Abstract
In the intense solar radiation of an alpine climate, small black bees often experience extremely high thoracic temperatures when they are foraging on flowers, but flies forage at lower temperatures. To explore the hypothesis that seed set could be depressed by transient dehydration of pollen at the high temperatures reached by hot bees foraging in sunshine, we compared the effectiveness of single visits by different pollinators to a bowl-shaped flower Potentilla lancinata in alpine meadows, SW China. The ratio of seed set to pollen transferred in individual flowers was monitored over 2 years, indicating that pollen deposited on stigmas by halictid bees produced lower seed set than pollen carried by flies. Scopal pollen applied to stigmas by hand gave good seed set, but in germination tests it burst more frequently than pollen from anthers, implying dehydration. Pollen grains freshly taken from the scopae of solitary bees foraging in sunshine were smaller than those taken from anthers or foraging bees in early-morning overcast conditions, implying dehydration. The effect was reversible: hand pollination showed that scopal pollen was no less effective than fly pollen after removal from the bee. Pollen carried by such bees foraging in intense sunlight in flowers became dehydrated, causing an osmotic mismatch between the pollen and the stigmas. Transient heat-induced dehydration of pollen represents a novel pathway by which climate warming may disrupt plant reproduction, and helps us understand why flies could be more effective pollinators than bees in cool, high-radiation arctic or high-altitude sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comparisons of different flower visitors in terms of pollinator performance are important to ecologists, conservation biologists, agronomists, plant breeders and evolutionary biologists (Wilson and Thomson 1991; Ne’eman et al. 2010). It has been proposed that flower visitors can be compared with respect to their proportionate contribution to plant reproduction by estimating pollinator effectiveness (King et al. 2013). Pollinator effectiveness is sometimes expressed in terms of seed set (e.g. Keys et al. 1995) or seedling germination rate (Herrera 2000), but for practical reasons, especially in multi-species surveys, it is often measured simply as the product of visit frequency and pollen grains deposited per visit, ‘pollinator importance’ (Reynolds and Fenster 2008). Thus King et al. (2013) and Ne’eman et al. (2010) recommend combining visit counts with single-visit studies followed by counts of pollen grains delivered to the stigma (single visit deposition). But pollination ecologists are well aware that not all pollen grains that reach a stigma achieve seed set. Well-known causes of failure include genetic effects (such as self-incompatibility), destruction of pollen by wetting (Mao and Huang 2009) or thermal stress (e.g. Zinn et al. 2010) and exposure to fungicides and other compounds that prevent subsequent germination (discussed in Supplementary material).
Here we explore a situation in which estimates of pollen transfer and seed set in the same flowers indicated that pollen transferred by halictid bees foraging on Potentilla lancinata resulted in lower seed set than pollen transferred by flies. The situation here was unusual because in the extreme radiation environment at high altitude, these small black bees foraging in pale, bowl-shaped flowers (Kevan 1975) achieved very high operative temperatures (up to 44.9 °C, Corbet and Huang 2016), whereas flies foraged when incident radiation was less intense and achieved lower body temperatures (Herrera 1997). We examine possible causes for the low seed set after pollen transfer by a hot bee in these unusual conditions.
Materials and methods
Flowers
Potentilla lancinata Cardot was abundant in a field station, the Shangri-La Alpine Botanic Garden in Yunnan Province, southwest China (27° 54′ 5″ N, 99° 38′ 17″ E, 3300 m a.s.l.), where we studied it in July and August 2014–2018. The bowl-shaped yellow flowers each have 20 anthers of an unusual flattened form with a peripheral line of dehiscence (Fig. 1). The gynoecium comprises numerous ovaries (mean ± se 97.4 ± 2.22, n = 170 in the dry year of 2015; 140.1 ± 3.16, n = 89 in 2016, when abundant rainfall resulted in unusually luxuriant growth), each with one style and a single ovule; thus fruit set is equivalent to seed set. We refer to the percentage of ovaries that developed into fruits as % seed set rather than % fruit set, for comparability with most other flowers in which seed set is a property of an individual flower, whereas fruit set is a property of a whole plant. The flowers are protandrous, at least partly self-compatible, and the female phase is recognizable by the darkened anthers (Fig. 1, Supplementary material, Figs. S1, S2).
