Abstract
Facultative ant–plant mutualisms are variable systems, shaped by a number of biotic and abiotic factors. Especially in tropical ecosystems, the generally assumed mutualistic benefits are often hard to prove. We studied the system Leea manillensis on the Philippine island Panay and its indirect defence mechanism against herbivory by producing extrafloral nectar therewith attracting ants. Unexpectedly, we found an ant-parasitoid wasp from the genus Chalcura (Eucharitidae) to have a strong influence on the system, on ants as well as on plants. The parasitoid not only altered the behaviour of interacting ant species, but also directly and indirectly affected the plants’ fitness. This study demonstrates how top-down effects may alter species interactions and have a massive effect on mutualisms and their beneficial outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interactions between organisms can be very complex and are often yet unexplored (Dáttilo et al. 2014), especially in tropical forests. Within ecosystems, members of different trophic levels are interacting with each other by regulatory forces. According to their main direction and frequency, these are described as top-down and bottom-up regulations (e.g. Power 1992; Oksanen and Oksanen 2000; Ernest and Brown 2001; Báez et al. 2006). The trophic cascade model combines both forces. These forces can flow up and down the trophic structure, and organisms on any level can have dramatic effects on the composition of the whole community (Leibold 1989; Schmitz 1993).
Compared with investigations of predation and competition as structuring forces, studies that address the roles of mutualism and parasitism in shaping communities are underrepresented. Mutualistic interactions between species are widespread in tropical forests, playing an important role in ecosystem functioning (Thompson 2006). For instance, ant–plant protection mutualisms have served as model systems to study mechanisms promoting species coexistence and trophic cascades, and they are known for many plants from different taxonomic groups (Heil and McKey 2003; Bronstein et al. 2006). Protection mutualisms can have far-reaching effects in food webs, because they involve not only direct (and often indirect) trophic exchanges between the two mutualists, but also interactions with additional trophic levels (Dyer 2008). In these associations, ants are defending a valuable food resource such as extrafloral nectar (EFN) and thus are indirectly protecting the entire plant or plant parts, respectively, against herbivores. Many studies demonstrated a positive effect of EFN on the fitness of the host plant, but others failed to find a benefit (O´Dowd and Catchpole 1983; Mody and Linsenmair 2004; Horvitz and Schemske 1984; Bronstein 1998, do Nascimento and Del-Claro 2010). The different results of these studies might be caused by the fact that in general these associations are characterized by a low specificity and high variability, with plants attracting a broad range of ant species (Schemske 1982; Diaz-Castelazo et al. 2010) as well as other arthropods (Koptur 1992).
While ant–plant interactions have been a research focus in different tropical regions, from the Philippines even basic knowledge about ant–plant interactions is lacking. And still more basically, the Philippine ant fauna is very poorly explored (General and Alpert 2012), and an unknown number of species has probably already been lost, due to the fact that only about 11.2 % of primary forests were left in 2010 through large-scale anthropogenic destruction (FAO UN 2010). We found that the shrub Leea manillensis (Leeaceae) offering EFN and food bodies (FB) on its shoots was regularly visited by ants. However, none of the hitherto performed studies could prove any benefit derived by the plant due to the ants’ presence and their potential protection against herbivores, but the genus Leea has been reported to be involved in facultative mutualisms in other parts of Southeast and East Asia (Fiala and Linsenmair 1995; Meng et al. 2011). The original aim of our study was to investigate this yet unknown ant–plant association and its mutualistic quality. Unexpectedly, this system became complicated by a third trophic level—an ant-parasitoid wasp of the genus Chalcura (Hymenoptera: Eucharitidae: Eucharitinae). This wasp turned out to be abundant and to have a massive impact on plants as well as on ants.
Parasitoids are important organisms and abundant members of nearly all terrestrial communities, essentially contributing to the high biodiversity of tropical ecosystems (Godfray 2007). Their species richness (and therefore possible functional relevance) has been markedly underestimated in the tropics (see, e.g. Veijalainen et al. 2012; Hrcek et al. 2013). The Eucharitidae is the only insect family known to comprise only parasitoids specialized on ant brood (Heraty 1985; Heraty and Darling 1984; reviewed in Lachaud and Pèrez-Lachaud 2012). All members of this family, where the life cycle is known, develop as koinobiont, larval–pupal ectoparasitoids (Lachaud and Pèrez-Lachaud 2012). Females place their eggs away from the host on plant tissue. The active first-instar larvae (planidia) are responsible for gaining access to the larval ant host via various phoretic behaviours, involving attachment to ant workers or intermediate hosts carried into the colony by ant workers (Heraty 2000). Ants tend to intensively forage on plants, especially when a food source as honeydew or EFN is given (Blüthgen and Fiedler 2004). Pérez-Lachaud et al. (2006) gave a description of the life cycle of Kapala iridicolor (Eucharitidae) from the Neotropics, which oviposits on undeveloped flower buds of Melampodium divaricatum (Asteraceae), possessing extrafloral nectaries at the abaxial side of its leaves. Observations of Carey et al. (2012) suggest that the parasitoid Orasema simulatrix (Eucharitidae) oviposits almost exclusively in the near vicinity of the extrafloral nectaries of Chilopsis linearis (Bigoniaceae), ensuring to gain access to an ant colony. But so far, no attention has been paid to the parasitoids` influence on the whole system and on possible effects of the protective function of the ants for the plants.
