Abstract
The Asian citrus psyllid, Diaphorina citri, is currently the most important pest of citrus worldwide because it vectors ‘Candidatus Liberibacter’ spp., the etiological agents of lethal Huanglongbing (HLB). Reduction of D. citri populations is a key component of HLB management. Identifying potential D. citri attractants, such as volatiles, could be useful for behavioral management of this insect. This could, for instance, enhance catches of adults on monitoring traps. The overall aim of this study was to investigate the response of D. citri to volatiles characteristic of preferred citrus hosts. An ancillary objective was to formulate a synthetic blend of volatiles based on preferred host odors that could enhance monitoring traps. Volatile collections of two preferred citrus species, Duncan grapefruit (Citrus paradise) and sweet orange (Citrus sinensis), were performed in aeration chambers and analyzed by gas chromatography-mass spectrometry. Based on the grapefruit volatile profile, a synthetic blend consisting in d-limonene (0.62 mg), methyl N-methylanthranilate (0.19 mg), β-ocimene (0.11 mg), β-elemene (0.02 mg), and β-caryophyllene (0.02 mg) was prepared in 9.9 mL of dichloromethane and evaluated for attractiveness, as well as the individual compounds, and compared with a positive control of natural volatiles collected from Citrus sinensis. Behavior of D. citri adults was evaluated in two-choice behavioral assays comparing response to lures with different combinations and dosages of the volatiles deployed on yellow sticky traps. Individually, neither d-limonene nor methyl N-methylanthranilate affected behavior of D. citri. However, these volatiles increased catches of D. citri, as compared with solvent control, when presented as components of the synthetic grapefruit blend, as did the natural citrus odor positive control. Importantly, catch of D. citri appeared to vary with the release rate of the identified grapefruit volatile blend, which was attractive at a 0.1 mg loading dosage, but repellent at a dosage only 1 log step higher.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), is the most serious pest of citrus worldwide, due primarily to its role as a vector of “Candidatus Liberibacter asiaticus” (Las) (Grafton-Cardwell et al. 2013). The bacterium causes the highly destructive citrus disease, citrus greening, also called Huanglongbing (HLB), which has no cure (Bové 2006). Worldwide, control of D. citri has been one of the three critical components of HLB management, which also includes planting pathogen-free nursery stock and removing the inoculum by destroying infected trees (Bové 2006). Due to the difficulty in detecting early Las infection in trees and the rapid spread of HLB, management programs in America have focused on vector control (Grafton-Cardwell et al. 2013).
The management strategy for D. citri has been based mainly on the use of insecticides, leading to an increase of annual insecticide applications (Boina and Bloomquist 2015). This strategy has many associated drawbacks, including D. citri insecticide resistance, negative consequences for beneficial fauna and for the environment, and destabilizing changes in the biological balance of agro-ecosystems (Beloti et al. 2017; Chen et al. 2017; Qureshi and Stansly 2010). Thus, the development of effective alternatives to control D. citri is still a matter of critical importance. Much research effort is currently aimed at finding potential attractants and repellents for D. citri.
Colored sticky traps are widely used for detection and population monitoring of D. citri, but their effectiveness varies with population density. For example, Miranda et al. (2018) showed that, taking into account costs of material and labor, sticky traps were an effective method for detecting D. citri under low pest densities; whereas, the opposite results were reported by Godfrey et al. (2013) under similar pest densities, when comparing multiple methods of pest monitoring. Hence, effective lures to increase D. citri captures are sorely needed. Using host-plant volatiles could increase the attractiveness of yellow sticky traps for monitoring the activity of D. citri adults and facilitate development of attract-and-kill technologies (Aksenov et al. 2014; Coutinho-Abreu et al. 2014; Yan et al. 2015). However, progress toward the development of these strategies for practical use has been slow with variable results (Grafton-Cardwell et al. 2013).
