).
The invasive Central American tree, Tecoma stans (L.) Juss ex Kunth var. stans (Bignoniaceae) commonly known as yellow bells, was targeted for biological control in South Africa in 2005 (Madire et al. 2011a, b). Tecoma stans has invaded much of South Africa, as well as neighbouring countries in southern Africa and other countries in the world. Increasing infestations of T. stans during the past 20 years have been a concern in South Africa, leading to the initiation of the biological control programme. The programme has resulted in the release of two insect agents, the leaf-feeding beetle Mada polluta Mulsant (Coleoptera: Coccinellidae) and a leaf-mining fly Pseudonapomyza sp. (Diptera: Agromyzidae) in 2013 and 2014, respectively. Mada polluta has since established and become abundant at a few sites in KwaZulu-Natal (KZN) and the Eastern Cape (EC) provinces, while very small populations of Pseudonapomyza sp. have been recorded in KZN, EC, Limpopo and Mpumalanga provinces. Due to the severity of T. stans invasions in South Africa, it has been argued that a suite of agents is required to attack various parts of the plant, including the root system and the reproductive organs (Madire et al. 2011a). The Mexican root-feeding flea beetle Heikertingerella sp. (Chrysomelidae: Galerucinae: Alticini) was introduced into quarantine in South Africa for assessment as an additional biocontrol agent for T. stans. While the adult flea beetles can cause extensive leaf damage, the larvae feed on the roots of the plant, often reducing growth (Madire et al. 2021). Host specificity testing has demonstrated that Heikertingerella sp. is suitable for release in South Africa (Madire et al. 2021). Research on plant-mediated interactions should form part of pre-release evaluation protocols to assist in decision-making about which agents to introduce in classical biological control programs, in order to achieve the greatest impact on invasive weeds (Milbrath and Nichols 2014).
Assuming that Heikertingerella sp. will eventually be cleared for release, this study examined the consequences of the simultaneous release of Heikertingerella sp. and M. polluta on the performance of each agent species on their shared host and on the growth of the target plant. When released from their natural enemies in the introduced range, biocontrol agents may compete with other agents that share the same host (Harley and Forno 1992; Sheppard and Woodburn 1996). Such competitive interactions for the same resources may be mediated by their host plant through changes in food quality or induced defences in response to herbivore attack (Denno et al. 1995; Gerber et al. 2007), with negative consequences for one or all agents. Below-ground herbivores can be effective agents by causing substantial damage to roots, which may have a more severe impact on plant fitness than above-ground damage (Gerber et al. 2007; Johnson and Cushman 2007). However, interactions between below-ground herbivores and their host plant could have a profound influence on above-ground herbivores (e.g. Simelane 2006), and this effect could be positive or negative for Heikertingerella sp. and M. polluta.
In this study, we assessed the interactions between M. polluta and Heikertingerella sp. by examining their survival and reproductive success when confined alone and in combination on potted T. stans plants in cages. We also assessed the individual and combined impact of both herbivores on leaf damage, leaf density and plant height.
Materials and methods
Laboratory conditions
This study was conducted in quarantine glasshouse at the Agricultural Research Council-Plant Health and Protection, Roodeplaat facility in Pretoria, South Africa (25° 36′ 8780″ S; 28° 21′ 9230″ E). The temperature and relative humidity during the trial was set at 28–33 °C and 47–60%, respectively. This study was conducted under natural light conditions during summer and under a LD 16:8 photoperiod during winter. The winter photoperiod was maintained using 50 W/LED 4000 K/230 V LED floodlights (Spazio lighting). Tecoma stans plants were propagated from seeds collected in the field using river sand only as the growth medium. After the seeds had germinated, the seedlings were transplanted into 2 l pots containing a standard growing mixture of one part each of top soil, river sand, compost and vermiculite. These plants were watered twice a day and Wonder Nitrogen, Phosphorus and Potassium fertilizer (2:3:2 [14%]) was applied every 3 weeks to promote plant growth. Plants were maintained until they were 1-year old and then used in the trial. Heikertingeralla sp. and M. polluta cultures that provided individuals for this study were reared on T. stans under the same conditions in the quarantine glasshouse, in gauze-covered cages (0.55 × 0.55 × 0.95 m).