Up to now, studies on ant–plant protection mutualisms mediated through a plant-derived food source have usually only regarded direct interactions between ants and herbivorous insects (but see, e.g. Rudgers 2004; Pires and Del-Claro 2014 and references therein). However, as our study shows, interactions can be much more complex. To test whether Leea manillensis is indeed involved in mutualistic interactions with ants, we studied food production, ant visitors, and their possible benefits for the plant. As a further trophic level was involved in our study system, we also needed to analyse the biology of the ant-parasitoid and its interference with the ant–plant relationship. In this study, we report about the parasitoid, its impact on the fitness of Leea manillensis, and the entire ant–plant system and show the effects propagated through three different trophic levels.
Materials and methods
Study site
The study site was located in the midwestern Philippines on the island Panay, within the ‘Northwest Panay Peninsula Natural Park’ in the north of the island, an area of mountainous secondary and primary lowland rainforest around the research station ‘Sibaliw’ (11°49′9.76″N, 121°57′37.95″E). The station was established by the Philippine NGO ‘PhilinCon’ and Eberhard Curio in 1997 on an abandoned settlement area, with the youngest secondary forest being about 35 years old. The study was carried out in secondary as well as primary forest habitats.
Study species
Leea manillensis (Leeaceae) is a large erect shrub with height up to 6 m, terminal inflorescences, and large stipular structures that enclose the next generation of shoots. It is distributed widespread throughout the Philippines and parts of Southeast Asia (Molina et al. 2013) and was formerly included in L. guineensis (Ridsdale 1976). The species is shade-tolerant, growing in the understory, as well as in forest gaps (and edges) in primary and secondary forests, with especially high abundances in bright, young-aged secondary growth forests. Photographs of Leea manillensis are shown in Online Resource 1.
We checked the plants’ shoots for the occurrence of extrafloral nectaries and food bodies, ants, or any other visitors. Nectar sugar content was measured after 5-h exclusion of ants from nectaries. Ant exclusion was achieved via the application of grease at the basis of the shoot. Droplets were taken with a glass capillary (KG01, A. Hartenstein laboratory supplies: 100 × 0.9 mm, wall thickness 0.1 mm), and sugar content in per cent was measured using a hand-held refractometer (Hand-Held Refractometer RHB-32ATC).
Parasitoid wasps
As we found an ant-parasitoid wasp from the genus Chalcura (Eucharitidae) to have a strong influence on the system, all the experimental shoots were regularly surveyed for wasps, planidia, and any abnormalities in growth or habitus. Planidia were taken to the field station in vials for further observations and photographs.
The parasitoid wasp was determined to genus level by Stefan Schmidt at the Bavarian Zoological State Collection, Munich (ZSM), and voucher specimens of wasps (females, males, planidia) were deposited at the ZSM.
The Eucharitidae (Chalcidoidea) is a small family with a worldwide distribution and divided into three subfamilies Oraseminae, Eucharitinae, and Gollumielinae. All of its members, where the host is known, parasitize ant brood (Pérez-Lachaud et al. 2006; Lachaud and Perez-Lachaud 2012). Species are found in all zoogeographical regions, but most are confined to the tropics. Eucharitids attack ants in at least 21 genera, distributed across the subfamilies Formicinae, Myrmeciinae, Myrmicinae, and Ponerinae (Heraty 1994). Some genera show a very broad host range, with species found on several different genera, or even on different subfamilies of ants. Most genera, however, are restricted to a single genus of ants (Heraty 1994).