Pollen counts
After petal fall on each flower about ten styles were carefully removed from each experimental flower (except alternate-numbered open-pollinated controls in 2015) in the bagging experiments, and mounted directly in a drop of cotton blue lactophenol (2015) or aniline blue (0.1% aniline blue in K3PO4 buffer, 2016–2018) on a microscope slide. On bagged flowers, we removed the bag briefly to take the styles and immediately replaced the bag before there was time for an insect visit. Style removal had no significant effect on seed set (Supplementary material). The stigmas were examined for conspecific pollen. Given that each ovary yields only one seed, instead of counting the pollen grains on stigmas in each flower we estimated the percentage of stigmas in each flower bearing at least one pollen grain (or, in 2018, the percentage of styles with at least one pollen tube). We estimated the percentage of stigmas bearing pollen, the percentage seed set (see below) and the ratio of % seed set to % stigmas bearing pollen (the seed:pollen ratio).
Bagging experiments
To examine the effects on pollen transfer, seed set and seed:pollen ratio of autogamy, self pollination, cross pollination and different pollen vectors, we bagged flower buds with 0.5 mm mesh bags (in 2015 and 2018) and/or (in 2016) with tetrahedra made from wire-edged florists’ ribbon (50 mm wide, 0.3 mm mesh), held closed with fine copper wire. Each of 15 (in 2016) and 30 (in 2017 and 2018) plants had six (2016) or seven (2017, 2018) flower buds bagged in bud and one tagged but left exposed to pollinators (open-pollinated control). On each plant one flower remained bagged until seed set to test for autogamy, and the other bagged flowers were hand pollinated, by brushing the stigmas with the anthers of a male-phase flower from the same plant (self pollination), or at least one male-phase flower from another plant (cross pollination), or the pollen-laden scopa of a halictid bee or a fly (caught foraging on P. lancinata and retained in an individual polythene bag) or used for single-visit studies (see below). The bags were replaced immediately. Any remaining bagged flowers were used to replace accidentally damaged flowers or to supplement the numbers of flowers pollinated with scopal pollen. Cases where flowers, bags or labels were damaged by livestock or trampling are excluded from the analysis. Single-visit studies were performed in 2015–2018. A bagged flower was unbagged when the anthers had darkened and the stigmas were assumed to be receptive (Supplementary material). In 2015 and 2016 most visits were videoed with a Sony Handicam HDR-CX405B video camera until an insect had visited the flower and departed, when the bag was immediately restored. The nature and duration of each visit was observed directly and confirmed from the video. Flowers that received more than one visit are excluded from the analysis. In 2018 single visits by bees and flies were also observed on flowers shaded under an opaque white polythene canopy which would diffuse incident radiation, reducing the intensity of radiation focused on the flower centre.
When fruits were swollen, 11–21 days after bagging, each flower head was cut off and preserved in FAA. Swollen fruits and undeveloped ovaries were counted to estimate the percentage seed set.
Pollen viability
To test the viability of pollen from halictid scopae and from anthers, we mounted it in MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), in which viable grains stained dark (Rodriguez-Riano and Dafni 2000), and also tested germination.
