Abstract
Biological control agents can be used as a complementary control measure that can be combined with resistant host plants to control pests. In this study, the effects of different canola cultivars (Karaj-1, Karaj-2, Karaj-3, Licord, Okapi, Opera, RGS003, Sarigol, Talaye and Zarfam) on the performance and life table parameters of the cabbage aphid, Brevicoryne brassicae, and its parasitoid, Diaeretiella rapae, were determined under laboratory conditions. Total fecundity of the cabbage aphid differed with cultivar, with the highest value (59.41 nymphs per female) of this parameter observed on Opera and the lowest (1.67) observed on RGS003. The highest and lowest intrinsic rates of increase (r) of the cabbage aphid were observed on Opera (0.331 day−1) and RGS003 (− 0.242 day−1) cultivars, respectively, suggesting these to be the most susceptible and most resistant cultivars to this pest. However, because the aphid did not settle and feed well on RGS003, it was not possible to determine demographic parameters for its parasitoid. Consequently, the Okapi cultivar, which was the most resistant cultivar to the cabbage aphid after RGS003, was used in this study to assess the parasitoid wasp. The parasitoid’s intrinsic rate of increase (r) varied from 0.426 day−1 on the susceptible cultivar (Opera) to 0.341 day−1 on the resistant canola cultivar Okapi. Aphid performance decreased 93% on the resistant canola cultivar, while parasitoid performance decreased only 20% on the resistant cultivar compared to more susceptible cultivar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cabbage aphid, Brevicoryne brassicae (Hemiptera: Aphididae) is one of the most serious pests of cruciferous plants such as canola, Brassica napus L., worldwide. This pest causes both direct damage by sucking plant sap and indirect damage by transmitting plant viruses (Blackman and Eastop 2000; Schliephake et al. 2000). Aphid infestations retard plant growth, resulting in canola grain yield losses of 9–77% (Butin and Raymer 1994; Ellis et al. 1996). Aphid infestations can cause up to an 11% decline in the oil content of the oilseed plants that survive (Kelm and Gadomski 1995). Until recently, pesticide application has been the primary method of controlling aphid populations, although such widespread use has adverse effects on the environment and nontarget organisms (Furk and Hines 1993; Saldo and Szpyrka 2009). The development of insecticide resistance by B. brassicae has been reported in Kenya (Oduor et al. 1997), and adverse effects of such pesticides on natural enemies highlights the need for less insecticide-dependent methods of pest control (Verkerk et al. 1998). Canola integrated pest management (IPM) programs sometimes make use of resistant host plant varieties, combining them with biological control agents of different pests (Stern et al. 1959; Maxwell and Jennings 1931; Soufbaf et al. 2012; Nikooei et al. 2015a, b, 2017; Fathipour and Mirhosseini 2017). However, basic research exploring the complex interactions among plants, herbivorous insects and their natural enemies are not always available (Sarfraz et al. 2008). One of the cabbage aphid’s biological control agents is Diaeretiella rapae (Hymenoptera: Braconidae, Aphidiinae), which is perhaps the most important agent for natural control of the cabbage aphid (Elliot et al. 1994; Pike et al. 1999; Jankowska and Wiech 2003). This wasp is found in most regions of the world and is the most common natural enemy of the cabbage aphid. Here, we evaluated the influence of different canola cultivars on biological parameters of the cabbage aphid under laboratory conditions to determine the most resistant and susceptible canola cultivars to this aphid. We also determined the impact of the level of resistance of these cultivars on the performance of D. rapae to assess the total impact of host plant resistance on aphid population growth.
Materials and methods
Plant cultivation
Seeds of canola including two genotypes, Karaj-1 and Karaj-2, and eight commercial cultivars extensively used by Iranian canola growers, Karaj-3, Licord, Sarigol, Talay, Zarfam, RGS003 (a spring cultivar with an average seed yield 2000–2500 kg/ha that is cultivated in hot regions of Iran), Okapi and Opera (winter cultivars with an average seed yield 4000–4500 kg/ha that are cultivated in cold regions of Iran), were obtained from the Seed and Plant Improvement Research Institute in Karaj, Iran. They were planted in 20-cm-diameter plastic pots filled with a mixture of field soil and compost in a greenhouse located at the Faculty of Agriculture, Tarbiat Modares University, Tehran. All cultivars were grown under greenhouse conditions at 25 ± 5 °C, 60 ± 10% RH and a 16:8-h L:D photoperiod without any fertilizer or pesticides. Fully expanded young leaves of canola cultivars were used for the experiments when plants were 5 weeks old and still did not have flowers.