Experiments on herbivory
The study was conducted in 2011 during 3 months of the rainy season from 15 August to 21 November. The initial aim was to study the system Leea manillensis and its defence mechanism against herbivory by producing extrafloral nectar (EFN) therewith attracting ants. A total of 223 plants were found in the study area—primary and secondary forests—and marked individually. Of these, 213 shoots were used for a short-term experiment on herbivory. All of these plants were monitored regularly. With a shoot expanding, the main experiment began, and plants were monitored visually every 3 days for a period of 21 days. We recorded the presence or absence of ants, ant species, and abundance on the shoot, as well as the number of other arthropods feeding on the plant tissue or at the EFN. After the 21-day period, the shoots were cut, dried between sheets of paper, and photographed (Canon® Digital IXUS 50) on scale paper for further analyses related to leaf area loss due to herbivory.
To analyse leaves for possible ant herbivore defence, leaf area loss was measured for each shoot using Photoshop® (Adobe® Photoshop® CS5 Extended, version 12.0.4 × 64). Leaf area loss was expressed as percentage of missing leaf area as well as lost area in cm2. As leaves do not shrink by more than 5 % during the process of drying, data from dried leaves give a reliable estimate of missing leaf area (Heil 2004). A complete loss of the shoot due to herbivory, which was a frequent incidence, was recorded as 100 % leaf area loss. Shoots with only intermittent ant association were not used for further experiments on herbivory.
Results
Leea manillensis and its associations with ants
The plant produced FB and EFN on its shoots that were mainly visited by different ant species, but also by a variety of other arthropods. Up to eight elongated extrafloral nectaries are found on the shoots’ stipule and two to six elongated extrafloral nectaries at the nodes above the stipule (Online Resource 1). When the young leaves become mature, the extrafloral nectaries stop nectar production. All of the shoots possessed nectary glands. However, production was very variable as many shoots did not produce FB or EFN at all. Food bodies usually occurred only in the first days after shoot expansion and predominantly around the shoots’ stipule and on the internode above. We assume the production of EFN and FB to be dependent on a combination of abiotic and biotic factors, but our study did not emphasize any clear pattern of dependency in that respect. Plant size did not play an important role, as we observed even seedlings to produce EFN and attract ants. Plants growing under low light conditions attracted ants as well as plants growing in forest gaps (unpublished observations).
Out of 223 marked plants, a total of 80 plants were visited by ants, whereas on 90, ants were never observed at the shoots’ nectaries. On 53 plants, ants were only rarely found at the nectaries. Only 36 % of the marked plants of Leea manillensis showed a permanent association with ants. On constantly ant-free plants also, no other visitors of the extrafloral nectaries were observed, suggesting a general inactivity of the nectary glands. In contrast, on shoots with regular ant occupation, droplets of EFN occurred on the nectaries soon after ant exclusion. The average sugar content of the EFN droplets after 5-h ant exclusion was 16.6 % (n = 13).
Ant activity was mainly concentrated on the biggest nectary glands at the stipule and to a lesser extent at the extrafloral nectaries positioned directly at the nodes. Occasionally, ants were observed feeding on extrafloral nectaries located at the underside of the leaves, but only on fresh shoots or in combination with—and probably connected to—recent leaf damage.
Over the study period of 3 months, a total of 26 ant species were observed visiting the extrafloral nectaries on the shoots of Leea manillensis. Sixteen ant species out of six genera showed a constant assemblage on the nectaries (from shoot expansion until maturity of leaves), whereas the rest visited the nectaries only temporarily. Most common, and assembling in the highest abundances with a maximum number of 65 ants, was a single unidentified species of the genus Dolichoderus. This species was only found on the shoots of Leea in secondary forest. In declining order in terms of the number of shoots occupied during the study, we found the genera Technomyrmex (two species), Crematogaster (five species), Tetramorium (three species), Polyrhachis (four species,) and Camponotus (one species). In general, about 10–20 ants assembled at the extrafloral nectaries, except of big ants from the genera Polyrhachis and Camponotus, where normally less than five individuals were assembled. During heavy rain showers, ants used to leave the shoots or seek shelter beneath the plants’ leaves. In general, only one species occupied a shoot at a time. Only rarely, two or three species at a time were observed using the same food source on the shoots. Ants of the genus Polyrhachis were the most dominant, but also the genus Dolichoderus and species of Crematogaster rank among the dominant species. In rare cases of two or three ant species at a time, the dominant species defended the nectaries against submissive species, mostly small species of the genera Tetramorium and Crematogaster, which were frequently observed to sneak in. Occasionally, the ant species composition on a plant changed, sometimes even several times over the study period. Most of the ant species associated with Leea manillensis showed a diurnal as well as nocturnal activity pattern. Species of the genus Polyrhachis showed a strictly diurnal activity, while the single species of the genus Camponotus was observed only at night-time. Some photographs of Leea manillensis and associated ant species are shown in Online Resource 1.