Preliminary experiments indicated that the optimal concentration of sucrose for in vitro germination of pollen taken from anthers was 20% (g sucrose per 100 g solution, Bolten et al. 1979), and germination was greatly increased by addition of 0.1% boric acid. A 5-µL droplet of 20% sucrose with 0.1% boric acid was placed on a coverslip and pollen was dabbed onto it from a dehiscing anther or a scopa from a halictid that had been caught foraging on P. lancinata in sunshine, killed by crushing the thorax and immediately sealed into a polythene bag and transferred to the laboratory as quickly as possible. In 2016 the coverslip was inverted over the cavity of a perspex ring (10 mm internal diameter, 2 mm high Incurring, Polysciences Inc., Warrington, PA 18976, USA) on a slide. The ring was sealed to the coverslip and the slide with liquid paraffin or cooking oil (Corbet and Plumridge 1985). 10 µL of the same germination solution was put into the cavity of the ring to control the relative humidity to which the hanging drop was exposed. In 2016 the experimental set-up was complete within 5 h 30 min of catching the bee. In 2017 a 5-µL droplet of sucrose solution was placed on a concave slide and pollen was dabbed onto it from a dehiscing anther or a scopa from a halictid that had been caught foraging on P. lancinata in sunshine and killed by crushing the thorax. The 2017 experiments were set up directly in the field and petroleum jelly was used to seal coverslips onto the slides to prevent rehydration of pollen.
After 18 h at room temperature we scored 100 grains from each coverslip or slide for germination (pollen tube exceeding the diameter of the grain) and bursting (usually after germination). At the end of the experiment the concentration of the hanging drop was measured with a sucrose refractometer modified for small volumes (Bellingham & Stanley, Tunbridge Wells, UK). Drops with a final sucrose concentration > 24% were excluded from the analysis.
Temperature measurements
To examine the effects of intrafloral microclimate on the operative temperatures of bees in 2015 we used an Omega RDXL4SD 4-channel SD card data logger. The operative temperature of a bee in a flower (the temperature of a dead bee in the centre of a flower) was measured with a fine (0.09 mm diameter) type K thermocouple thrust into the thorax of a freshly-killed Lasioglossum female (Corbet and Huang 2016). In 2018 flower temperatures were measured with a thermocouple ca. 2 mm diameter.
Pollen grain volume
To see whether pollen grains in the scopae of halictids foraging in sunshine were dehydrated, in 2017 we collected pollen (1) from anthers, at 10.30–11.30 h in overcast conditions; (2) from halictid scopae, at 10.30–11.30 h soon after the bees had first become active, before there had been any sunshine to dehydrate the pollen; (3) from scopae of halictids foraging at 1300–1500 h in intense sunlight, at least 20 min after the start of a sunny interval and (4) from anthers in the sun. The pollen was put into cedar oil on a side immediately to prevent rehydration, and brought back to the laboratory for measurement.
Plant names follow e-flora of China (http://www.efloras.org) and bee names follow (Michener 2000).
Data analysis
Seed set and pollen on stigmas were compared among natural and bagging treatments and to examine the pollination performance of flies and halictids using a generalized linear model (GLM) with binary distribution and logistic-link function, while seed: pollen ratios were analysed with normal distribution and identity-link function. To compare the size of pollen grains in anthers and scopae at different humidities, we used a GLM with normal distribution and identity-link function. Analyses of GLM were conducted in IBM SPSS 20.0 and data are presented as mean ± standard error. A generalized linear mixed model (GLMM) was used to examine effects of year and pollination treatments (related to Tables 1 and 2) with individual plants as random variables; the results are presented in the supplemental material Table S1.
Results
Insect visitors
Insects foraging on flowers of Potentilla lancinata included small solitary bees (including species of Lasioglossum and Hylaeus); flies (including bombyliids, sarcophagids, muscoids, syrphids and calliphorids); ants; evanioids; solitary vespoid and crabronine wasps; thrips; minute flies and parasitic hymenopterans and small numbers of butterflies. The solitary bees took nectar, applying their mouthparts to the nectaries, and female halictids used their mandibles to extract pollen from the anthers (see Supplementary material). In 2014 and 2015 we saw few bumblebees and no honeybees on P. lancinata, but in 2016, a year of abundant rainfall and luxuriant growth, bumblebees were abundant foraging for nectar. Honeybees occasionally visited in 2016–2018. Insect visitors were categorized as large female halictids (n = 10 in 2016) (c. 9 mm long), large flies (over 3 mm long, including muscoids, calliphorids, sarcophagids, bombyliids and syrphids) (n = 10 in 2016) and other insects.
