Abstract
Microbial symbionts have come to be recognized as agents in the speciation of their eukaryote hosts. In this study, we asked if bacterial symbionts are, or were in the past, involved in the speciation of the gall-inducing aphid Slavum wertheimae (Hemiptera: Aphididae). This aphid is specific to the tree Pistacia atlantica, which has a fragmented distribution among mesic and xeric habitats, leading to corresponding fragmentation of the aphid population. Previous studies revealed genetic differentiation among populations of the gall-inducing aphid, suggesting cryptic allopatric speciation. Pistacia atlantica trees show no such variation. By means of diagnostic PCR, we screened several populations of S. wertheimae from mesic and xeric sites in Israel for the presence of nine known aphid symbionts: Arsenophonus, Hamiltonella, Regiella, Rickettsia, Rickettsiella, Serratia, Spiroplasma, Wolbachia, and X-type, as well as Cardinium, known to be a reproductive manipulator. Only one symbiont, Wolbachia, was detected in S. wertheimae. Wolbachia was found in all the aphids of the mesic populations, compared to 26% in the aphids from the xeric populations. Multilocus Sequence typing of Wolbachia revealed new haplotypes in the fbpA and coxA genes in both the mesic and xeric populations. Phylogenetic analysis showed that Wolbachia of S. wertheimae is closely related to Wolbachia strains from assorted hosts, mostly lepidopterans, but only distantly related to Wolbachia strains from other aphid species. We conclude that the cryptic speciation of mesic and xeric populations of S. wertheimae was likely driven by geographical isolation rather than by Wolbachia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many arthropods maintain symbiotic relationships with microorganisms that affect host development, reproduction, and survival. Some symbiont–host interactions are mutually obligate, with the symbiont producing essential nutrients lacking in the host’s diet, while the host provides the symbiont with other nutrients which the microorganism cannot produce. Obligate symbionts (also termed “primary symbionts”) are maternally transmitted, and consequently the phylogenies of the parties are typically congruent (Moran et al. 2008; Douglas 2015). Other, facultative symbionts (FS), are maternally transmitted but can also be transmitted horizontally among host lineages, therefore the phylogenies of hosts and FS are typically incongruent. While FS are generally not considered critical for host development and reproduction, multiple vital functions of FS revealed in recent years indicate important roles in the hosts’ ecology and evolution. Some FS contribute directly to their host’s fitness in various ways, such as conferring resistance to pathogens and natural enemies or enhancing fecundity, thereby indirectly promoting their own fitness (reviewed in Oliver and Martinez 2014; Douglas 2015; McLean et al. 2016).
Fascinatingly, some FS use a different strategy to promote their fitness: they manipulate the reproduction of the host in ways that lead to increased proportions of female progeny, which will transmit the symbiont to the next generation, at the expense of males and/or females that do not carry the symbiont. These reproductive manipulations include parthenogenesis, male killing (male embryos die before hatching), feminization (genetic males develop as females), and cytoplasmic incompatibility (CI) (a cross between a symbiont-infected male and an uninfected female is incompatible, leading to a decrease in the proportion of uninfected individuals in the population) (Zchori-Fein and Bourtzis 2012).
To date, Arsenophonus, Cardinium, Rickettsia, Spiroplasma, and Wolbachia have been found to cause one or more of these reproductive manipulations. According to a recent analysis (Weinert et al. 2015), Wolbachia, which infects about 50% of arthropod species, is by far the most studied symbiont and the only one known to induce all four types of manipulations (Zug and Hammerstein 2015; Correa and Ballard 2016).
Theoretical studies corroborated by empirical evidence show that reproductive manipulations reduce gene flow between sympatric/parapatric populations, leading to pre- or post-zygotic reproductive isolation and accelerating speciation over time (reviewed in Engelstädter and Hurst 2009; Brucker and Bordenstein 2012; Vavre and Kremer 2014; Bennett and Moran 2015). Symbiont-induced parthenogenesis results in the production of asexual females alongside the original sexual population; with time, asexual females accumulate mutations in genes that are involved in sexual reproduction, leading to asexual speciation. Bidirectional CI is another possible speciation mechanism: individuals acquire different strains of the reproductive manipulator, resulting in reciprocal incompatibility in both cross directions (Bordenstein et al. 2001; Brucker and Bordenstein 2012).