Host finding behavior in psyllids is complex and comprises several sensory modalities (Patt and Setaḿou 2010). Many studies have shown that stimuli emitted by flushing shoots may play an important role in the detection, location, and evaluation of potential host plants by D. citri (Martini et al. 2014a; Patt et al. 2011; Patt and Setaḿou 2010; Stockton et al. 2016; Wenninger et al. 2009). The role of odor cues for D. citri orientation to host plants has been extensively investigated and reviewed (Mann et al. 2011; Patt and Setaḿou 2010; Sétamou et al. 2012; Wenninger et al. 2009). While host-plant odor itself may not function as an orientation cue, it may enhance psyllid responsiveness to visual cues (Wenninger et al. 2009). D. citri responsiveness to the odor emitted by flushing shoots may depend on several different odor characteristics, such as the qualitative and quantitative composition of the volatiles, as well as, their release rate (Aksenov et al. 2014; Patt and Setaḿou 2010). However extensively researched, most of these studies have been carried out under controlled conditions, and D. citri behavior is still not understood completely.
The overall aim of this study was to investigate the response of D. citri to odors of preferred hosts, under less artificial conditions, for potential contribution to behavioral management of this insect. Volatile organic compounds (VOCs) emitted by two preferred citrus genotypes, sweet orange (Citrus sinensis) and Duncan grapefruit (Citrus paradisi) (Alves et al. 2014; Liu and Tsai 2000; Sétamou et al. 2008), were characterized by gas chromatography-mass spectrometry. Based on the volatile profile of Duncan grapefruit, synthetic volatiles were evaluated as a blend and compared with its major individual components comprising the blend (d-limonene and methyl N-methylanthranilate). Volatiles were evaluated as lures deployed with yellow sticky traps in two-choice cage experiments. We identified a synthetic blend of volatiles that increased catch of D. citri compared to solvent control. In addition, our investigation revealed new insights regarding the influence of volatile host odors on D. citri host preference behavior.
Materials and methods
Host plants
VOCs were collected from Las-free, 8–10 month-old (20–25 cm tall) sweet orange (Citrus sinensis) and Duncan grapefruit (Citrus paradisi) potted seedlings. These were maintained in a greenhouse at the Estación Experimental INIA Salto Grande, Instituto Nacional de Investigación Agropecuaria, Salto, Uruguay (− 31.32°, − 57.94°). Plants were maintained at 27–28 °C, with 60–65% relative humidity and a 14:10 (L:D) photoperiod without exposure to insecticides.
Insects
Diaphorina citri used in the cage experiments were obtained from weekly field collections and maintained on orange jasmine, Murraya paniculata (L.) Jack., in a greenhouse at 27 ± 1 °C, 63 ± 2% RH and under a L14:D10 photoperiod at the University of Florida Citrus Research and Education Center in Lake Alfred, Florida. Illumination in the greenhouse was supplemented with linear fluorescent 54 W lights (F54W/T5/865/ECO, GE lighting, Nela Park, OH). The culture was established in 2000 from field populations collected in Polk Co., FL, USA (28.0′N, 81.9′W) before confirmation of Las in Florida.
Volatile collection and analysis
Leaf VOCs were obtained from potted undamaged seedlings enclosed in glass chambers (37.5 cm height, 24.0 cm diam.) sealed with Teflon lids. VOCs emitted were adsorbed during 48 h on 50 mg of Haysep-Q 80/100 mesh, using a stream of charcoal-filtered humidified air (300 mL/min). The flow was achieved pumping air to the system through a N035 At.18 bomb (KNF, Germany), and air suctioning, after air passed the adsorbent, through Personal Air Sampling Apex, Casella Cel. During the duration of VOC collection, plants were kept at 22 ± 2 °C, 45 ± 5% RH and under a 14:10 h light : dark photocycle. A total of 6 replicates (1 pot containing 2 seedlings comprised a replicate) and two blank controls where performed. Collections were made on two different collection dates. The VOCs were eluted with 1 mL bi-distilled hexane (J. K. Baker) and concentrated to 100 µL under a stream of Nitrogen for gas chromatography coupled to mass spectrometry (GC–MS) analysis. GC–MS analyses were done using a QP-2010 Shimadzu GC–MS, equipped with a non-polar (AT-5 MS) column (Alltech, 30 m × 0.25 mm, 0.25 µm), and operated with a constant carrier (He) flow of 1 mL/min. The oven temperature was programmed as follows: the initial temperature was 40 °C (1 min), then increased to 280 °C at 5 °C/min, and held for 1 min at 280 °C. The injector temperature was 250 °C and the interphase temperature 280 °C. Injections (1 µL) were in the splitless mode, and mass spectra were acquired from m/z 28 to 350 (70 eV, scan mode). For retention index calculations, a mixture of n-alkanes (100 ppm each, in hexane) was injected in the splitless mode immediately after the samples. Compounds were identified tentatively through comparison of their retention indexes and mass spectra with the ones reported in the database Adams and NIST (Adams 2007; NIST Mass Spec Data Center 2017), as well as comparison with retention indexes and mass spectra of synthetic standards injected in the same conditions. Differences in content of compounds were determined by comparison of relative areas.