Insect culture
The cabbage aphid and its parasitoid wasp D. rapae used in the experiments were collected from a cabbage field on the campus of the Faculty of Agriculture, Tarbiat Modares University, Tehran. The initial colony of cabbage aphids was reared on cabbage (Snow-March cultivar) under laboratory conditions to keep aphids from adapting to canola. All experiments and rearing of the aphid and parasitoid were conducted in a growth chamber at 25 ± 1 °C, 60 ± 5% RH and a 16:8-h L:D photoperiod. The aphid populations were reared on the leaves of ten canola cultivars separately for two generations to adapt to the new host plants before use in the experiments. The laboratory colony of the parasitoid was reared on cabbage aphid feeding on cabbage, and before the experiments, parasitoids were reared for one generation on cabbage aphid fed on the respective canola cultivars for their treatments.
Experimental design
Leaf discs (2.80 cm diameter) were taken from leaves of each cultivar and placed upside down on water-soaked cotton inside plastic petri dishes (6 cm diameter and 1.5 cm height). To prevent the escape of aphids, the leaf margin was surrounded by a Kleenex strip. A circular hole (2 cm diameter) was cut in the lid of each petri dish and covered with fine mesh gauze for ventilation. To obtain similarly aged cabbage aphid nymphs, 50 adult aphids were transferred from the colony of each cultivar onto a leaf disc of the same cultivar. After 24 h, each nymph laid by these adults was transferred on one leaf disc in each petri dish and a total of 70 nymphs were examined for each treatment. To keep the leaves fresh and to maintain adequate saturation of the cotton strips, water was added daily to each dish, and leaf discs were also exchanged for new ones daily. The duration and mortality of immature stages were observed and recorded daily using a stereomicroscope. After adult emergence, daily observations were made of aphid longevity, fecundity and mortality until the death of the last individual in each group. To determine the demographic parameters of D. rapae, transparent plastic containers (5 × 12 × 14 cm) with a hole (5 cm diameter) on their lids covered with fine mesh gauze were used to hold complete leaves of a canola cultivar infested with 100 2nd to 4th instar cabbage aphid nymphs. A piece of water-soaked cotton was wrapped around the petioles of detached leaves to prevent desiccation, and the leaves were changed daily with new ones. To obtain adult parasitoids to start the experiments, one mated female of D. rapae was released separately into each container. After 24 h, female wasps were removed, and the exposed aphids were transferred into a growth chamber until all parasitized aphids mummified and became apparent; from these mummies, 100 parasitized aphids were randomly selected as a cohort for each canola cultivar. Mummified aphids were checked daily until adult parasitoid emergence, after which females were coupled with males obtained from the same cultivars. The leaves of each canola cultivar infested with 100 individuals of the preferred instars (2nd to 4th instars) of the cabbage aphid were exposed to each pair of D. rapae for 24 h. It should be noted that although the main purpose of the current study is to evaluate and compare the effect of host quality on parasitoid performance, to minimize the effect of host density on parasitoid performance, to some extent controlling the number of leaves and consequently aphid density was attempted. Parasitoids were transferred into a new experimental unit each day until the death of all the female parasitoids, and exposed nymphs were maintained in the growth chamber. A paper strip coated with a thin layer of honey was placed in each experimental unit as food for the adult parasitoids. These experimental units were checked daily using a stereomicroscope, and the time period from oviposition to mummy formation and from mummy formation to adult emergence was recorded, along with adult mortality and the number of eggs deposited by females.