In the very changeable alpine climate, the visitor spectrum depended on the weather. During sunny intervals visits by solitary bees outnumbered those of other insects. For example, a sequence of 30 five-min counts of insects visiting two flowers, one in the male phase and one in the female phase on 2 days in August 2014 yielded 83 and 79 visits respectively, a mean of 0.54 visits per flower per minute, of which 89% were made by solitary bees. In a sequence of 27 five-min counts of insects visiting a patch of 43 flowers in August 2015 the predominant visitors were large flies during cloudy periods but halictid bees during sunny intervals, and a similar pattern was seen in 2018 (Fig. 2).
Visits by different groups of insects in successive 5-min periods to a patch of flowers (43 flowers in August 2015), expressed as a percentage of the total number of visits in each 5-min period, plotted against A flower temperature, and B mean operative temperature of a dead halictid in a flower during that period
What role do different pollinator types play in pollen delivery and seed set?
Bagging experiments showed that the flowers were partially self-incompatible, and insect visits were required for full seed set. Autogamy and self pollination resulted in low seed set, and a low seed: pollen ratio (Fig. 3; Table 1). The seed: pollen ratio from self pollination was not significantly higher than that resulting from autogamy, and it was lower than that produced by cross pollination or open pollination. In 2016 and 2018 the seed set and seed: pollen ratio of cross-pollinated flowers was not significantly lower than that of open-pollinated flowers, indicating that bagging had no significant effect on seed set other than exclusion of pollinators.
Comparisons of mean (± s.e.m.) % stigmas with pollen (top), % seed set (middle) and seed/pollen ratio (bottom) in P. lancinata flowers after various hand-pollination treatments or open pollination in 2016 (open bars) and 2018 (filled bars). Bars marked with different letters are significantly different at p < 0.05. The seed: pollen ratio does not differ significantly between scopal pollen and fly pollen in either year. (See Table 1)
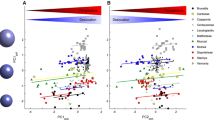
In 2015, and in both exposed and shaded flowers in 2018, similar amounts of pollen were transferred by single visits by female halictid bees or flies, but bee visits resulted in remarkably low seed set and seed: pollen ratio, indicating that pollen transferred by halictids was less effective than pollen transferred by flies (Fig. 4; Table 2). The seed:pollen ratios resulting from hand pollination with halictid scopal pollen and fly-borne pollen were not significantly different from one another (Fig. 3; Table 1).
Comparisons of mean (± s.e.) % stigmas with pollen (top), % seed set (middle) and seed:pollen ratio after single visits by halictid bees or flies, in exposed flowers and under a translucent polythene canopy in 2015 (open bars) and 2018 (filled bars). Bars marked with different letters are significantly different at p < 0.05. (See Table 2)
Tests of viability of scopal pollen confirmed that halictid scopal pollen had not been killed. When the viability of scopal pollen from halictid bees was tested by staining with MTT and counting 100 grains on each of 3 (in 2016) or 30 (in 2017) slides per treatment, the percentage of dark-staining pollen grains was not lower in scopal pollen than in pollen taken directly from an anther (Table 3). Further evidence for the viability of scopal pollen came from germination tests; in 20% sucrose with 0.1% boric acid, scopal pollen grains germinated, but preliminary observations indicated that pollen grains from a scopa were more likely to burst (usually at the tip of the pollen tube) than pollen grains from an anther. When pollen from the scopa of freshly-killed halictid females was compared with pollen taken direct from dehiscing anthers, germination rates did not differ significantly, but a higher percentage of scopal pollen grains burst (Table 3). This suggests disturbance of the water relations of pollen in the scopa of a foraging halictid, and the finding that hand pollination with scopal pollen yielded good seed set (Fig. 3) suggests that the disturbance is reversible.