Preparation of stimuli
A synthetic blend mimicking the volatile emissions of Duncan grapefruit was prepared (grapefruit blend). Because the Duncan grapefruit volatile profile was observed to be less complex than the one from sweet orange (see “Results” section), it was selected for preparation of a synthetic blend for behavioral analysis. The five compounds that constituted about 96% of the composition of the VOCs profile (relative percentages) were selected: d-limonene 62%, methyl N-methylanthranilate (MNMA) 19%, β-ocimene 11%, β-elemene 2% and β-caryophyllene 2% (Table 2). A synthetic volatile lure treatment based on the profile of grapefruit was evaluated at two dosages, 0.1 (referred to as GB/10) and 1.0 mg (referred to as GB), released from polyethylene vials, described below, that were attached to yellow sticky traps. The attractiveness of d-limonene and MNMA alone was also evaluated. In all cases, a 100 µL total of corresponding dilution was loaded into vials and dicloromethane (DCM) was used as solvent. Pure compounds were obtained from Sigma-Aldrich y TCI Chemicals America. Purity of compounds used were as follows: d-limonene 97%, MNMA > 95%, β-ocimene ≥ 90% (mixture of isomers), β-elemene ≥ 98% and β-caryophyllene ≥ 80%. Actual amounts of each compound loaded into release vials and all treatments evaluated are summarized in Table 1.
To create a positive control treatment for behavioral evaluation, VOCs were obtained from sweet orange over a 48-h collection period according to the methods described above. The volatile lure consisted of a solution obtained by eluting the adsorbent columns with 1 mL of DCM. Sweet orange was used for this purpose because the seedlings were available at the time when behavioral assays were being conducted. DCM alone was used as a solvent control.
Two-choices cage experiment
To allow air flow inside the experimental arena, white-mesh cages (90 × 60 × 55 cm) were used. At each opposite side of length of the cages, 8 × 8 cm yellow sticky traps were placed, one containing 100 µL of the stimulus solution (Table 1), and the other 100 µL of DCM only. Volatiles were released from polyethylene vials (BEEM 1001 Capsules for Embedding, Size 00). The loading of 100 µL on the vials was established according to the loading of stimulus applied in olfactometry experiments in the authors’ previous research (Aksenov et al. 2014; Martini et al. 2014b). Forty adult D. citri were released in the middle of each cage and 24 h later, the number of adults captured on each trap was recorded. Cages were distributed randomly in a glass greenhouse (8.0 × 9.60 × 6.30 m aprox.) used for plant production, mostly citrus seedlings culture, and one treatment was assigned to each cage. A randomized complete block design was used, where each treatment was evaluated once per date, and the whole experiment was repeated 10 times on consecutive days with each date considered as a block. For each replicate, treatments were randomly assigned to different positions inside the cages to avoid positional bias. All tests were conducted at 35 ± 6 °C, 69 ± 21% RH, and a 12:12 L:D photoperiod.