Life history of the study organisms
Adults of the root-feeding flea beetle Heikertingerella sp. feed on the leaves of T. stans and create small, irregular round holes by scraping the leaf epidermis through to the mesophyll, eventually causing extensive damage. The females deposit eggs onto the soil surface of potted plants. Heikertingerella sp. larvae feed on the secondary roots and develop on the core of the primary roots, eventually pupating in the soil until adult emergence. The flea beetle has a generation time from adult to adult of 49–67 days (Madire et al. 2021).
Both adults and larvae of the lady beetle M. polluta feed on the leaves of T. stans. Adults feed on the upper surface of the leaves, whereas the larvae feed on the under surface. Female M. polluta deposit their eggs in clusters on the under surface of the leaves and all larval instars develop on the leaves until pupation. This lady beetle has a generation time of ca. 36 days (Madire 2013).
Experimental design
Sixteen 1-year old T. stans plants of similar stem height, ranging from 18 to 20 cm tall (Mean ± SE = 19.56 ± 0.16; n = 16) and leaf density, ranging from 16 to 29 leaves (Mean ± SE = 19.94 ± 0.80; n = 16) were selected from the nursery for the experiment. Plants were sprayed with water and cleaned to remove any unwanted insects or contaminants before they were moved to the quarantine glasshouse. Individual plants were placed in separate gauze-covered cages (0.55 m × 0.55 m × 0.95 m) prior to their exposure to the insects. The four treatments included controls (with no insects), five mating pairs of Heikertingerella sp. only, five mating pairs of M. polluta only and a combination of Heikertingerella sp. and M. polluta that included three mating pairs of each beetle species. Insect densities were chosen based on the results of preliminary assays conducted to determine the range of insect densities in which considerable damage was observed on the growth and development of the plant. Newly emerged adults (P1) were used in this study and each treatment was replicated four times. After 20 days, the surviving P1 adults of Heikertingerella sp. and M. polluta were counted and removed from the plants in all treatments, while their immature stages (i.e., eggs, larvae and pupae) were allowed to develop to adulthood over 60 days and then recorded. To determine the effect of the treatments on the two agents, we compared the percentage survival of P1 adults over the 20-day period and the number of emerging F1 progeny over the 60-day period, between the individual and combined exposures. To determine the response of the host plants to each treatment, we compared adult foliar damage, leaf density and plant height between the controls and the three beetle treatments after the 60-day period.
Data analysis
The statistical analyses were conducted using IBM SPSS version 26.0. Since the datasets did not meet the assumptions of normality, generalized linear modelling was used to determine the effect of treatment on the numbers of surviving P1 adults, F1 progeny, leaves damaged, leaves produced and the size of the plants. The models that analysed count data incorporated a Poisson distribution (corrected for over-dispersion) with a log link function. The model that analysed plant size data incorporated a Tweedie distribution (corrected for over-dispersion) with a log link function. Significance (p < 0.05) was assessed using Likelihood ratio chi-square statistics because of the small sample sizes. When treatment had a significant influence, post-hoc paired comparisons (Fisher’s Least Significant Difference) were performed on the means.
Results
Survival of P1 adults of Heikertingerella sp. and M. polluta
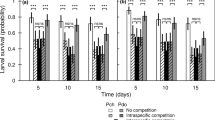
The percentage survival of the P1 adults of M. polluta and Heikertingerella sp. did not differ between the single and combined treatments during the 20-day period (χ2 = 1.100; df = 3; p = 0.777). The percentage survival of M. polluta in both single and combined treatments was slightly higher than that of Heikertingerella sp. in the same treatments, with 73% and 71% of M. polluta adults surviving in single and combined treatments, respectively, compared to 67% and 68% of Heikertingerella sp. adults surviving in the same treatments (Fig. 1).