Changes in pollen hydration result in changes in pollen grain volume (Corbet and Plumridge 1985). Pollen grains taken directly from the scopae of bees foraging in full sun were smaller than those collected in overcast conditions from anthers or foraging bees, and pollen grains from sunlit anthers were smaller than those from anthers in overcast conditions (Fig. 5).
Discussion
Why did flowers receiving single visits from female halictids set so little seed? It was not due to poor pollen delivery, because bee-visited flowers had no less pollen but a lower ratio of seed set to pollen-bearing stigmas than fly-visited flowers (Fig. 4). It was unlikely to be due to a higher proportion of self pollen, because the effect was transient and reversible; scopal pollen was no less effective than fly pollen when transferred by hand. Furthermore, bee visits were much more frequent than fly visits, and bees often flew from plant to plant (Corbet and Huang 2016). The transience of the effect means that genetic effects or destruction of pollen by wetting, overheating or exposure to toxic compounds could be ruled out. The low seed set was not due to the pollen being killed by heat stress or by contamination with mandibular gland secretion (this hypothesis is discussed in Supplementary material), because pollen from scopae was viable by the MTT test and was capable of germinating in vitro. Another possibility is that heterogamous pollen in the bees’ scopae was not released on subsequent flower visits, which therefore transferred only self pollen to the stigmas. We consider this unlikely because the pollen is loosely held in the scopae, and because there is marked herkogamy in P. lancinata (described in Supplementary material), so that when stigmas are receptive very little pollen is available in the same flower, but bees transfer large amounts.
In the medium that supported germination of fresh pollen, pollen grains from scopae were more likely to burst, usually after germination (Table 3). We suggest that failure of seed set was due to an osmotic mismatch between the stigma and pollen that had been exposed to low relative humidity in contact with the body of a hot bee, which in sunlit flowers could reach much higher operative temperatures than the stigmas (Corbet and Huang 2016).
For a given ambient absolute humidity (kg water per cubic metre), relative humidity (the ratio of the partial pressure of water vapour in the air at a specific temperature to the saturated vapour pressure at that temperature) falls as temperature rises, making it difficult to disentangle effects of temperature and humidity on pollen germination (Loupassaki et al. 1997). Low relative humidity affects the germination and bursting of pollen. In oilseed rape, germination and bursting of pollen depend on the relationship between the osmolarity of the germination medium and the relative humidity to which the pollen has previously been exposed (Corbet and Plumridge 1985). Thus high temperature can affect pollen hydration status, which in turn affects viability, germinability and propensity to burst.
Exposure to low relative humidity causes pollen grains to lose water and decrease in volume (Fig. 5), consistent with previous observations (Nepi et al. 2001; Corbet and Plumridge 1985; Bassani et al. 1994), and can cause bursting after germination in a hypotonic germination medium (Corbet and Plumridge 1985; Yates and Sparks 1989). Low relative humidity can also render pollen inviable as tested by the fluorochromatic reaction (Bassani et al. 1994), or prevent germination (Yates and Sparks 1989; Loupassaki et al. 1997). Studies of environmental effects on pollen performance commonly consider effects on viability and/or germination. Our findings suggest that environmental effects may reversibly suppress seed set even after adequate germination.
Given that elevation of temperature at a given absolute humidity results in reduction of relative humidity, a local increase in temperature, caused by incident radiation reflected into the centre of a high-albedo flower raising the temperature of a low-albedo foraging bee, would effectively lower the relative humidity to which scopal pollen is exposed in the boundary layer next to the bee’s body, causing the pollen grains to lose water and increasing the proportion of grains bursting in a hypotonic germination medium or on a stigma. This effect could be reversible; when the hydration status is restored by equilibration to a higher relative humidity the pollen might germinate without bursting. Pre-incubation rehydration at high RH restores germinability (e.g. Luza and Polito 1987; Yates and Sparks 1989). For pollen of Brassica napus an equilibration period of 2 h was effective (Corbet and Plumridge 1985), but a scopal pollen mass might equilibrate more slowly than an array of individual pollen grains.