In the current study, we focused on bacterial symbionts of the aphid Slavum wertheimae Hille Ris Lambers (Hemiptera: Aphididae: Eriosomatinae: Fordini), that induces galls in the Mt. Atlas mastic tree, Pistacia atlantica Desfontaines (Sapindales: Anacardiaceae). All aphid species harbor the obligate symbiont Buchnera aphidicola, which synthesizes amino acids lacking in the phloem diet (with the exception of several aphid species within the subfamily Cerataphidinae, in which Buchnera has been replaced by a yeast-like symbiont; Vogel and Moran 2013). Additionally, aphids are facultatively associated with an array of bacterial symbionts having diverse effects on the fitness of their aphid hosts, including a single example of male killing by Spiroplasma (Skaljac 2016; Simon et al. 2011; Zytynska and Weisser 2016).
Pistacia atlantica has a disjunctive Irano-Turanian distribution extending from central Asia through the Levant and North Africa as far as the Canary Islands. In the Pleistocene, when the climate was cooler, P. atlantica was more continuously distributed, but climatic changes in the region during the Pleistocene and Holocene left isolated populations in unconnected suitable habitats (Danin 1999). Consequently, the tree is fragmentally distributed in Israel from the mesic climate in the north to the xeric climate of the southern Negev desert highlands (~600 vs. <100 mm mean annual precipitation, respectively) (Fig. 1). The mesic and xeric populations of P. atlantica, although geographically separated and phenotypically distinct (Fig. 1), are genetically alike (Inbar and Kark 2007; Avrani et al. 2012). Pistacia atlantica is an obligate host to several species of gall-inducing aphids, including S. wertheimae, readily identified by the distinctive red, cauliflower-shaped galls on the lateral buds. A single tree can host numerous galls. Galls are induced in the spring, each gall by a single female; subsequently the aphids feed on the phloem sap within the gall and reproduce parthenogenetically for multiple generations until the fall, at which time, a winged aphid generation is released from the galls and disperses to other branches or nearby trees. The winged aphids then give birth to sexual aphids, which mate and lay eggs that will diapause throughout the winter and will hatch in the spring (Wool and Bogen 1999; Wool 2004). Since this aphid is specific to P. atlantica, the distributions of the two species are linked. However, unlike the host tree populations, the mesic and xeric aphid populations, although morphologically indistinguishable, differ genetically in their sequences of the mitochondrial genes COI and COII and their AFLP fingerprint profile, resulting in two distinct phylogenetic groups (genetic distances of 49 AFLP loci ranged between 0.09 and 0.141) and suggesting cryptic speciation. There were no such differences between aphids within each region (Avrani et al. 2012).
The presence and identity of bacterial symbionts in S. wertheimae have never been explored. Therefore, the goals of our research were (1) to study which facultative symbionts are hosted by S. wertheimae; (2) to study whether the mesic or xeric populations of S. wertheimae are differentially associated with reproductively manipulative bacterial symbionts. Such an association, if found, could suggest that symbionts play, or at least played in the past, a role in the cryptic speciation of the aphids, and/or have other adaptive effects on the aphid hosts.
Materials and methods
Gall aphid collections
Specimens of S. wertheimae were collected from 9 sites in Israel, including seven locations in the north (mesic) and two in the south (xeric) (Fig. 1). Slavum wertheimae, like most other aphid species, reproduces parthenogenetically; all individuals within a single gall are thus genetically identical. Therefore, all aphids within a gall were separated from the gall tissue and their DNA was extracted together, as a pool, using DNeasy Blood and Tissue Kit (Qiagen, GmbH). Hence the unit of replication in this study comprises DNA extracted from the aphids inhabiting a single gall. The numbers of trees and galls that were sampled at each site are detailed in Table S1.
PCR protocols
We screened the aphids’ DNA for the presence of ten facultative bacterial symbionts using genus-specific primers, as detailed in Table 1. Nine of the symbionts are known from aphids, and five are known as reproductive manipulators in various hosts. PCR products were visualized in 1% agarose gels, and the identity of selected amplification products was verified by Sanger sequencing (McLab Laboratories, San Francisco, CA, USA). Each set of PCRs included a relevant positive control sample (an extraction of a symbiont-infected pea aphid, Acyrthosiphon pisum, supplied by Dr. Kerry Oliver, University of Georgia, Athens, GA, USA). Where no symbiont was detected, the presence and quality of DNA was re-verified by amplifying a fragment of the aphid’s COI gene as described in Avrani et al. (2012).
The differences in symbiont frequencies in the mesic versus xeric populations were analyzed by the Pearson Chi-square test using SPSS 19.0 software.