Mean (± S.E.) percentage of P1 adults of Heikertingerella sp. and M. polluta that survived in single and combined treatments during a 20-day period. Heiker only = Heikertingerella sp. alone; Mada only = M. polluta alone; Heiker combined and Mada combined = both beetles in combination. Bars with the same letter are not significantly different (Fisher’s Least Significant Difference)
Emergence of F1 adult progeny of Heikertingerella sp. and M. polluta
There were significant differences in the number of F1 adult progeny emerging from single and combined treatments of Heikertingerella sp. and M. polluta (χ2 = 63.547; df = 3; p < 0.001). The numbers of adult progeny produced by both beetle species in the single treatments were significantly higher than those produced in the combined treatments (Fig. 2). When confined alone on T. stans, M. polluta produced 46% more adult progeny than when confined with Heikertingerella sp. Similarly, Heikertingerella sp. produced 65% more adult progeny when confined alone than when confined with M. polluta (Fig. 2).
Mean (± S.E.) number of Heikertingerella sp. and M. polluta F1 adult progeny that emerged from single and combined treatments. Heiker only = Heikertingerella sp. alone; Mada only = M. polluta alone; Heiker combined and Mada combined = both beetles in combination. Bars with different letters are significantly different (Fisher’s Least Significant Difference)
Leaf feeding damage and leaf density
There were significant differences in leaf damage between single and combined exposures of T. stans to Heikertingerella sp. and M. polluta (χ2 = 101.099; df = 2; p < 0.001). Exposure to M. polluta only and a combination of both species caused significantly more leaf damage than exposure to Heikertingerella sp. only, with no significant difference between the M. polluta only and combined treatments (Fig. 3).
Exposure to Heikertingerella sp. alone, M. polluta alone and a combination of the two beetle species significantly reduced leaf density on T. stans relative to the control (χ2 = 44.964; df = 3; p < 0.001). Exposure to each of Heikertingerella sp. and M. polluta alone reduced leaf density by 20% and 29%, respectively, while exposure to a combination of both species reduced leaf density by 43% (Fig. 4).
Mean (± S.E.) number of leaves produced by Tecoma stans in response to exposure to Heikertingerella sp. alone (Heiker only), Mada polluta alone (Mada only) and both beetles in combination (Heiker + Mada). Bars with different letters are significantly different (Fisher’s Least Significant Difference)
Plant height
Single and combined exposures to Heikertingerella sp. and M. polluta caused significant reductions in plant height (χ2 = 62.890; df = 3; p < 0.001) in relation to the control. However, there were no significant differences in plant height between the three beetle exposure treatments. Plant height in the Heikertingerella sp. only, M. polluta only and combined treatments were reduced by 20.3%, 20.8% and 21.3%, respectively, relative to the control (Fig. 5).
Discussion
Although there seems to be a trade-off between the additive effect of Heikertingerella sp. and M. polluta on T. stans and the reduction in their reproductive success, the two beetle species had a greater impact when combined than when confined individually. On their own, Heikertingerella sp. and M. polluta reduced leaf density by 20% and 29%, respectively, but together caused a 43% reduction as a result of higher levels of leaf damage. Reductions in plant height through insect attack were similar for the single and combined treatments relative to the control. Although it is uncertain how Heikertingerella sp. and M. polluta will affect weed density, our study suggests that they could complement each other in the field, particularly since the larvae of Heikertingerella sp. are root feeders.