Consistent with previous investigations (Herrera 1997), we observed that halictids were active in warm, sunny conditions, whereas flies were active in cooler weather (Fig. 2). We propose that pollen carried in the scopae of halictids foraging during periods of intense solar radiation had been exposed to a microclimate that caused an osmotic mismatch between pollen and stigma, whereas pollen on the bodies of flies, foraging in less extreme conditions, had not. If the effectiveness of transferred pollen, in terms of subsequent seed set, varies through the day in relation to ambient microclimate, the relative effectiveness of different pollen vectors may depend on the microclimatic conditions in which they forage. In studies of single-visit pollen deposition, observations are sometimes limited to periods of fine weather (e.g. Pisanty et al. 2016); but in climates where incident radiation is strong, pollination may be more effective in cooler or more humid conditions, and pollen vectors active at such times, such as flies, may be responsible for a larger proportion of seed set than conventional fine-weather studies suggest (Herrera 1997; Inouye 2015). Flower reorientation movements (with the corolla facing upward at sunset and downward after sunrise) in wild tobacco reduce anther temperature in the daytime, protecting pollen from overheating and losing viability (Haverkamp et al. 2018). It has been suggested that the effect of pre-exposure humidity on pollen hydration may influence the pollinating success of different pollen vectors in relation to the microclimatic conditions to which they expose the grains (Corbet and Plumridge 1985). It would be interesting to relate the seed: pollen ratio to short-term variations in microclimate (and corresponding variation in foraging activity of different pollinators) through a day, especially in the extreme case of a pale bowl-shaped flower in the intense radiation at high altitude.
The expectation that pollen delivery to the stigma is an indication of pollinator effectiveness depends on the assumption that the pollen is conspecific, compatible and viable, and the stigma is receptive (Ne’eman et al. 2010). For practical reasons these conditions cannot always be tested, especially in extensive studies involving multiple species (e.g. King et al. 2013; Ballantyne et al. 2015). But our findings suggest that a further criterion deserves attention: that the water relations of the pollen at the time of transfer are compatible with those of the stigma.
Flies can be important pollinators in arctic or cold regions (Tiusanen et al. 2016) or at high altitude (Song et al. 2014). Rosbakh et al. (2018) recently pointed out that studies of the regeneration niche (Grubb 1977) should include pre-pollination stages, including temperature effects on pollen performance (Haverkamp et al. 2018). Climate change may be expected to influence the effectiveness of pollen carried by insects that forage in the intense radiation of alpine habitats, particularly in low-albedo bowl-shaped flowers like Potentilla and Ranunculus, in which bees may reach very high operative temperatures (Kevan 1975; Totland 1994, 1996). Fluctuations of weather may potentially influence pollen performance and consequently plant reproductive success, but the deleterious effects of rapid changes of temperature and/or humidity on plant fitness remain little known (Corbet 1990).
References
Ballantyne G, Baldock K, Willmer P (2015) Constructing more informative plant-pollinator networks: visitation and pollen deposition networks in a heathland plant community. Proc R Soc B 282(1814):20151130
Bassani M, Pacini E, Franchi GG (1994) Humidity stress reponse in pollen of anemophilous and entomophilous species. Grana 33:146–150
Bolten AB, Feinsinger P, Baker HG, Baker I (1979) On the calculation of sugar concentration in flower nectar. Oecologia 41:301–304
Corbet SA (1990) Pollination and the weather. Israel J Bot 39:13–30
Corbet SA, Huang SQ (2016) Small bees overheat in sunlit flowers: do they make cooling flights? Ecol Entomol 41:344–350
Corbet SA, Plumridge JR (1985) Hydrodynamics and the germination of oil-seed rape pollen. J Agric Sci (Cambridge) 104:445–451