Further arthropods
During the daily monitoring, besides ants and the parasitoid wasp, a number of other arthropod species were frequently observed on the shoots of Leea manillensis. Of these, the most common visitors were weevils (Curculionidae), peacock flies (Tephritidae), a variety of Orthopterans, planthoppers (Fulgoroidea; Auchenorrhyncha), leaf beetles (Chrysomelidae), and spiders (Aranaeae). Peacock flies were only feeding at the EFN, spiders were also seen hunting. The rest, besides feeding on the EFN, was mainly observed damaging the young leaves. Ants used to tolerate most of these species. In particular, weevils were tolerated even at the extrafloral nectaries. Some photographs of further arthropod species on Leea manillensis are shown in Online Resource 4.
Ant association and herbivory
Two different kinds of shoot damage became apparent during the study. On one hand, there were single feeding incidents by a range of herbivores, sometimes lasting over several days. This type of damage was mainly caused by Orthopterans and leaf beetles with whole parts of the leaves missing, or by weevils with leaves perforated. However, these feeding events rarely caused damage exceeding 20 % leaf area loss. In contrast, in 25 % of the shoots (54 of 213), the loss of the whole shoot was observed caused by one or more unidentified herbivores. In many cases, we realized this to have occurred over night. Apparently, this kind of damage had the most important impact on Leea manillensis.
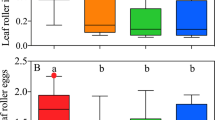
Ant-free shoots suffered a significantly greater loss of leaf area than ant-associated shoots (Fig. 1 ). The total shoot loss, recorded as 100 % of leaf area lost, played a significant role. 57 % of the plants not associated with ants suffered from a total shoot loss, compared to only 8 % of ant-associated plants. A re-analysis without the samples of complete shoot loss revealed no differences in general damage in both groups (Fig. 2).
Loss of per cent leaf area in ant-associated versus ant-free shoots. Data from Fig. 1 re-analysed, but cases with complete shoot loss excluded. Then, no longer resulting in difference in leaf damage (Mann–Whitney U test: p = 0484.)
The parasitoid wasp of the genus Chalcura: observations and experimental results
Oviposition
Female wasps of the genus Chalcura were frequently observed to place their eggs into the stipule of the plants shoots close to the stipules’ extrafloral nectaries. These large stipular structures enclose and protect the next generation of leaves in an early developmental stage. Whenever the arrival of a female wasp was observed, the wasps never directly landed on the stipule, but on a leaf close to it. After a period of a few minutes, meanwhile probably observing the shoot, the wasps directly walked or flew to the stipule.
During these observed ovipositions, which lasted about 1 h up to maximum of 2 h, the female wasp placed masses of eggs directly into the closed stipule without changing the position. During the 3 months of monitoring Leea manillensis, a total of 136 females were observed to oviposit into the stipules of 66 plants, indicating single plants to be utilized repeatedly and often simultaneously by two, three, or even four females (Online Resource 2). On 25 out of 66 plants over a period of a few days up to 2 weeks, wasps repeatedly were observed to oviposit into the plants stipule. Experimentally opening those stipules, we found the undeveloped young leaves to be embedded in masses of eggs (Online Resource 2).
Out of 136 observations of oviposition, 128 took place on ant-free shoots. Only eight wasps were observed on ant-occupied shoots. Four of them were either not noticed or ignored, one wasp was killed and three were chased away by assembling ants. Occasionally, female wasps were observed to feed at the extrafloral nectaries.
Infestation with planidia and loss of leaf area
Out of 213 experimental shoots, a total of 53 % were infested with planidia (n = 114). The parasitoid wasp had a massive effect on Leea manillensis, its association with ants, its shoot development and survival. Only 19 % of shoots infected with planidia were visited by ants, compared to 68 % of uninfected shoots (Fig. 4). Shoots infested by planidia had a significantly higher loss of leaf area than planidia-free shoots. Again, the total loss of the shoot due to herbivory was responsible for most of the differences between the two groups. In planidia-infested shoots, the incidence of a complete shoot loss was almost 10 times higher than in those not infested, with 38 % compared to only 4 % (Figs. 3 and 4).
Observations on the wasp biology and its influence on ants
Shoot development was strongly affected on planidia-infested plants. In 38 % of the observed plants, the planidia hatched synchronically with the shoot expanding, with no visible harm to the young leaves. In 62 % of infested shoots, the mass eclosion of planidia apparently affected the stipules’ tissue. In these cases and probably depending on shoot size, its stage of development and the number of planidia hatching inside the shoots suffered from different kinds of shoot damage (Online Resource 3).