Data analysis
The life history data of all individuals were analyzed using the age-stage, two-sex life table theory (Chi and Liu 1985; Chi 1988). The age-stage specific survival rate (s xj ) (where x = age in days and j = stage), age-stage specific fecundity (f xj ), age-specific survival rate (l x ), age-specific fecundity (m x ), intrinsic rate of increase (r), finite rate of increase (λ = e r), gross reproductive rate (GRR), net reproductive rate (R 0) and mean generation time (T) were all calculated (Khanamani et al. 2013; Safuraie-Parizi et al. 2014; Goodarzi et al. 2015). The equations of the two-sex life table and the differences from the female-based life table are found in Fathipour and Maleknia (2016).The standard errors of the population parameters were estimated by a bootstrap procedure (Efron and Tibshirani 1993; Huang and Chi 2013) with 40,000 samples.
Population parameters were calculated using the TWOSEX-MSChart program (Chi 2015). Comparison of the durations of different life stages of B. brassicae on the ten canola cultivars and D. rapae on one susceptible and one resistant canola cultivar was made using one-way ANOVA and t tests, respectively. Multiple comparisons among population growth parameters of different treatments were carried out using the paired bootstrap test (Maleknia et al. 2016; Talaee et al. 2017).
To calculate the reduction percentages of the aphid and parasitoid performance on the susceptible and resistant canola cultivars, the intrinsic rate of increase (r) of the aphid and parasitoid on these cultivars was used. Of course, since the most resistant cultivar for the aphid (RGS003) was not used for assessment of the parasitoid performance, the second most resistant cultivar was used to calculate both reduction percentages. Percentages were calculated as follows:
Results
Effect of canola cultivars on aphid life stage duration
The canola cultivar significantly influenced the duration of different life stages of B. brassicae (Table 1). We found the longest first and third nymphal instar periods to be on Sarigol and the shortest on Karaj-3 and Opera, respectively. Nymphs reared on Karaj-3 and RGS003 had the longest second nymphal instar period while those reared on Opera had the shortest. The longest fourth nymphal instar period was in aphids reared on RGS003 while the shortest was on Opera. Developmental time of the whole immature stage was also affected by different canola cultivars, with the longest observed on RGS003 and the shortest on Opera.
Adult longevity and total aphid life span also differed among cultivars, with those reared on Opera having the longest adult and total life span, while those reared on RGS003 and Okapi had the shortest adult and total life spans, respectively. Both reproductive period and fecundity of B. brassicae were significantly influenced by different canola cultivars (Table 2). The longest adult pre-reproductive period (APOP) (the duration from adult emergence to first nymphal deposition of B. brassicae) was on RGS003 while the shortest was on Licord. Furthermore, the total pre-reproductive period (TPOP) (the duration from first instar nymphs to first nymphal deposition) was significantly different among canola cultivars, with the longest period found in aphids reared on RGS003 and the shortest found on Opera. The reproductive period of the cabbage aphid was also significantly affected by canola cultivar, with the longest and shortest periods observed on Opera and RGS003 cultivars, respectively.
Aphid survival rate and fecundity
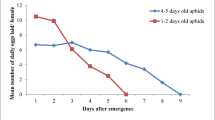
We found that the canola variety affected the ability of B. brassicae to survive and reproduce under laboratory conditions (Figs. 1, 2). Aphid reproduction of the first female on Opera (a susceptible variety) occurred at 5 days, while on the more resistant varieties on RGS003 and Talaye reproduction did not begin before 8 days. Aphid reproduction was highest (7.0 nymphs per day) on the susceptible variety Opera, while it was lowest (1.40) on the resistant variety RGS003. Total fecundity of the cabbage aphid also differed with cultivar, with the highest value (59.41 nymphs per female) of this parameter observed on Opera and the lowest (1.67) observed on RGS003.
Aphid population growth parameters
Population growth parameters of B. brassicae were significantly affected by different canola cultivars under laboratory conditions (Table 3). The values of the intrinsic rate of increase (r), the finite rate of increase (λ), gross reproductive rate (GRR) and net reproductive rate (R 0) of B. brassicae were highest on the cultivar Opera and lowest on RGS003. The longest and shortest mean generation times (T) were found on Sarigol and Okapi, respectively.
Overall, Opera and RGS003 were found to be the most susceptible and resistant cultivars to the cabbage aphid, respectively. However, the low population of aphids that survived on RGS003 made determining the demographic parameters of the aphid’s parasitoid on this cultivar impossible. Therefore, the next most resistance cultivar, Okapi, was used to assess in its place.