Statistical analysis
The pooled number of psyllids caught on each trap during the 10 replicates of the choice tests were analyzed with Chi square tests for within-group comparisons. Beforehand, we carried out heterogeneity chi-squared tests to ensure that data from each replicate were homogenous (Zar 2009). In the case of non homogenous data (d-limonene), paired t tests with square root transformed data were performed. The counts of insects per trap, considering the total catches per replicate, were subjected to a generalized linear model (GLM) with binomial distribution and treatment means were compared using Tukey’s test at an α = 0.05. Relative amounts of monoterpenes, sesquiterpenes and oxygenated monoterpenes (compound families) between sweet orange and Duncan grapefruit were compared by analysis of variance (ANOVA) followed by Tukey’s test at an α = 0.05. Tests were run with R statistical software (0.99.892 version – © 2009–2016 RStudio, Inc.) (RStudioTeam 2015) and Infostat statistical software (Di Rienzo et al. 2011).
Results
Volatile collection and analysis
Profiles of leaf VOCs from the two genotypes exhibited quantitative and qualitative differences (Fig. 1; Table 2). There were 28 compounds in the profiles of sweet orange and 9 in Duncan grapefruit (Fig. 1; Table 2). VOCs from both genotypes were primary monoterpene hydrocarbons (58 ± 6 and 76 ± 5% in sweet orange and Duncan grapefruit, respectively). Among these monoterpenes, limonene was the most abundant one in both species (41 ± 10% and 67 ± 6%, respectively; Table 2). Furthermore, high relative amounts of methyl N-methylanthranilate were found in both genotypes (33 ± 5% in sweet orange and 21 ± 5% in Duncan grapefruit). Oxygenated monoterpenes (present only in sweet orange, 1.5 ± 0.4%) and sesquiterpene hydrocarbons were also detected in lower proportions (1.8 ± 0.4% in sweet orange, 3 ± 1% in Duncan grapefruit). No significant differences in relative amounts of the above-mentioned compound families were found between genotypes (ANOVA, Tukey test p > 0.05, Fig. 1). Miscellaneous compounds found in lower abundance (aldehyde, ketone, aromatic, hydrocarbons, and unknown compounds) were more prevalent in sweet orange (5 ± 1%) than in Duncan grapefruit (0.6 ± 0.4%).
Relative percentage of compounds grouped by families. Results are shown as media ± standard error. MT monoterpene hydrocarbons, MA methyl anthranilates, ST sesquiterpene hydrocarbons, oxyg MT oxygenated monoterpenes. The remaining percentage is accounted by other compounds belonging to other chemical classes (see Table 1). ns not significant differences between both genotypes (ANOVA, p > 0.05 in all cases; oxyg MT were not detected in Duncan grapefruit)
Cage experiments
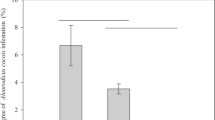
Significantly more D. citri were captured on traps baited with 0.1 mg of the synthetic grapefruit blend than on traps with the solvent control (χ2 = 10.45, d.f. = 1, p = 0.001) (Fig. 2). Catch of D. citri were increased by two-fold on traps baited with this dosage of the synthetic grapefruit blend as compared with the control. Similarly, significantly more D. citri were captured on traps baited with natural VOCs from sweet orange than on the solvent control (χ2 = 5.76, d.f. = 1, p = 0.016); representing a 1.5-fold increase in capture relative to the control (Fig. 2). d-Limonene and MNMA stimuli did not affect the catches of D. citri relative to solvent baited control traps (paired t test, p = 0.472 and: χ2 = 0.94, d.f. = 1, p = 0.334, respectively).
Diaphorina citri catches (%) in choice experiments. White bars indicate the percentage of D. citri caught on control traps. Gray bars indicate percentage of D. citri caught on traps baited with the indicated stimulus. Lines outside the bars show the standard deviation. Asterisks (*) outside bars indicate differences between treatment and control (χ2 test, *p < 0.05, **p < 0.01). GB indicates grapefruit blend at 1.0 mg dosage. GB/10 indicates grapefruit blend at 0.1 mg dosage. MNMA is methyl N-methyl anthranilate (mg applied are detailed in Table 1). The positive control is a natural volatile mixture collected from Citrus sinensis
Catch of D. citri was significantly lower on traps baited with the synthetic grapefruit blend at the 1.0 mg dosage as compared with solvent baited controls (χ2 = 10.33, d.f. = 1, p = 0.001), indicating this volatile blend was repellent at the higher dosage. Captures of D. citri on traps baited with the synthetic grapefruit blend at the 0.1 mg loading dosage and traps baited with the natural sweet orange odor (positive control) were not statistically different from one another, but both were higher than that captured on the GB (Fig. 3, GLM, p < 0.05).