Wolbachia characterization
We used the multilocus sequence typing (MLST) protocol (http://pubmlst.org/Wolbachia) (Baldo et al. 2006) to characterize the strain/s of Wolbachia found in our samples. MLST is a robust classifying system that provides strain typing based on variation in five conserved housekeeping genes (gatB, coxA, hcpA, ftsZ, and fbpA), and is the current standard for Wolbachia identification. Wolbachia was sequenced from 16 samples from various mesic populations and from all (n = 6) infected xeric samples, following the protocol specified in the MLST website. PCR reactions were performed in volumes of 25 µl, of which 5 µl was used to verify a single product in 1% agarose gels and the remaining 20 µl was used for direct Sanger sequencing (MCLAB Laboratories, San Francisco, CA, USA). The sequences’ chromatograms were visualized, checked manually, and aligned using MEGA6 software; the consensus sequences obtained were then deposited in the MLST database.
To infer the phylogenetic relationships between Wolbachia from S. wertheimae and from other hosts, two phylogenetic analyses were performed on the MLST alleles sequence alignment (using MEGA7 software; Kumar et al. 2016).
-
1.
Fifty-eight sequences from the MLST database were used for the first analysis, including all STs that have at least one allele in common with S. wertheimae’s Wolbachia (n = 35), as well as representative STs from a variety of host taxa (Table S2). A single strain belonging to Supergroup A from a hemipteran host was included for rooting the trees. This analysis was performed on the concatenated sequences of the five MLST alleles (2073–2079 bp).
-
2.
In the second analysis, we added sequences from two aphid species (Augustinos et al. 2011)—Cinara cedri and the gall-inducing aphid, Baizongia pistaciae—which were not deposited in the MLST database. The data of these sequences lacked the ftsZ allele in C. cedri and the coxA and hcpA alleles in B. pistaciae (Table S2). Due to the missing data, this analysis was done on concatenated sequences of the gatB and fbpA alleles (792–798 bp), which are the only two alleles with available sequences for all the hosts.
The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura–Nei model as implemented in the MEGA software (Tamura and Nei 1993). Initial tree(s) for the heuristic search were obtained by applying the Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach and then selecting the topology with superior log likelihood value. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. Branch support was assessed by 1000 bootstrap replications.
Results
The only bacterial symbiont that was detected in our samples is Wolbachia, which was found in 100% of the mesic aphid samples (n = 25 galls), compared to 26% of the xeric samples (n = 23 galls) (χ2 1=28.6; p < 0.001). The COI gene fragment was successfully amplified in all samples that were negative for all symbionts, confirming that the results were not false negatives.
Characterization of Wolbachia
All Wolbachia-carrying individuals tested, from both mesic and xeric populations, carried a single and identical strain of Wolbachia. Comparison of our consensus sequences to the MLST database revealed new haplotypes in two genes—fbpA and coxA; the allelic profile of the Wolbachia isolate from S. wertheimae is therefore novel as well. The new sequences were deposited in the MLST database and assigned the sequence type (ST) number 460 (Table 2). Each of the other 3 alleles (i.e., those that were not new to the database-gatB, hcpA, and ftsZ) has been reported in the past from various hosts. The combination of these 3 alleles was found in a single additional ST in the database, from the lepidopteran Jalmenus evagoras (ST#154). The hcpA allele of S. wertheimae is shared by 34 other STs, of which 22 were isolated from 82 different lepidopteran hosts (Table S2). The ftsZ and gatB alleles of S. wertheimae are found in 8 other STs (seven Lepidopterans and one mite) and 4 other STs (two Lepidopterans, one Hemipteran, and one Dipteran), respectively. All the STs that had at least one allele in common with S. wertheimae’s Wolbachia belong to Supergroup B.
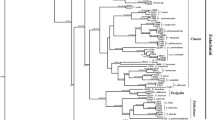
Maximum Likelihood phylogenetic analysis of the gatB and fbpA concatenated sequences (the only two alleles with available sequences for all the samples included in the analysis; see methods and materials section for details) revealed that the Wolbachia strain from S. wertheimae is clustered on a relatively recently evolved branch that includes many strains from Lepidopteran host species, as well as from two hemipterans and two dipterans (Fig. 2a). Wolbachia of the two other aphid species—C. cedri and B. pistaciae—clustered together as an out-group and were thus distantly related to Wolbachia from S. wertheimae. Wolbachia strains from other host species that belong to the suborder Sternorrhyncha (the suborder to which S. wertheimae belongs) are placed on various, earlier, branches of the tree (Fig. 2a). The Maximum Likelihood phylogenetic analysis that was done on the concatenated sequences of all five alleles (but without C. cedri and B. pistaciae) showed a similar pattern, with the closest relative being the butterfly J. evagoras, but here the specific branch of S. wertheimae included more hemipteran hosts, including one from the Sternorrhyncha, Diaphorina citri (Fig. 2b). It should be noted that some branches of the tree are poorly supported (<50% bootstrap support). Phylogenetic analyses of each allele by itself resulted in similar results, although in the analysis of the coxA allele, Wolbachia of S. wertheimae is located on more basal branches of the tree (data not shown).