Duration of parasitoid life stages
The duration of different parasitoid life stages differed among cultivars, except for the pupal period, total longevity of adult females and total life span of both sexes, which did not differ (Table 4). Duration of the egg-larval stage was significantly longer on the aphid-resistant cultivar than on the susceptible one. The shortest development time of the immature stages of the parasitoid was also observed on the susceptible cultivar. Cultivar also had a significant effect on the longevity of adult male parasitoids, which was longest on the susceptible cultivar.
Since female parasitoids laid their eggs on the first day of after mating, the duration of the adult pre-oviposition period (APOP) was considered zero on all cultivars. The total pre-oviposition period (TPOP) was significantly influenced by cultivar, however, and this period was significantly shorter on susceptible cultivar than on resistant one. A longer parasitoid oviposition period and higher total fecundity were also observed on the susceptible cultivar.
Parasitoid survival rate, age-specific and age-stage-specific fecundity
Age-specific survivorship (l x ) (the probability that a newborn individual will survive to age x) at the time of adult emergence of the parasitoid wasp was 1 on the susceptible and 0.97 on the resistant cultivar (Figs. 3, 4). According to the results of age-specific fecundity (m x ), the peaks of progeny production (highest daily fecundity) were 32.87 (at the age of 9 days) and 19.2 (10 days) on B. brassicae reared on the susceptible and resistant cultivars, respectively. The age-stage-specific fecundity (f xj ) curve showed the start of oviposition on susceptible and resistant canola cultivars occurred at 8 and 10 days, respectively. Furthermore, the highest daily fecundity of the parasitoid wasp on the above-mentioned cultivars was 80 and 38.4 eggs, respectively, which occurred at 8 and 10 days, respectively.
Parasitoid population growth parameters
All of the population growth parameters of the parasitoid wasp, except the gross reproductive rate (GRR), were significantly influenced by the canola cultivar (Table 5). The intrinsic rate of increase (r), finite rate of increase (λ) and net reproductive rate (R 0) of the parasitoid wasp were significantly higher on the susceptible cultivar than on the resistant one. The mean generation time (T) was also found to be longer on the resistant than on the susceptible cultivar.
Discussion
We found significant differences among canola cultivars in both cabbage aphid performance and, for two cultivars (most susceptible and second-most resistance), the biological parameters of its parasitoid D. rapae. The developmental time of B. brassicae was longer on some cultivars, such as RGS003, than on others, with the effect that different life stages of the aphid, especially nymphal stages, have longer exposure to parasitoids such as D. rapae. In this way, resistant cultivars can enhance the effectiveness of parasitoids in a pest management program. However, this scenario can also be interpreted in other ways as the lower quality of resistant cultivars may decrease the tendency of parasitoids to visit the plants. The considerable variation among the cultivars we tested in their effect on the duration of different life stages of the aphid might be due to differences in plant quality, in terms of either the nutrients required by B. brassicae or the levels of secondary metabolites that impede aphid growth (Jahan et al. 2014; Bashir et al. 2013; Mirmohammadi et al. 2009).
The intrinsic rate of increase (r) that can be estimated using female-based or two-sex procedures (Fathipour and Maleknia 2016) is the most important life table parameter for evaluating the resistance level of plants to herbivorous arthropods (Safuraie-Parizi et al. 2014). The r value is usually affected by several factors, including fecundity, survival rate and development, and any changes in these factors result in variation in the value of r as an index of population growth (Khanamani et al. 2014). In the present study, the highest and lowest value of r of the cabbage aphid was obtained on Opera and RGS003, respectively. The higher reproductive potential of the aphid on Opera compared with other cultivars tested showed it to be the most suitable cultivar for B. brassicae. On the other hand, RGS003 showed the highest antibiosis resistance against the cabbage aphid, and, due to the long development time, the low survival rate of immature stages as well as the very low fecundity of the aphid on this cultivar, it was recognized as the most unsuitable cultivar for this pest in this study, making it the recommended cultivar for any IPM strategy for controlling cabbage aphid. Such considerable variation among different canola cultivars has also been seen in the feeding response of Plutella xylostella (Soufbaf et al. 2010a, b; Fathi et al. 2011; Nikooei et al. 2015b; Fathipour and Mirhosseini 2017; Kianpour et al. unpublished data), Helicoverpa armigera (Karimi et al. 2012) and Spodoptera exigua (Goodarzi et al. 2015). In agreement with our findings, Soufbaf et al. (2010a) reported Opera as the most favorable and RGS003 as the most unfavorable host plant for P. xylostella. On the other hand, Nikooei et al. (2015b) reported that these two cultivars, both with high levels of glucosinolate, were more suitable hosts for P. xylostella than other tested cultivars (mostly genetically manipulated). In another study, the RGS003 cultivar was reported to be the most resistant canola cultivar to P. xylostella (Kianpour et al. unpublished data). Finally, both cultivars were reported to be susceptible to H. armigera (Karimi et al. 2012) but unfavorable host plants for S. exigua (Goodarzi et al. 2015).