Emerging planidia had a body length of about 0.13 mm and were found sitting in a typical lurking erected position on the expanding shoot, most often in high abundances, concentrating on the area around the stipule and its nectaries (see Online Resource 2). In this early stage, often dead ants were found hanging on strands attached to the shoot. Those ants always had planidia on them. This observation was made for most of the common ant visitors. In the following days of observation, the number of planidia on the shoot declined, either due to them getting carried away by foraging ants or other visitors, or by actively spreading over the whole plant by moving along strands. The infestation with planidia caused a complete loss of ant association after a short time. Ants then actively avoided the shoot.
Planidia taken to the field station in vials survived up to 3 weeks without food intake. Ants placed into the vials immediately showed an intensified, but apparently inefficient cleaning behaviour. After a short time, first locomotive dysfunctions were observed, followed by immobility connected with a typical twitch of legs and antennae. In the final state, ants did not show any vital signs at all. With fresh planidia and small ant species of the genera Crematogaster, Technomyrmex, or Dolichoderus, this state was reached in 10–30 min. In big ant species like Polyrhachis, it took up to 1 h. The planidia were preferentially situated at the soft parts of the ants between the segments, probably feeding and apparently gaining body mass. Apart from that, there were always some larvae showing the typical lurking position on the ant’s bodies. Photographs of the ant-parasitoid wasp and effects on Leea manillensis and associated ants are given in Online Resources 2 and 3.
Discussion
Ants, EFN, and herbivory
Our results show that Leea manillensis consists of a highly variable system, shaped by a number of factors. We could confirm that it has mutualistic interactions with ants attracted by extrafloral nectaries and food bodies. Ants provided a certain degree of protection. However, the indirect defence mechanism via ants turned out to be very fragile, dependent on different conditions that influenced the magnitude of the beneficial effect of ants on plants (see also Rico-Gray et al. 2012). We found no evidence of any specific relationship between Leea and the attracted ants. Meng et al. 2011 describe similar conditions in the species Leea glabra (Leeaceae) growing in South China. The broad range of ant species observed to visit the extrafloral nectaries is consistent with a pattern typically found in many facultative ant–plant associations and indicating a low specificity (Fiala et al. 1994; Kessler and Heil 2011). Not only did we find a broad range of ant species, but also a high intraspecific variability in the abundance of ants assembling at the extrafloral nectaries. Only 36 % of the observed shoots showed a permanent presence of ants perhaps due to varying EFN production of the plants. In general, variability is quite common in facultative ant–plant systems (Horvitz and Schemske 1984), and different abiotic and biotic factors may have massive effects on species interactions (Thompson 1999), directly influencing the outcome of ant–plant–herbivore mutual effects and determining the benefit for the plant (e.g. Ness et al. 2006; Rosumek et al. 2009; Pires and Del-Claro 2014).
Nonetheless, myrmecophily in plants evidentially is an adaptive mechanism of defence (e.g. Heil and McKey 2003). In this study, we are able to show that ant attraction by the production of EFN on shoots significantly increased the chances of shoot survival of Leea manillensis. Certainly, herbivory pressure is an important parameter for Leea and a critical selective factor as reported for many plant species (e.g. Vasconcelos 1991; Karban and Agrawal 2002). While the average herbivore damage on the shoots, with <20 % leaf area loss, was probably tolerable for the plant, the phenomenon of complete shoot loss played a considerable role: 57 % of the shoots without notable ant occupation suffered a total shoot loss, in contrast to only 8 % in ant-occupied shoots. In Leea manillensis, the large stipule offers the biggest nectaries and is the part of the shoot with the highest rate of ant visitation. The optimal defence theory (McKey 1974) predicts that a plant should optimize its defensive investment according to the organs’ current and future value and its likelihood of attack (reviewed in Heil 2008). In Leea, the defensive investment and its focus on the shoots’ stipule corresponds with its high risk of attack. Beyond that, the successful attraction of ants to the stipules offered protection to an even more important enemy, not only directly harming the shoots, but also indirectly by affecting the defensive function of the ants for Leea manillensis.
Influence of the parasitoid wasp
The ant-parasitoid wasp focused on the stipules of Leea manillensis for egg deposition. A broad range of plant hosts and different types of plant tissue have been reported as a depot for eggs in other Eucharitid ant-parasitoids: seeds, flower buds, flower stems or young fruits, leaves or young shoots (Heraty 1985, 1994; Varone and Briano 2009; Carey et al. 2012). In Leea manillensis, the stipule that encloses the next generation of shoot is the spatial focus of investment into defensive mechanisms via EFN and FB. Obviously not general herbivores are the most damaging threats for Leea manillensis in the study area, but the specialized ant-parasitoid.