Phylogenetic analyses of Wolbachia, using the Maximum Likelihood method. a A tree constructed from concatenated sequences of the gatB and fbpA alleles, which were available for all host species included in the analysis (i.e., 58 sequences from the MLST database + sequences of two aphid species, from Augustinos et al. 2011). There were a total of 792 positions in the final dataset. The tree with the highest log likelihood (−3492.9020) is shown. b A tree constructed from concatenated sequences of all the five alleles. This analysis involved 58 sequences (i.e., without the sequences of the two aphid species from Augustinos et al. 2011, because 1–2 allele sequences are lacking). There were a total of 2071 positions in the final dataset. The tree with the highest log likelihood (−8480.9079) is shown. In both trees, bootstrap support values are shown next to the branches (only values ≥50%). See text for further details. Wolbachia of S. wertheimae, obtained in this study, is highlighted in yellow and marked with a green dot (dot). The two other aphid species are marked with a green symbol (open circle); hosts from the suborder Sternorrhyncha are marked with a green triangle symbol (open triangle). Hosts’ orders are color coded: Lepidoptera—red, Hemiptera—green, Diptera—maroon, Hymenoptera—blue, Orthoptera—purple, Coleoptera—gray, Acari—black (STs 442 and 447—order not specified in the database). Hosts’ species, family, and order names, as well as the Wolbachias’ MLST genes and Supergroups are detailed in Table S2
Discussion
The question of how new species evolve continues to intrigue evolutionary biologists and theoreticians. In the past, speciation was believed to occur mainly under conditions of allopatry; currently, it is widely accepted that speciation may take place sympatrically as well, as a consequence of differential adaptations among individuals in a given population (Bolnick and Fitzpatrick 2007; Butlin et al. 2008; Schluter 2009). In recent years, the ability of microbial symbionts, both reproductive manipulators and gut bacteria, to induce reproductive isolation through pre- and post-zygotic mechanisms has been demonstrated in several studies, and speciation is the inferred long-term consequence (e.g., Bordenstein et al. 2001; Brucker and Bordenstein 2012, 2013; Vavre and Kremer 2014; Gilbert et al. 2015).
The genetic profiles of populations of the gall-inducing aphid S. wertheimae along the Irano-Turanian distribution zone in Israel suggest allopatric cryptic speciation, as the aphids display two distinct phylogenetic groups, one distributed in the northern mesic region, and the second in the xeric southern zone (Avrani et al. 2012). Here, we found that all the mesic aphids carry Wolbachia compared to only 26% of the xeric ones. Is it possible, then, that Wolbachia played a role in the cryptic speciation of S. wertheimae? In light of our results we think this is unlikely because the genotype of Wolbachia in both regions is identical. If Wolbachia genotypes in the mesic and xeric populations were different, that could suggest the potential for bidirectional CI, but this is not the case here. Instead, an ecology-based speciation scenario seems more plausible. Current-fragmented populations of S. wertheimae represent the remains of a more continuous historical distribution of the host tree which was altered by climatic changes during the Pleistocene and Holocene, leading to the disjunction between northern and southern populations (Danin 1999). Thus, the most parsimonious explanation for the situation we see today is that all S. wertheimae populations originally harbored Wolbachia, until climate change caused a drastic shift in selection pressures that led to the loss of Wolbachia in the majority of the xeric aphids. If so, then probably the fitness cost of harboring Wolbachia is greater than the benefits, in the harsh xeric conditions. Indeed, Wolbachia are usually sensitive to heat, a fact that is often used to experimentally establish aposymbiotic insect populations (Li et al. 2014). In an alternative scenario, Wolbachia invaded the mesic and xeric aphid populations independently, after the populations became geographically isolated. This scenario seems less plausible, as the sequence type of Wolbachia is identical in mesic and xeric aphids. A third scenario is that Wolbachia-infected aphids disperse from the mesic to the xeric habitats, but this too is very unlikely because (1) mesic and xeric aphid populations differ genetically in both mitochondrial and nuclear genomes (this is the starting point of our study); (2) winged aphids have a very limited flight range (Wool and Bogen 1999), and although they may also disperse passively with winds, the probability of landing on one of very few host trees, at least 200 km away, is extremely low.