Glucosinolates are the most important secondary metabolites of cruciferous plants and an indicator of a plant’s chemical defense against higher trophic levels (Nikooei et al. 2015b). Various studies have found that different amounts of these compounds have different effects on the population growth, development time and survival rate of higher trophic levels. Although Nikooei et al. (2015b) suggested a similar level of glucosinolates in the most susceptible (Opera) and most resistant (RGS003) canola cultivars of our current study, the performance of the cabbage aphid on these two cultivars was quite different. This suggests that in our current study, the essential primary nutrients of plants were the important factors affecting the performance of the herbivorous insect and that glucosinolates, as one of secondary metabolites, had no role in the creation of such differences. Soufbaf et al. (2012) similarly found poor population growth and development of P. xylostella on RGS003 as a result of lower nitrogen in the leaves of this cultivar. However, further study is needed to examine the effects of each of these compounds on the performance of B. brassicae.
However, our results showed that plant cultivars significantly affected the performance of D. rapae. In agreement with our findings, Kalule and Wright (2002) also reported the plant cultivar is an important and influential factor affecting the performance of Aphidius colemani Viereck. Desneux and Ramirez-Romero (2009) also introduced D. rapae as a successful biological control agent against Myzus persicae (Sulzer) in winter canola fields because of the higher attraction of parasitoids to host-plant complex-related odor blends that in turn can depend on the plant variety (Kalule and Wright 2004). The R 0 value of the parasitoid in the current study was different from the value reported by Tazerouni et al. (2013) on Diuraphis noxia. This difference was probably a result of different food sources taken up by the parasitoid. The higher value of R 0 in the current study indicated that B. brassicae is a more suitable host than D. noxia for the parasitoid. This conclusion is supported by Kalule and Wright (2002) who found that the performance of this parasitoid differed with aphid host species and A. colemani performed better on M. persicae than on B. brassicae. Our results showed that adult females attack host nymphs during almost the entire duration of their longevity, but their fecundity declines with increasing age.
We found the performance of the parasitoid wasp D. rapae and of its herbivorous host B. brassicae to be profoundly affected by different canola cultivars. Cabbage aphid performance decreased 93% on the resistant canola cultivar compared with the susceptible one, while parasitoid performance was only reduced by 20% on the resistant cultivar compared with the susceptible one. This suggests that combining resistant canola cultivars with biological control from D. rapae would be a viable IPM strategy. A higher influence of the host plant on the herbivore compared with the parasitoid could be due to the herbivore being directly in connection with substances existing in the plant, while the parasitoid is indirectly affected by plant substances that pass through the body of the herbivore.