The additive impact of Heikertingerella sp. and M. polluta implies that releases of both species as biocontrol agents could be more effective than the release of a single species. This supports the notion that weed biocontrol success rates improve with releases of multiple agents (Denoth et al. 2002; Seastedt et al. 2007). For example, the release of an undescribed leafhopper (Hemiptera: Cicadellidae), formerly referred to as Zygina sp., and the rust fungus Puccinia myrsiphylli (Thuem.) Winter (Pucciniaceae) against Asparagus asparagoides (L.) Druce (Asteraceae) in Australia had an additive impact on various plant growth parameters (Turner et al. 2010). Furthermore, the combined impact of the leaf- and stem-mining Neurostrota gunniella Busck (Lepidoptera: Gracillariidae) and the fungus Phloeospora mimosa pigra H.C. Evans & Carrion (Ascomycotina) in reducing the leaf density of Mimosa pigra L. (Mimosaceae) in Australia was higher than that caused by each species on its own (Paynter and Hennecke 2001).
Stiling and Cornelissen (2005) concluded that multiple releases of biocontrol agents against insect and plant pests decreased pest abundance by 27.2% more, when compared to single-species releases. While our study revealed an antagonistic interaction between Heikertingerella sp. and M. polluta, with fewer F1 progeny produced in combination than in isolation, this is unlikely to diminish their combined negative effect on the target weed. For example, despite decreased populations of the thistle-head weevil Rhinocyllus conicus Froelich (Coleoptera: Curculionidae) in the presence of the rosette weevil Trichosirocalus horridus Panzer (Coleoptera: Curculionidae), their combined attack reduced viable seed production in Carduus nutans L. (Asteraceae) by 59%, while the presence of R. conicus alone caused a 45% reduction (Milbrath and Nechols 2004). Several other studies (Crawley 1983; Kinsmann and Platt 1984; Marquis 1984; Strauss 1991; Karban and Strauss 1993; Poveda et al. 2003) have also demonstrated that releases of multiple biocontrol agents may be needed to inflict sufficient damage on target weed populations.
The interaction between Heikertingerella sp. and M. polluta deserves further investigation under field conditions, since our laboratory trials may have exacerbated interference between the two species, more than would otherwise occur under unconfined conditions. Although F1 progeny production by both beetle species was reduced by competition, Heikertingerella sp. was more affected. The shorter developmental period of M. polluta (36 days) (Madire 2013) and its potential for rapid population increase might have reduced food quality for Heikertingerella sp., thereby negatively affecting its performance due to a longer developmental period (49–67 days) (Madire et al. 2021). However, unlimited food resources under field conditions are likely to mitigate any niche overlap (i.e. leaf feeding) between the two beetle species, particularly since biocontrol agents often avoid plant tissues infested by competitors in the field (e.g. Rayamajhi et al. 2006), thereby reducing competition. Furthermore, the size of both the potted plants and the cage might have limited the development of the root system and the foliage of T. stans, thereby affecting both species; particularly Heikertingerella sp. which displays longer larval development and feeding activity (Brown and Gange 1990; Masters et al. 1993). Buccellato et al. (2019) also found that the results of glasshouse trials on agent interactions were not predictive of the field results, and attributed this to variation in biotic and abiotic environmental factors which are excluded in the controlled conditions of a glasshouse trial. Thus uncertainty makes it difficult to select the best possible agent for a target weed (i.e. “silver bullet”) and biocontrol programmes thus often tend towards the “cumulative stress” approach (e.g. Dauer et al. 2012).
Although the two beetle species have been found in similar habitats in their native range in Central America, populations of M. polluta appear to peak earlier in the season than those of Heikertingerella sp. This could allow resource partitioning over time (Denno et al. 1995) and promote co-existence between the two agents in the field. However, an increase in the intensity of herbivory by early-season M. polluta could cause rapid deterioration of the host plants, with adverse effects on the performance of late-season Heikertingerella sp. (e.g. Hunter 1990; Denno et al. 1995; Kaplan and Denno 2007). Nonetheless, we speculate that unlimited food resources under field conditions in South Africa are likely to dampen the effects of competition between early- and late-season feeding agents (Rayamajhi et al. 2006).