The hatching planidia had a massive impact on the whole ant–plant–herbivore system. In our study, about 53 % of the plants’ shoots were observed to be infested with planidia. All of the common ant visitors at the extrafloral nectaries strictly avoided the shoots after the eclosion of the wasps’ larvae. That might explain why we noticed planidia to leave the shoot and disperse. The avoidance of shoots and the observed aggressive behaviour of ants towards adult wasps indicate that the ants were able to recognize this parasitoid as a threat. On 81 % of the planidia-infested shoots, ants were never observed to visit the extrafloral nectaries. Our observations suggest that this avoidance finally led to a cessation of EFN secretion in most of the shoots. Hence, the avoidance of planidia directly resulted in a lasting lack of ants on the shoots, which then caused an almost 10-fold increase in complete shoot losses compared to the unaffected shoots.
On the other hand, the presence of ants had a great effect on the behaviour of the parasitoid wasp. Low presence of ants enhanced the risk of egg deposition on the plant, often leading to a vicious circle hard to overcome. Successful ovipositions into the stipule in the presence of ants were very rare. Half of all observed attempts in the presence of ants were interrupted due to very aggressive ant behaviour.
In the Eucharitidae, a high host specificity is assumed as well as a certain amount of co-evolution with a particular ant host subfamily (Heraty 2002). We found no evidence that the parasitoid wasp on Leea manillensis had its focus on a specific ant species, genus, or subfamily. In the contrary, we observed a broad range of ant species to get attacked and killed by the planidia, but have no information about the effect of the parasitoid on the ant colony level. As far as it is known, hatching planidia are carried into the colony via foraging ants (Pérez-Lachaud et al. 2006; Lachaud and Perez-Lachaud 2012). On Leea, the number of larvae on the shoots declined over time, and we observed planidia to spread over the whole plant along strands. We assume but do not know whether they were carried away by ant foragers. So far, the attack of adult ants has not been reported for the family Eucharitidae. In our experiments with planidia and different ant species, all of the ants died quite fast. On Leea, we found foraging ants killed and attached to leaves and stems. Ants tend to keep foraging trails free of dead nestmates to avoid disease transmission (e.g. Wilson et al. 1958). Besides lurking on the shoot and other plant parts, lurking on corpses might be another way to ensure a contact with foraging ants. Access to ant colonies might also occur via alternate hosts (Heraty et al. 2004). As we observed the planidia to attack all kinds of insects in the vial experiments, they might indeed use a number of different taxa of insect prey as a transport vehicle. However, the killing of potential host ants foraging on the plant does not fit to what is known so far about phoretic behaviour of the Eucharitidae. At this point, we could only speculate what strategies planidia might use to gain access to the ant host colony.
A growing number of studies provide support for top-down influence on ant community, coexistence, and structure in the form of behavioural responses to parasitoids, although they were yet only reported from dipteran parasitoids (e.g. Feener 1981; Morrison 2000; LeBrun 2005, Feener 2000). Parasitoids not only pose the direct threat of mortality but also provoke a behavioural response of ants, consisting of an alteration of their foraging behaviour. This interaction modification (Wootton 1994) not only affects the defensive function of the ants for the plants, but may also change interactions among competing ant species, in which the presence of the parasitoid indirectly affects the abundance of species. Further investigations should focus on the effects of this parasitoid on different ant species visiting the plant. The unexpectedly strong influence of this parasitoid on the ants and plant in our study shows for a first time that top-down processes might be very important for different trophic levels in facultative ant–plant mutualisms.