Comparison of D. citri response to synthetic volatile odors. Results are shown as percentage of insects caught after 24 h on baited traps. Boxes show the 1st and 3rd quartiles; vertical lines indicate maximum and minimum. Medians with the same letter indicate no significant differences (GLM, Tukey–Kramer p > 0.05). GB indicates grapefruit blend at 1.0 mg dosage. GB/10 indicates grapefruit blend at 0.1 mg dosage. The positive control is a natural volatile mixture collected from Citrus sinensis. Gray area indicates response to cue(s) causing no effect on D. citri behavior (χ2 test, p > 0.05)
Discussion
The profile of citrus VOCs has been investigated extensively and reported to contain a mixture of monoterpene and sesquiterpene hydrocarbons and oxygenated monoterpenes (Beloti et al. 2017; Killiny and Jones 2017; Patt and Setaḿou 2010; Robbins et al. 2012). Our results were consistent with previous reports using similar in vivo methodologies of VOCs collection (Patt and Setaḿou 2010; Robbins et al. 2012) and indicated high proportions of monoterpene hydrocarbons (Fig. 1) in both genotypes. Our results also indicated that limonene was the most abundant compound in both profiles (Table 2). This compound has also been described as a predominant citrus leaf volatile by other authors (Killiny and Jones 2017; Mann et al. 2012; Robbins et al. 2012). An important difference in our investigation was the relatively high amount of methyl N-methylanthranilate found in both species as compared with previous studies. This compound has been reported in essential oils from sour orange (Citrus aurantium L.) (Aragão da Hora Almeida et al. 2015) and leaf volatiles analyzed through solvent extraction (Gancel et al. 2003) but not, to our knowledge, as a citrus leaf volatile from Duncan grapefruit or sweet orange obtained from in vivo collections (Beloti et al. 2017; Killiny and Jones 2017; Patt and Setaḿou 2010; Robbins et al. 2012). Both Duncan grapefruit and sweet orange VOCs profiles reported here were similar with respect to the families of compounds characterizing both profiles (Fig. 1). However, differences were found with respect to complexity of each profile and certain specific compounds that distinguished the two profiles. Despite these differences, the synthetic blend mimicking Duncan grapefruit profile (GB/10) and the natural sweet orange VOCs showed similar attractiveness to D. citri in cage experiments (Fig. 3). Indeed in PCA analysis of VOCs from multiple genotypes, these two species have been grouped together (Hijaz et al. 2016).
When evaluated at the lower 0.1 mg dosage, the synthetic volatile blend from Duncan grapefruit, GB/10, was roughly equivalent to a natural volatile collection from sweet orange (the positive control, Fig. 3) with regard to enhancing catch of D. citri on yellow monitoring traps. However, there was substantial variance in the results obtained, which can be visualized in the dispersion of the data represented in the boxplots in Fig. 3. The variation in D. citri response to the synthetic volatile blend as compared with the natural sweet orange odor positive control suggests possible hypotheses for future investigation.