Blossey and Hunt-Joshi (2003) also argued that the performance of root-feeding herbivores could be compromised if food quality and quantity is reduced by aboveground herbivores, which is likely to be exacerbated on potted plants under confined conditions. However, such events are likely to be rare under field conditions (Hunt-Joshi and Blossey 2005), emphasizing the need to confirm these results with field trials, once Heikertingerella sp. is approved for release from quarantine. Indeed, entire defoliation of purple loosestrife Lythrum salicaria L. (Lythraceae) shoots by the leaf-feeding beetle Galerucella calmariensis L. (Coleoptera: Chrysomelidae) in field cages had no negative impact on leaf herbivory by adults of the root-feeding weevil Hylobius transversovittatus Goeze (Coleoptera: Curculionidae) (Hunt-Joshi and Blossey 2005). While additional long-term data under field conditions are needed, our data suggest that the simultaneous release of Heikertingerella sp. and M. polluta appears likely to complement the biocontrol programme against T. stans.
Availability of data and materials
All data generated or analyzed during this study are included in the published article (Interaction data, Microsoft Excel worksheet).
References
April V, Robertson MP, Simelane DO (2011) Interaction between Uroplata girardi (Coleoptera: Chrysomelidae) and Ophiomyia camarae (Diptera: Agromyzidae) on a shared host Lantana camara (Verbenaceae). Environ Entomol 40(5):1123–1130. https://doi.org/10.1603/EN11027
Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48:521–547. https://doi.org/10.1146/annurev.ento.48.091801.112700
Brown VK, Gange AC (1990) Insect herbivory below ground. Adv Ecol Res 20:1–58. https://doi.org/10.1016/S0065-2504(08)60052-5
Buccellato L, Byrne MJ, Fisher JT, Witkowski ETF (2019) Post-release evaluation of a combination of biocontrol agents on Crofton weed: testing extrapolation of greenhouse results to field conditions. Biocontrol 64:457–468. https://doi.org/10.1007/s10526-019-09946-0
Crawley MJ (1983) Herbivory, the dynamics of animal-plant interactions. Blackwell Scientific Publications, Oxford
Crowe M (2003) Ecological interactions between insect herbivores and their host plant in a weed biocontrol system. MSc thesis. University of Lethbridge, Faculty of Art and Science, Lethbridge, Alberta
Dauer JT, McEvoy PB, Van Sickle J (2012) Controlling a plant invader by targeted disruption of its life cycle. J Appl Ecol 49:322–330. https://doi.org/10.1111/j.1365-2664.2012.02117.x
Denno RF, McClure MS, Ott JR (1995) Interspecific interactions in phytophagous insects: competition revisited and resurrected. Annu Rev Entomol 40:297–331. https://doi.org/10.1146/annurev.en.40.010195.001501
Denoth M, Frid L, Myers JH (2002) Multiple agents in biological control: improving the odds? Biol Control 24:20–30. https://doi.org/10.1016/S1049-9644(02)00002-6
Gerber E, Hinz HL, Blossey B (2007) Interaction of specialist root and shoot herbivores of Alliaria petiolata and their impact on plant performance and reproduction. Ecol Entomol 32:357–365. https://doi.org/10.1111/j.1365-2311.2007.00875.x
Harley KLS, Forno IW (1992) Biological control of weeds: a handbook for practitioners and students. Inkata Press, Sydney
Hoffmann JH, Moran VC (1998) The population dynamics of an introduced tree, Sesbania punicea, in South Africa, in response to long-term damage caused by different combinations of three species of biological control agents. Oecologia 114(3):343–348. https://doi.org/10.1007/s004420050456
Hunter MD (1990) Differential susceptibility to variable plant phenology and its role in competition between two insect herbivores on oak. Ecol Entomol 15:401–408
Hunt-Joshi TR, Blossey B (2005) Interactions of root and leaf herbivores on purple loosestrife (Lythrum salicaria). Oecologia 142:554–563. https://doi.org/10.1007/s00442-004-1747-4