Grubb PJ (1977) The maintenance of species richness in plant communities: the importance of the regeneration niche. Biol Rev 52:107–145
Haverkamp A, Li X, Hansson BS, Baldwin IT, Knaden M, Yon F (2018) Flower movement balances pollinator needs and pollen protection. Ecology. https://doi.org/10.1002/ecy.2553
Herrera CM (1997) Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos 78:601–611
Herrera CM (2000) Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology 81:15–29
Inouye DW (2015) Flies and flowers III: ecology of foraging and pollination. J Pollinat Ecol 16:115–133
Kevan PG (1975) Suntracking solar furnaces in high arctic flowers: significance for pollination and insects. Science 189:723–726
Keys RN, Buchmann SL, Smith S (1995) Pollination effectiveness and pollination efficiency of insects foraging Prosopis velutina in south-eastern Arizona. J Appl Ecol 32:519–527
King C, Ballantyne G, Willmer PG (2013) Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods Ecol Evol 4:811–818
Loupassaki M, Vasilakakis M, Androulakis I (1997) Effect of pre-incubation humidity and temperature treatment on the in vitro germination of avocado pollen grains. Euphytica 94:247–251
Luza J, Polito VS (1987) Effects of desiccation and controlled rehydration on germination in vitro of pollen of walnut (Juglans spp.). Plant Cell Environ 10:487–492
Mao YY, Huang SQ (2009) Pollen resistance to water in 80 angiosperm species: flower structures protect rain-susceptible pollen. New Phytol 183:892–899
Michener C (2000) The bees of the world. The Johns Hopkins University Press, Baltimore
Ne’eman G, Jurgens A, Newstrom-Lloyd L, Potts S, Dafni A (2010) A framework for comparing pollinator performance: effectiveness and efficiency. Biol Rev 85:435–451
Nepi M, Franchi G, Pacini E (2001) Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216:171–180
Pisanty G, Afik O, Wajnberg E, Mandelik YD (2016) Watermelon pollinators exhibit complementarity in both visitation rate and single-visit pollination efficiency. J Appl Ecol 53:360–370
Reynolds R, Fenster C (2008) Point and interval estimation of pollinator importance: a study using pollination data of Silene caroliniana. Oecologia 156:325–332
Rodriguez-Riano T, Dafni A (2000) A new procedure to assess pollen viability. Sex Plant Reprod 12:241–244
Rosbakh S, Pacini E, Nepi M, Poschlod P (2018) An unexplored side of regeneration niche: seed quantity and quality are determined by the effect of temperature on pollen performance. Front Plant Sci 9:1036
Song B, Chen G, Stöcklin J, Peng DL, Niu Y, Li ZM, Sun H (2014) A new pollinating seed-consuming mutualism between Rheum nobile and a fly fungus gnat, Bradysia sp., involving pollinator attraction by a specific floral compound. New Phytol 203:1109–1118
Tiusanen M, Hebert PDN, Schmidt NM, Roslin T (2016) One fly to rule them all—muscid flies are the key pollinators in the Arctic. Proc R Soc B 283:20161271
Totland Ø (1994) Intraseasonal variation in pollination intensity and seed set in an alpine population of Ranunculus acris in Southwestern Norway. Ecography 17:159–165
Totland Ø (1996) Flower heliotropism in an alpine population of Ranunculus acris (Ranunculaceae): effects on flower temperature, insect visitation, and seed production. Am J Bot 83:452–458
Wilson P, Thomson JD (1991) Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72:1503–1507
Yates I, Sparks D (1989) Hydration and temperature influence in vitro germination of pecan pollen. J Am Soc Hortic Sci 114:599–605
Zinn K, Tunc-Ozdemir M, Harper J (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exper Bot 61:1959–1968
Acknowledgements
We thank Director Z.-D. Fang for making available the facilities of the Shangri-La Alpine Botanic Garden, and Jim Cane, John Hobart and two referees for valued comments. This work was supported by the National Science Foundation of China (Grants Nos. U1402267, 31730012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stanislav Gorb.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Corbet, S.A., Chen, FF., Chang, FF. et al. Transient dehydration of pollen carried by hot bees impedes fertilization. Arthropod-Plant Interactions 14, 207–214 (2020). https://doi.org/10.1007/s11829-019-09726-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-019-09726-8