References
Báez S, Collins SL, Lightfoot D, Koontz TL (2006) Bottom-up regulation of plant community structure in an aridland ecosystem. Ecology 87:2746–2754
Blüthgen N, Fiedler K (2004) Competition for composition: lessons from nectar-feeding ant communities. Ecology 85:1479–1485
Bronstein JL (1998) The contribution of ant-plant protection studies to our understanding of mutualism. Biotropica 30:150–161
Bronstein JL, Ruben A, Geber M (2006) The evolution of plant-insect mutualisms. New Phytol 172:412–428
Carey B, Visscher K, Heraty JM (2012) Nectary use for gaining access to an ant host by the parasitoid Orasema simulatrix (Hymenoptera, Eucharitidae). J Hymenop Res 27:47–65
Dáttilo W, Marquitti FMD, Guimarães PR Jr, Izzo TJ (2014) The structure of ant-plant ecological networks: Is abundance enough? Ecology 95:475–485
Dìaz-Castelazo C, Guimarães PR Jr, Jordano P, Thompson JN, Marquis RJ (2010) Changes of a mutualistic network over time: reanalysis over a 10-year period. Ecology 91:793–801
do Nascimento EA, Del-Claro K (2010) Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a Neotropical savanna. Flora 205:754–756
Dyer LA (2008) The ecology of tri-trophic interactions in the tropics. In: Carson WP, Schnitzer SA (eds) Tropical forest community ecology. Blackwell Science, Oxford, pp 275–293
Ernest SM, Brown JH (2001) Homeostasis and compensation: the role of species and resources in ecosystem stability. Ecology 82:2118–2132
F.A.O. UN (2010) The food and agriculture organization of the United Nations’s. Global forest resources Assessment 2010. FAO Forestry paper, 163
Feener DH (1981) Competition between ant species: outcome controlled by parasitic flies. Science 214:815–817
Feener DH (2000) Is the assembly of ant communities mediated by parasitoids? Oikos 90:79–88
Fiala B, Linsenmair KE (1995) Distribution and abundance of plants with extrafloral nectaries in the woody flora of a lowland primary forest in Malaysia. Biodiv Cons 4:165–182
Fiala B, Grunsky H, Maschwitz U, Linsenmair KE (1994) Diversity of ant-plant interactions: protective efficacy in Macaranga species with different degrees of ant association. Oecologia 97:186–192
General DM, Alpert GD (2012) A synoptic review of the ant genera (Hymenoptera, Formicidae) of the Philippines. ZooKeys 200:1–111
Godfray HCJ (2007) Parasitoids. Encyclopedia of Biodiversity. Elsevier, Oxford. online version doi:10.1016/B0-12-226865-2/00218-2 ed. Simon A. Levin
Heil M (2004) Direct defense or ecological costs: responses of herbivorous beetles to volatiles released by wild lima bean (Phaseolus lunatus). J Chem Ecol 30:1289–1295
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Heil M, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Syst 34:425–453
Heraty JM (1985) A revision of the Nearctic Eucharitinae (Hymenoptera: Chalcidoidea: Eucharitidae). Proc Entomol Soc Ontario 116:61–103
Heraty JM (1994) Biology and importance of two eucharitid parasites of Wasmannia and Solenopsis. In: Williams DR (ed) Exotic ants. Biology, impact and control of induced species. Westview Press, Boulder, pp 104–120
Heraty JM (2000) Phylogenetic relationships of Oraseminae (Hymenoptera: Eucharitidae). Annu Entomol Soc Am 93:374–390
Heraty JM (2002) A revision of the genera of Eucharitidae (Hymenoptera: Chalcidoidea) of the world. Mem Am Entomol Inst 68:1–367
Heraty JM, Darling D (1984) Comparative morphology of the planidial larvae of Eucharitidae and Perilampidae (Hymenoptera: Chalcidoidea). Syst Entomol 9:30–328
Heraty J, Hawks D, Kostecki JS, Carmichael A (2004) Phylogeny and behaviour of the Gollumiellinae, a new subfamily of the ant-parasitic Eucharitidae (Hymenoptera: Chalcidoidea). Syst Ent 29:544–559
Horvitz CC, Schemske DW (1984) Effects of ants and an ant-tended herbivore on seed production of a Neotropical herb. Ecology 65:1369–1378
Hrcek J, Miller SE, Whitfield JB, Shima H, Novotny V (2013) Parasitism rate, parasitoid community composition and host specificity on exposed and semi-concealed caterpillars from a tropical rainforest. Oecologia 173:521–532
Karban R, Agrawal AA (2002) Herbivore offense. Annu Rev Ecol Syst 33:641–664
Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Funct Ecol 25:348–357
Koptur S (1992) Extrafloral nectary-mediated interactions between insects and plants. Insect Plant Interact 4:81–129
Lachaud J-P, Pèrez-Lachaud G (2012) Diversity of species and behavior of hymenopteran parasitoids of ants: a review. Psyche Article ID 134746. DOI:10.1155/2012/134746
LeBrun EG (2005) Who’s the top dog in ant communities? Resources, parasitoids, and multiple competitive hierarchies. Oecologia 142:643–652