First, the VOC profiles of the host-plant odors are characterized by likely important complexity, which may need to be retained in a synthetic volatile employed as an effective lure for D. citri monitoring. Sweet orange VOCs (the positive control), which maintain the complexity and the proportions of odors that D. citri encounters in its natural environment, showed a more consistent effect (never less than 50% of insects on treated trap) than the low dosage synthetic grapefruit blend (which has a minimum of 20% of catches on treated trap). Also, it is interesting that the VOC profiles of the two preferred genotypes investigated here (Alves et al. 2014; Liu and Tsai 2000; Sétamou et al. 2008) were characterized by relatively high proportions of d-limonene and MNMA and yet, at the evaluated doses, neither of the individual compounds increased attraction of D. citri to yellow sticky traps. Perhaps, this absence of effect on the behavioral preference of D. citri is indicative of the requirement of integrating a complex blend to elicit a behavioral response to host-plant odor from D. citri. It is also possible that some of the volatile and non-volatile compounds characterizing host-plant chemistry of D. citri interact additively or synergistically when perceived as blends [reviewed by Bruce and Pickett (2011)] by foraging psyllids as has been reported previously with D. citri (Patt and Setaḿou 2010). Collectively, our current data and results from previous work (Patt and Setaḿou 2010) strongly suggest that D. citri response toward host volatiles is mediated by odor blends rather than individual components comprising those blends.
In addition to blend complexity, the release rate of the identified grapefruit volatile blend appeared to significantly influence catch of D. citri on monitoring traps. The effect of the synthetic grapefruit volatile blend on captures of D. citri exhibited a typical biphasic response attracting psyllids at a lower (presumably more natural) release rate of odors (0.1 mg loading dosage), but repelling them at a higher dosage that was likely above a threshold for attraction (1.0 mg loading dosage) (Fig. 2). Similar findings with D. citri have been described previously. For example, Mann et al. (2012) found differential response of D. citri to varying release rates of d-limonene in olfactometer tests with attraction increasing in a dosage dependent manner. In our experiment, although d-limonene caught 1.4 times more D. citri than the respective untreated trap (Fig. 2), the variability in response among replicates prevented resolution of a statistical difference from the control. In addition, Martini et al. (2016) demonstrated that pre-exposing D. citri to unnaturally high concentrations of certain plant volatiles alters their subsequent ability of to select hosts based on odor preference. Finally, β-caryophyllene has been recently reported as a D. citri repellent at high doses (Alquézar et al. 2017); this could explain the repellent activity of the high dosage (1 mg) synthetic grapefruit blend, which included 2% of β-caryophyllene. In general terms, repellency of known insect attractants at abnormally high release doses is a well-known phenomenon (Dethier 1947). Therefore, optimization of the release rate of a semiochemical attractant lure for D. citri will likely be of significant importance and deserves further attention.
Our results reflect the complexity of D. citri behavior and in particular, the difficulty of manipulating psyllid behavior with volatile semiochemicals. The poor and variable response of D. citri to odors has been commonly observed in olfactometry (Alquézar et al. 2017), semi-field (Patt and Setaḿou 2010) and field trials of scented traps with both candidate attractants (Godfrey et al. 2013) and repellents (Kuhns et al. 2016). The weak response to natural VOCs has also been described for other psyllid species (Steinbauer 2016). In fact, to our knowledge, no efficient practical applications of D. citri chemical ecology research have been incorporated to management strategies yet, despite significant research effort (Grafton-Cardwell et al. 2013; Yan et al. 2015). The relative simplicity of D. citri antennae (Onagbola et al. 2009), may be suggestive that this species relies on other senses rather than olfaction when selecting host plants for nutrition, mate finding, and oviposition. Indeed, visual cues, which may be integrated with semiochemical cues for D. citri management, are known to strongly affect orientation behavior of D. citri (Patt et al. 2011; Stockton et al. 2016). Recent research on the psyllid species Cacopsylla pruni, proved that sap composition is a crucial factor explaining host-plant alternation of this insect (Gallinger and Gross 2018). This is another example of the importance of non-volatile plant compounds on host acceptance of psyllids. In this respect, the combined use of both visual and chemical attractants and repellents, and in the context of a push and pull strategy where a trap crop may provide a necessarily complete set of host selection cues, may be necessary for behavioral modification of this species. In fact, current research efforts in D. citri chemical ecology are trending toward these kinds of strategies, where release of selected behavior modifying semiochemicals for D. citri is up-regulated from natural plants (Alquézar et al. 2017).