Jimenez MM, Balandra MA (2007) Integrated control of Eichhornia crassipes by using insects and plant pathogens in Mexico. Crop Prot 26(8):1234–1238. https://doi.org/10.1016/j.cropro.2006.10.028
Johnson BE, Cushman JH (2007) Influence of a large herbivore reintroduction on plant invasions and community composition in a California grassland. Conserv Biol 21(2):515–526. https://doi.org/10.1111/j.1523-1739.200600610.x
Kaplan I, Denno RF (2007) Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol Lett 10(10):977–994. https://doi.org/10.1111/j.1461-0248.2007.01093.x
Karban R, Strauss SY (1993) Effects of herbivores on growth and reproduction of their perennial host, Erigeron glaucus. Ecology 74(1):39–46. https://doi.org/10.2307/1939499
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17(4):164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kinsman S, Platt WJ (1984) The impact of a herbivore upon Mirabilis hirsuta, a fugitive prairie plant. Oecologia 65(1):2–6. https://doi.org/10.1007/BF00384454
Madire LG (2013) Biology and host range of Mada polluta, a potential biological control agent of Tecoma stans in South Africa. Biocontrol Sci Technol 23(8):944–955. https://doi.org/10.1080/09583157.2013.809404
Madire LG, Wood AR, Williams HE, Neser S (2011a) Potential agents for the biological control of Tecoma stans (L.) Juss ex Kunth var stans (Bignoniaceae) in South Africa. Afr Entomol 19:434–442. https://doi.org/10.4001/003.019.0216
Madire LG, Simelane DO, Waladde S (2011b) Biology and host range of the leafminer, Pseudonapomyza sp. (Diptera: Agromyzidae), a potential biological control agent for Tecoma stans (Bignoniaceae) in South Africa. Biocontrol Sci Technol 21:1409–1421. https://doi.org/10.1080/09583157.2011.628121
Madire L, Simelane D, Olckers T (2021) Pre-release evaluation of Heikertingerella sp. as a potential biocontrol agent for Tecoma stans in South Africa. J Appl Entomol 145(1–2):65–72. https://doi.org/10.1111/jen.12837
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226(4674):537–539. https://doi.org/10.1126/science.226.4674.537
Masters GJ, Brown VK, Gange AC (1993) Plant mediated interactions between above- and below-ground insect herbivores. Oikos 66:148–151. https://doi.org/10.2307/3545209
McEvoy PB, Coombs EM (2000) Why things bite back: unintended consequences of biological weed control. In: Follet PA, Duan JJ (eds) Non target effects of biological control. Springer, Boston, MA, pp 167–194. https://doi.org/10.1007/978-1-4615-4577-4_11
Milbrath LR, Nechols JR (2004) Individual and combined effects of Trichosirocalus horridus and Rhinocyllus conicus (Coleoptera: Curculionidae) on musk thistle. Biol Control 30(2):418–429. https://doi.org/10.1016/j.biocontrol.2003.12.005
Milbrath LR, Nechols JR (2014) Plant-mediated interactions: considerations for agent selection in weed biological control programs. Biol Control 72:80–90. https://doi.org/10.1016/j.biocontrol.2014.02.011
Myers JH (1985) How many insects species are necessary for successful biocontrol of weeds? In: Delfosse ES (ed) Proceedings of the 6th international symposium on the biological control of weeds. Agriculture Canada, Canadian Government Printing Office, Ottawa, pp 77–82
Myers JH, Higgins C, Kovacs E (1989) How many insect species are necessary for the biological control of insects? Environ Entomol 18:541–547. https://doi.org/10.1093/ee/18.4.541
Paynter Q, Hennecke B (2001) Competition between two biological control agents, Neurostrota gunniella and Phloeospora mimosa-pigrae, and their impact on the invasive tropical shrub Mimosa pigra. Biocontrol Sci Technol 11:575–582. https://doi.org/10.1080/09583150120076139
Poveda K, Steffan-Dewenter I, Scheu S, Tscharntke T (2003) Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 135:601–605. https://doi.org/10.1007/s00442-003-1228-1