It will be technically difficult, perhaps even impossible, to test empirically whether Wolbachia manipulates the reproduction of S. wertheimae, since gall-inducing aphids are difficult to culture in the laboratory and complete only one sexual reproduction cycle every year. Nonetheless, comparing fitness parameters between Wolbachia-infected and Wolbachia-free S. wertheimae in xeric populations should be fairly doable and may help to clarify the role of this widespread symbiont in this aphid host.
Aphids often harbor a diverse array of facultative bacterial symbionts (Skaljac 2016; Zytynska and Weisser 2016), of which only Wolbachia was detected in S. wertheimae in our study. Wolbachia went unnoticed in aphids for many years; it was noted for the first time only in 2000 (Jeyaprakash and Hoy 2000). Since then, Wolbachia has been found in over a hundred species of aphids (Wang et al. 2009, 2014; Jones et al. 2011), including one gall-inducing aphid species, B. pistaciae, collected in Greece from Pistacia terebinthus (Augustinos et al. 2011). Unfortunately, MLST data of aphids’ Wolbachia are limited to two species only (!) with 1–2 alleles missing in each profile (Augustinos et al. 2011). The allelic profile of Wolbachia from our samples is new to the MLST database, as the sequences of the coxA and fbpA genes differ from all previously known sequences. The Wolbachia of S. wertheimae is nested within a clade that includes various hosts, all from Supergroup B (Fig. 2); therefore we infer that S. wertheimae’s Wolbachia belongs to Supergroup B as well. In contrast, Wolbachia from the gall-inducing aphid B. pistaciae and the aphid C. cedri were found here to be only distantly related to Wolbachia of S. wertheimae, corresponding with their Supergroups affiliations (A and M, respectively, Augustinos et al. 2011). The tree that was constructed with all the five MLST alleles (Fig. 2b) likely reflects the phylogenetic relationships more reliably, since it is based on the whole MLST alleles set (~2000 bp). In this tree (Fig. 2b), Wolbachia from other Sternorrhynchan hosts is located in the same clade (Diaphorina citri) or in a sister clade (Kerria lacca and Bemisia tabaci) of the tree, but the closest relatives were found in the butterfly J. evagoras and the spider-mite T. viennensis (Fig. 2b). Overall, these findings corroborate the notion that Wolbachia is transferred horizontally between species and subsequently diversifies (Correa and Ballard 2016), although it is hard to explain how (i.e., both the evolutionary and the ecological mechanisms) Wolbachia strains from distantly related hosts, like aphids and butterflies, are more similar than closely related hosts. Our findings coincide with the high divergence of Wolbachia in aphids found by Augustinos et al (2011), but in our system, although the aphids are subject to different selection pressures and Wolbachia’s infection rates are subject to drift in the xeric populations, Wolbachia has not diverged genetically (yet?) between the mesic and xeric populations. Obtaining MLST data from more aphid species will clarify the evolutionary history of Wolbachia in this important insect family.