References
Adams R (2007) Identification of essential oils components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream
Aksenov A, Martini X, Zhao W, Stelinski LL, Davis CE (2014) Synthetic blends of volatile, phytopathogen-induced odorants can be used to manipulate vector behavior. Front Ecol Evol. https://doi.org/10.3389/fevo.2014.00078
Almeida LA, Santos JZ, Soares-Filho WD, Bizzo HR, Silva JP, Vieira RF (2015) Chemical characterization of leaf essential oil from seven accessions of sour orange (Citrus aurantium L.). J Essent Oil Bear Plants 18:426–435
Alquézar B et al (2017) β-Caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci Rep 7:5639. https://doi.org/10.1038/s41598-017-06119-w
Alves GR, Diniz AJF, Parra JRP (2014) Biology of the huanglongbing vector Diaphorina citri (Hemiptera:Liviidae) on different host plants. J Econ Entomol 107:691–696
Beloti VH, Santos F, Alves GR, Bento JMS, Yamamoto PT (2017) Curry leaf smells better than citrus to females of Diaphorina citri (Hemiptera: Liviidae). Arthropod. https://doi.org/10.1007/s11829-017-9524-6
Boina DR, Bloomquist JR (2015) Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag Sci 71:808–823
Bové JM (2006) Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88:7–37
Bruce TJ, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72:1605–1611
Chen XD, Gill TA, Pelz-Stelinski KS, Stelinski LL (2017) Risk assessment of various insecticides used for management of Asian citrus psyllid, Diaphorina citri in Florida citrus, against honey bee, Apis mellifera. Ecotoxicology 26:351–359
Coutinho-Abreu IV, Forster L, Guda T, Ray A (2014) Odorants for surveillance and control of the Asian citrus psyllid (Diaphorina citri). PLoS ONE 9:e109236
Dethier VG (1947) Chemical insect attractants and repellents. Blakiston, Philadelphia
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2011) InfoStat. Córdoba, Argentina Patent
Gallinger J, Gross JU (2018) Unraveling the host plant alternation of Cacopsylla pruni—adults but not nymphs can survive on conifers due to phloem/xylem composition. Front Plant Sci 9:484. https://doi.org/10.3389/fpls.2018.00484
Gancel AL, Ollitrault P, Froelicher Y, Tomi F, Jacquemond C, Luro F, Brillouet JM (2003) Leaf volatile compounds of seven citrus somatic tetraploid hybrids sharing willow leaf mandarin (Citrus deliciosa Ten.) as their common parent. J Agric Food Chem 51:6006–6013
Godfrey KE, Galindo C, Patt JM, Luque-Williams M (2013) Evaluation of color and scent attractants used to trap and detect Asian citrus psyllid (Hemiptera: Liviidae) in urban environments. Fla Entomol 96:1406–1416
Grafton-Cardwell EE, Stelinski LL, Stansly PA (2013) Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu Rev Entomol 58:413–432
Hijaz F, Nehela Y, Killiny N (2016) Possible role of plant volatiles in tolerance against Huanglongbing in citrus. Plant Signal Behav. https://doi.org/10.1080/15592324.2016.1138193
Killiny N, Jones SE (2017) Profiling of volatile organic compounds released from individual intact juvenile and mature citrus leaves. J Plant Physiol 208:47–51
Kuhns EH, Hoyte XM, Stelinski A LL (2016) Repellent activity of botanical oils against asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects. https://doi.org/10.3390/insects7030035
Liu YH, Tsai JH (2000) Effects of temperature and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae. Ann Appl Biol 137:201–206
Mann RS, Rouseff RL, Smoot JM, Castle WS, Stelinski LL (2011) Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bull Entomol Res 101:89–97. https://doi.org/10.1017/s0007485310000222
Mann RS, Ali JG, Hermann SL, Tiwari S, Pelz-Stelinski KS, Alborn HT, Stelinski LL (2012) Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog 8:e1002610. https://doi.org/10.1371/journal.ppat.1002610