Rathcke BJ (1976) Competition and coexistence with a guild of herbivorous insects. Ecology 57:76–87. https://doi.org/10.2307/1936399
Rayamajhi MB, Van TK, Pratt PD, Center TD (2006) Interactive association between Puccinia psidii and Oxyops vitiosa, two introduced natural enemies of Melaleuca quinquenervia in Florida. Biol Control 37:56–67. https://doi.org/10.1016/j.biocontrol.2005.10.013
Seastedt TR, Knochel DG, Garmoe M, Shosky SA (2007) Interactions and effects of multiple biological control insects on diffuse and spotted knapweed in the Front Range of Colorado. Biol Control 42:345–354. https://doi.org/10.1016/j.biocontrol.2007.06.003
Sheppard AW, Woodburn TL (1996) Population regulation in insects used to control thistles: can we predict effectiveness. In: Floyd RB, Sheppard AW, De Barro PJ (eds) Frontiers of population ecology. CSIRO Publications, Canberra, pp 277–290
Simelane DO (2006) Effect of herbivory by Teleonemia scrupulosa on the performance of Longitarsus bethae on their shared host, Lantana camara. Biol Control 39:385–391. https://doi.org/10.1016/j.biocontrol.2006.07.013
Stiling P, Cornelissen T (2005) What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol Control 34(3):236–246. https://doi.org/10.1016/j.biocontrol.2005.02.017
Strauss SY (1991) Direct, indirect, and cumulative effects of three native herbivores on a shared host plant. Ecology 72:543–558. https://doi.org/10.2307/2937195
Strong DR, Lawton JH, Southwood TRE (1984) Insects on plants: community patterns and mechanisms. Blackwell Science, Oxford
Turner PJ, Morin L, Williams DG, Kriticos DJ (2010) Interactions between a leafhopper and a rust fungus on the invasive plant Asparagus asparagoides in Australia: a case study of two agents being better than one for biological control. Biol Control 54:322–330. https://doi.org/10.1016/j.biocontrol.2010.06.005
Acknowledgements
We thank the National Resource Management Programme of the Department of Environmental Affairs and the Agricultural Research Council-Plant Health & Protection for funding this project. Colleagues of the ARC-PHP, including S. Neser, F. Heystek, K. Mawela and M. Netshiluvhi, provided assistance during field collections of both beetles in Mexico, while K. Sehlola and S. Mokwena provided technical assistance during the study.
Funding
This study was funded by the National Resource Management Programme of the Department of Environmental Affairs and by the Agricultural Research Council-Plant Health & Protection (ARC-PHP).
Author information
Authors and Affiliations
Contributions
LM: Investigation; Data curation; Formal Analysis; Writing—original draft. DS: Conceptualization; Funding Acquisition; Project Administration; Supervision; Writing—review & editing. TO: Formal Analysis; Supervision; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This study complies with good scientific practice.
Consent to participate
All three authors have agreed to the publication of the current version of this manuscript.
Consent for publication
The authors of this study would like to submit this manuscript entitled, ‘Competitive interactions between the root-feeding Heikertingerella sp. and foliage-feeding Mada polluta on the invasive Tecoma stans’ to be considered for publication in the journal, Arthropod—Plant Interactions.
Additional information
Handling Editor: Livy Williams.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madire, L.G., Simelane, D.O. & Olckers, T. Competitive interactions between the root-feeding Heikertingerella sp. and foliage-feeding Mada polluta on the invasive Tecoma stans. Arthropod-Plant Interactions 15, 265–271 (2021). https://doi.org/10.1007/s11829-021-09814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09814-8