References
Bashir F, Azim MN, Akhter N, Muzaffar G (2013) Effect of texture/morphology of host plants on the biology of Brevicoryne brassicae (L.) (Homoptera: Aphididae). Int J Curr Res 2:178–180
Blackman RL, Eastop VP (2000) Aphids on the world crop pests. Wiley, London
Butin GD, Raymer PL (1994) Pest status of aphids and other insects in winter canola in Georgia. J Econ Entomol 87:1097–1104
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34
Chi H (2015) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/. Accessed 5 Oct 2015
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin 24:225–240
Desneux N, Ramirez-Romero R (2009) Plant characteristics mediated by growing conditions can impact parasitoid’s ability to attack host aphids in winter canola. J Pest Sci 82:335–342
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, New York
Elliot NC, Reed DK, French BW, Kindler SD (1994) Aphid host effects on the biology of Diaeretiella rapae. Southwest Entomol 19:279–283
Ellis PR, Singh R, Pink DAC, Lynn JR, Saw PL (1996) Resistance to Brevicoryne brassicae (L.) in horticultural brassica. Euphitica 88:85–96
Fathi SAA, Bozorg-Amirkalaee M, Sarfaraz RM (2011) Preference and performance of Plutella xylostella (L.) (Lepidoptera: Plutellidae) on canola cultivars. J Pest Sci 84:41–47
Fathipour Y, Maleknia B (2016) Mite predators. In: Omkar (ed) Ecofriendly pest management for food security. Elsevier, San Diego, pp 329–366
Fathipour Y, Mirhosseini MA (2017) Diamondback moth (Plutella xylostella) management. In: Reddy GVP (ed) Integrated management of insect pests on canola and Other Brassica oilseed crops. CABI, Croydon, pp 13–43
Furk C, Hines CM (1993) Aspects of insecticide resistance in the melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae). Ann Appl Biol 123:9–17
Goodarzi M, Fathipour Y, Talebi AA (2015) Antibiotic resistance of canola cultivars affecting demography of Spodoptera exigua (Lepidoptera: Noctuidae). J Agric Sci Technol 17:23–33
Huang YB, Chi H (2013) Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): with an invalidation of the jackknife technique. J Appl Entomol 137:327–339
Jahan F, Abbasipour H, Askarianzadeh AR, Hassanshahi GH, Saeedizadeh AA (2014) Biology and life table parameters of Brevicoryne brassicae (Hemiptera: Aphididae) on cauliflower cultivars. J Insect Sci 14(284):1–6
Jankowska B, Wiech K (2003) Occurrence of Diaeretiella rapae (M’Intosh) (Aphidiidae) in the cabbage aphid (Brevicoryne brassicae L.) colonies on the different crucifere crops. Sodininkystėirdaržininkystė 22:155–163
Kalule T, Wright DJ (2002) Effect of cabbage cultivars with varying levels of resistance to aphids on the performance of the parasitoid, Aphidius colemani (Hymenoptera: Braconidae). Bull Entomol Res 92:53–59
Kalule T, Wright DJ (2004) The influence of cultivar and cultivar-aphid odours on the olfactory response of the parasitoid Aphidius colemani. J Appl Entomol 128:120–125
Karimi S, Fathipour Y, Talebi AA, Naseri B (2012) Evaluation of canola cultivars for resistance to Helicoverpa armigera (Lepidoptera: Noctuidae) using demographic parameters. J Econ Entomol 105:2172–2179
Kelm M, Gadomski H (1995) Occurrence and harmfulness of the cabbage aphid, Brevicoryne brassicae (L.) on winter rape. Mater Ses Inst Ochr Rosl 5:101–103
Khanamani M, Fathipour Y, Hajiqanbar H (2013) Population growth response of Tetranychus urticae to eggplant quality: application of female age-specific and age-stage, two-sex life tables. Int J Acarol 39:638–648
Khanamani M, Fathipour Y, Hajiqanbar H, Sedaratian A (2014) Two-spotted spider mite reared on resistant eggplant affects consumption rate and life table parameters of its predator, Typhlodromus bagdasarjani (Acari: Phytoseiidae). Exp Appl Acarol 63:241–252
Maleknia B, Fathipour Y, Soufbaf M (2016) How greenhouse cucumber cultivars affect population growth and two-sex life table parameters of Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 42:70–78
Maxwell FG, Jennings PR (1931) Breeding plants resistant to insects. Wiley, Hoboken