Martini X, Kuhns EH, Hoyte A, Stelinski L (2014a) Plant volatiles and density-dependent conspecific odors are used by asian citrus psyllid to evaluate host suitability on a spatial scale. Arthropod 8:453–460
Martini X, Pelz-Stelinski KS, Stelinski LL (2014b) Plant pathogen-induced volatiles attract parasitoids to increase parasitism of an insect vector. Front Ecol Evol. https://doi.org/10.3389/fevo.2014.00008
Martini X, Willett DS, Kuhns EH, Stelinski LL (2016) Disruption of vector host preference with plant volatiles may reduce spread of insect-transmitted plant pathogens. J Chem Ecol 42:357–367
Miranda MP, dos Santos FL, Bassanezi RB, Montesino LH, Barbosa JC, Sétamou M (2018) Monitoring methods for Diaphorina citri Kuwayama (Hemiptera: Liviidae) on citrus groves with different insecticide application programmes. J Appl Entomol 142:89–96
NIST Mass Spec Data Center (2017) Mass spectra. In: Linstrom PJ, Mallard WG (eds) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg
Onagbola EO, Boina DR, Hermann SL, Stelinski LL (2009) Antennal sensilla of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of Diaphorina citri (Hemiptera: Psyllidae. Ann Entomol Soc Am 102:523–531
Patt JM, Setaḿou M (2010) Responses of the asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ Entomol 39:618–624
Patt JM et al (2011) Multimodal cues drive host-plant assessment in Asian Citrus Psyllid (Diaphorina citri). Environ Entomol 40:1494–1502
Qureshi JA, Stansly PA (2010) Dormant season foliar sprays of broad-spectrum insecticides: an effective component of integrated management for Diaphorina citri (Hemiptera: Psyllidae) in citrus orchards. Crop Protection 29:860–866. https://doi.org/10.1016/j.cropro.2010.04.013
Robbins PS, Stelinski ART, Lapointe LL SL (2012) Volatile profiles of young leaves of rutaceae varying the suceptibility to the Asian citrus psyllid (Hemiptera: Psyllidae. Fla Entomol 95:774–776
RStudioTeam (2015) RStudio: integrated development for R. RStudio. Boston, MA Patent
Sétamou M, Flores D, French JV, Hall DG (2008) Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J Econ Entomol 101:1478–1487
Sétamou M, Sanchez A, Patt JM, Nelson S, Jifon J, Louzada E (2012) Diurnal patterns of flight activity and effects of light on host finding behavior of the Asian citrus psyllid. J Insect Behav 25:264–276
Steinbauer MJ (2016) Effects of eucalypt nutritional quality on the bog gum-victorian metapopulation of Ctenarytaina bipartita and implications for host and range expansion. Ecol Entomol 41:211–225
Stockton DG, Martini X, Patt JP, Stelinski LL (2016) The influence of learning on host plant preference in a significant phytopathogen vector, Diaphorina citri. PLoS ONE 11:e0149815
Wenninger EJ, Stelinski LL, Hall DG (2009) Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ Entomol 38:225–234
Yan H, Jiwu Zeng J, Zhonga G (2015) The push–pull strategy for citrus psyllid control. Pest Manag Sci 71:893–896
Zar JH (2009) Biostatisical analysis, 5th edn. Prentice Hall, Upper Saddle River
Acknowledgements
The authors would like to thank the financial supporting agencies: CISC (Comisión Sectorial de Investigación Científica from Universidad de la República) - Grant CSIC-Grupos, INIA (Instituto Nacional de Investigación Agropecuaria); ANII - Graduate scholarship and travel grant for María Eugenia Amorós, and PEDECIBA (Programa para el desarrollo de las Ciencias Básicas, Uruguay). Also thanks to Verónica Galván, Abel Rodríguez and Andrés González for the collaboration with this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Anna-Karin Borg-Karlson.
Rights and permissions
About this article
Cite this article
Amorós, M.E., Pereira das Neves, V., Rivas, F. et al. Response of Diaphorina citri (Hemiptera: Liviidae) to volatiles characteristic of preferred citrus hosts. Arthropod-Plant Interactions 13, 367–374 (2019). https://doi.org/10.1007/s11829-018-9651-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-018-9651-8