Leibold MA (1989) Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am Nat 134:922–949
McKey D (1974) Adaptive patterns in alkaloid physiology. Am Nat 108:305–320
Meng L-Z, Martin K, Liu J-X, Chen J (2011) Young leaf protection in the shrub Leea glabra in south-west China: the role of extrafloral nectaries and ants. Arthropod-Plant Interact 6(1):59–65
Mody K, Linsenmair KE (2004) Plant-attracted ants affect arthropod community structure but not necessarily herbivory. Ecol Entomol 29:217–225
Molina JE, Wen J, Struwe L (2013) Systematics and biogeography of the non-viny grape relative Leea (Vitaceae). Bot J Linn Soc 171:354–376
Morrison LW (2000) Mechanisms of Pseudacteon parasitoid (Diptera: Phoridae) effects on exploitative and interference competition in host Solenopsis ants (Hymenoptera: Formicidae). Ann Entomol Soc Am 93:841–849
Ness JH, Morris WF, Bronstein JL (2006) Integrating quality and quantity of mutualistic service to contrast ant species protecting Ferocactus wislizeni. Ecology 87(4):912–921
O´Dowd DJ, Catchpole EA (1983) Ants and extrafloral nectaries: no evidence for plant protection in Helichrysum spp.—ant interactions. Oecologia 59:191–200
Oksanen L, Oksanen T (2000) The logic and realism of the hypothesis of exploitation ecosystems. Am Nat 155:703–723
Pérez-Lachaud G, Heraty JM, Carmichael A, Lachaud J (2006) Biology and behavior of kapala (Hymenoptera: Eucharitidae) attacking Ectatomma, Gnamptogenys, and Pachycondyla (Formicidae and Ponerinae) in Chiapas, Mexico. Ann Entomol Soc Am 99:567–576
Pires LP, Del-Claro K (2014) Variation in the outcomes of an ant-plant system: fire and leaf fungus infection reduce benefits to plants with extrafloral nectaries. J Insect Sci 14(1):84
Power ME (1992) Top-down and bottom-up forces in food webs: do plants have primacy. Ecology 73(3):733–774
Rico-Gray V, Díaz-Castelazo C, Ramírez-Hernández A, Guimaraes PR Jr, Holland JN (2012) Abiotic factors shape temporal variation in the structure of an ant-plant network. Arthropod-Plant Interact 6:289–295
Ridsdale CE (1976) A revision of the tribe Cephalantheae (Rubiaceae). Blumea 23:177–188
Rosumek BF, Fernando FAO, Neves FDeS, Barbosa NPDeU, Diniz L, Oki Y, Pezzini F, Fernandes GW, Cornelissen T (2009) Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160:537–549
Rudgers JA (2004) Enemies of herbivores can shape plant traits: selection in a facultative ant-plant mutualism. Ecology 85(1):192–205
Schemske DW (1982) Ecological correlates of a neotropical mutualism: ant assemblages at Costus extrafloral nectaries. Ecology 63:932–941
Schmitz OJ (1993) Trophic exploitation in grassland food chains: simple models and a field experiment. Oecologia 93:327–335
Thompson JN (1999) Specific hypotheses on the geographic mosaic of coevolution. Am Nat 153:1–14
Thompson JN (2006) Mutualistic webs of species. Ecology 312:372–373
Varone L, Briano J (2009) Bionomics of Orasema simplex (Hymenoptera: Eucharitidae), a parasitoid of Solenopsis fire ants (Hymenoptera: Formicidae) in Argentina. Biol Control 48:204–209
Vasconcelos HL (1991) Mutualism between Maieta guianensis Abl., a myrmecophytic melastome, and one of its ant inhabitants: ant protection against insect herbivores. Oecologia 87:295–298
Veijalainen A, Wahlberg N, Broad GR, Erwin TL, Longino JT, Sääksjärvi IE (2012) Unprecedented ichneumonid parasitoid wasp diversity in tropical forests. Proc Biol Sci 279:4694–4698. doi:10.1098/rspb.2012.1664
Wilson EO, Durlach NI, Roth LM (1958) Chemical releasers of necrophoric behavior in ants. Psyche 65:108–114
Wootton JT (1994) Predicting direct and indirect effects: an integrated approach using experiments and path analysis. Ecology 75:151–165
Acknowledgments
The study was generously supported by the DAAD (German Academic Exchange Service). We are very grateful to the Philippine NGO PhilinCon and the staff of the research station Sibaliw for their support, especially Benjamin S. Tacud jr. and Junmar E. Jamangal. We thank the PASU Rhodel B. Lababit (DENR) for the generous permission to work at the NW Panay Natural Park. We also thank Stefan Schmidt (ZMS) for the determination of the parasitoid wasp.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Heikki Hokkanen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schwitzke, C., Fiala, B., Linsenmair, K.E. et al. Eucharitid ant-parasitoid affects facultative ant-plant Leea manillensis: top-down effects through three trophic levels. Arthropod-Plant Interactions 9, 497–505 (2015). https://doi.org/10.1007/s11829-015-9391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-015-9391-y