References
Augustinos AA, Santos-Garcia D, Dionyssopoulou E et al (2011) Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS One 6:e28695
Avrani S, Ben-Shlomo R, Inbar M (2012) Genetic structure of a galling aphid Slavum wertheimae and its host tree Pistacia atlantica across an Irano-Turanian distribution: from fragmentation to speciation?. Tree Genet Genom 8: 811–820
Baldo L, Hotopp JCD, Jolley KA et al (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Bennett GM, Moran NA (2015) Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci 112: 10169–10176
Bolnick DI, Fitzpatrick BM (2007) Sympatric speciation: models and empirical evidence. Annu Rev Ecol Evol Syst 38: 459–487
Bordenstein SR, O’Hara FP, Werren JH (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409:707–710
Brucker RM, Bordenstein SR (2012) Speciation by symbiosis. Trends Ecol Evol 27:443–451
Brucker RM, Bordenstein SR (2013) The hologenomic basis of speciation: gut bacteria cause hybrid lethality in the genus Nasonia. Science 341: 667–669
Butlin RK, Galindo J, Grahame JW (2008) Sympatric, parapatric or allopatric: the most important way to classify speciation?. Philos Trans R Soc Lond B Biol Sci 363: 2997–3007
Correa CC, Ballard JWO (2016) Wolbachia associations with insects: Winning or losing against a master manipulator. Front Ecol Evol 3:153
Danin A (1999) Sandstone outcrops: a major refugium of Mediterranean flora in the xeric part of Jordan. Isr J. Plant Sci 47:179–187
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Engelstädter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40: 127–149
Ferrari J, West JA, Via S, Godfray HCJ (2012) Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 66: 375–390
Gilbert SF, Bosch TCG, Ledón-Rettig C (2015) Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet 16:611–622
Heddi A, Grenier A-M, Khatchadourian C et al (1999) Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci 96: 6814–6819
Inbar M, Kark S (2007) Gender-related developmental instability and herbivory of Pistacia atlantica across a steep environmental gradient. Folia Geobot 42: 401–410
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol 9:393–405
Jones RT, Bressan A, Greenwell AM, Fierer N (2011) Bacterial communities of two parthenogenetic aphid species cocolonizing two host plants across the Hawaiian Islands. Appl Environ Microbiol 77:8345–8349
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Li YY, Floate KD, Fields PG, Pang BP (2014) Review of treatment methods to remove Wolbachia bacteria from arthropods. Symbiosis 62:1–15
McLean AHC, Parker BJ, Hrček J et al (2016) Insect symbionts in food webs. Philos Trans R Soc Lond B Biol Sci 371:20150325
Moran NA, Russell JA, Koga R, Fukatsu T (2005) Evolutionary relationships of three new species of enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol 71:3302–3310
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Ann Rev Genet 42:165–190
Nakamura Y, Kawai S, Yukuhiro F et al (2009) Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl Environ Microbiol 75:6757–6763
Oliver KM, Martinez AJ (2014) How resident microbes modulate ecologically-important traits of insects. Curr Opin Insect Sci 4: 1–7
Russell JA, Weldon S, Smith AH et al (2013) Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol Ecol 22:2045–2059
Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323:737–741
Simon J-C, Boutin S, Tsuchida T et al (2011) Facultative symbiont infections affect aphid reproduction. PLoS One 6:e21831
Skaljac (2016) Bacterial Symbionts of Aphids (Hemiptera: Aphididae). In: Vilcinskas E (ed) Biology and Ecology of Aphids, CRC press, Boca Raton, pp 100–125
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Taylor GP, Coghlin PC, Floate KD, Perlman SJ (2011) The host range of the male-killing symbiont Arsenophonus nasoniae in filth fly parasitioids. J Invertebr Pathol 106:371–379
Tsuchida T, Koga R, Shibao H et al (2002) Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11:2123–2135
Vavre F, Kremer N (2014) Microbial impacts on insect evolutionary diversification: from patterns to mechanisms. Curr Opin Insect Sci 4: 29–34
Vogel KJ, Moran NA (2013) Functional and evolutionary analysis of the genome of an obligate fungal symbiont. Genom Biol Evol 5:891–904
Wang Z, Shen Z-R, Song Y et al (2009) Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. Eur J Entomol 106: 49–55
Wang Z, Su X-M, Wen J et al (2014) Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Sci 21: 313–325
Weinert LA, Araujo-Jnr E V., Ahmed MZ, Welch JJ (2015) The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc B 282: 20150249
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol 49:175–192
Wool D, Bogen R (1999) Ecology of the gall-forming aphid, Slavum wertheimae, on Pistacia atlantica: population dynamics and differential herbivory. Isr J Zool 45:247–260
Zchori-Fein E, Bourtzis K (2012) Manipulative tenants: bacteria associated with arthropods. CRC Press, Boca Raton
Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol Rev 90:89–111
Zytynska SE, Weisser WW (2016) The natural occurrence of secondary bacterial symbionts in aphids. Ecol Entomol 41: 13–26
Acknowledgements
We would like to thank Moshe Inbar, Einat Zchori-Fein, and three anonymous reviewers for critical comments on earlier versions of the manuscript. We also thank Kerry Oliver for supplying us specimens for positive controls. The study was funded by an internal research grant from “Oranim” College of Education, and in part by the Israel Science Foundation (Grant No. 276/14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: John F. Tooker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amit, L., Ben-Shlomo, R. & Chiel, E. Are microbial symbionts involved in the speciation of the gall-inducing aphid, Slavum wertheimae?. Arthropod-Plant Interactions 11, 475–484 (2017). https://doi.org/10.1007/s11829-017-9495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9495-7