Mirmohammadi SH, Allahyari H, Nematolahi MR, Saboori A (2009) Effect of host plant on biology and life table parameters of Brevicoryne brassicae (Hemiptera: Aphididae). Ann Entomol Soc Am 102:450–455
Nikooei M, Fathipour Y, Javaran MJ, Soufbaf M (2015a) How different genetically manipulated brassica genotypes affect life table parameters of Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 108:515–524
Nikooei M, Fathipour Y, Javaran MJ, Soufbaf M (2015b) Influence of genetically manipulated Brassica genotypes on parasitism capacity of Diadegma semiclausum parasitizing Plutella xylostella. J Agric Sci Technol 17:1743–1753
Nikooei M, Fathipour Y, Javaran MJ, Soufbaf M (2017) Genetically manipulated Brassica genotypes affect demography and performance of Diadegma semiclausum parasitizing Plutella xylostella. J Appl Entomol 141:161–171
Oduor GL, Löhr B, Seif AA (1997) Seasonality of major cabbage pests and incidence of their natural enemies in central Kenya. In: Sivapragasam A, Loke WH, Hussan AK, Lim GS (eds) Proceedings of the 3rd international workshop, management of diamondback moth and other crucifer pests. 29 October–1 November 1996, Kuala Lumpur
Pike KS, Stary P, Miller T, Allison D, Graf G, Boydston L, Miller R, Gillespie R (1999) Host range and habitats of the aphid parasitoid Diaeretiella rapae (Hymenoptera: Aphididae) in Washington State. Environ Entomol 28:61–71
Safuraie-Parizi S, Fathipour Y, Talebi AA (2014) Evaluation of tomato cultivars to Helicoverpa armigera using two-sex life table parameters in laboratory. J Asia Pac Entomol 17:837–844
Saldo S, Szpyrka E (2009) Ecotoxicological view of protection of apple orchards against insect pests in Poland. Pestycydy/Pesticides 1:15–26
Sarfraz M, Dosdall LM, Keddie BA (2008) Host plant genotype of the herbivore Plutella xylostella (Lepidoptera: Plutellidae) affects the performance of its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Biol Control 44:42–51
Schliephake E, Graichen K, Rabenstein F (2000) Investigation on the vector transmission of the beet mild yellowing virus (BMYV) and the turnip yellows virus (TYV). Z Pflanzenk Pflanzen 107:81–87
Soufbaf M, Fathipour Y, Karimzadeh J, Zalucki MP (2010a) Bottom-up effect of the different host plants on Plutella xylostella (Lepidoptera: Plutellidae): a life-table study on canola. J Econ Entomol 103:2019–2027
Soufbaf M, Fathipour Y, Karimzadeh J, Zalucki MP (2010b) Development and age-specific mortality of diamondback moth on brassica host plants. The pattern and causes of mortality under laboratory conditions. Ann Entomol Soc Am 103:574–579
Soufbaf M, Fathipour Y, Zalucki MP, Hui C (2012) Importance of primary metabolites in canola in mediating interactions between a specialist leaf-feeding insect and its specialist solitary endoparasitoid. Arthropod Plant Interact 6:241–250
Stern V, Smith R, van den Bosch R, Hagen K (1959) The integration of chemical and biological control of the spotted alfalfa aphid: the integrated control concept. Hilgardia 29:81–101
Talaee L, Fathipour Y, Talebi AA, Khajehali J (2017) Screening of potential sources of resistance to Spodoptera exigua (Lepidoptera: Noctuidae) in 24 sugar beet genotypes. J Econ Entomol 110:250–258
Tazerouni Z, Talebi AA, Rakhshani E, Zamani AA (2013) Comparison of life table parameters of Russian wheat aphid, Diuraphis noxia, and its parasitoid, Diaeretiella rapae under constant temperatures. Appl Entomol Phytopath 81:1–10
Verkerk RHJ, Neugebauer KR, Ellis PR, Wright DJ (1998) Aphids on cabbage: tritrophic and selective insecticide interactions. Bull Entomol Res 88:343–349
Acknowledgements
We would like to thank the Department of Entomology, Tarbiat Modares University, for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Miriama Malcicka.
Rights and permissions
About this article
Cite this article
Karami, A., Fathipour, Y., Talebi, A.A. et al. Canola quality affects second (Brevicoryne brassicae) and third (Diaeretiella rapae) trophic levels. Arthropod-Plant Interactions 12, 291–301 (2018). https://doi.org/10.1007/s11829-017-9576-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9576-7