Abstract
Parasitic loranthus [Taxillus chinensis (DC.) Danser] is an important medicinal plant that produces recalcitrant seeds that are sensitive to dehydration. Desiccation tolerance is critical for the survival of recalcitrant seeds in low-moisture environments. Clarifying how these seeds respond to desiccation is important for long-term conservation. Thus, the viability, germination, microstructure, and antioxidant enzyme activities of dehydrated parasitic loranthus seeds were investigated. Diverse organelles were degraded or deformed during dehydration treatments. Additionally, superoxide dismutase and catalase activities gradually decreased in response to desiccation stress. A proteomic analysis involving TMT-labeling and LC–MS/MS were performed. A total of 1479 proteins were identified, of which 141 were differentially expressed proteins (DEPs) at 16 and 36 h after initiating the dehydration treatments. A functional annotation based on gene ontology revealed that the DEPs were mainly localized in chloroplasts and were related to energy metabolism, responses to stimuli, and the regulation of biological processes. A KEGG pathway enrichment analysis determined that several of the identified proteins were associated with signal transductions, photosynthesis, and glycolysis/gluconeogenesis. The results suggest that the efficient removal of excessive ROS amounts may be crucial for promoting parasitic loranthus seed germination under dehydration stress conditions. A series of candidate dehydration stress-related proteins were identified and may be relevant for enhancing the dehydration tolerance of the recalcitrant seeds. To the best of our knowledge, this is the first study to elucidate the possible molecular mechanisms underlying the sensitivity of recalcitrant parasitic loranthus seeds to dehydration via a proteomic analysis involving TMT-labeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic loranthus [Taxillus chinensis (DC.) Danser], which is also known as ‘Sang ji sheng’, grows by parasitizing other plants. Moreover, it is mostly distributed in southern China and is an important medicinal plant in the pharmaceutical industry (Moghadamtousi et al. 2014; Wei et al. 2020). Specifically, its branches and leaves are widely used in traditional Chinese medicines for treating rheumatism, hypertension, and obesity as well as for preventing miscarriages (Wang et al. 2008; Ding et al. 2013). A previous study revealed that parasitic loranthus extracts by ethanol, ethyl acetate and aqueous solution, containing triterpenes, lectins, polysaccharides, and alkaloids (Wong et al. 2012).
Seeds can be divided into the following three main categories based on their dehydration tolerance: orthodox, intermediate, and recalcitrant (Roberts 1973) . Recalcitrant seeds are sensitive to dehydration and unable to survive the low-moisture conditions required for long-term storage (Berjak and Pammenter 2013) . Parasitic loranthus produces recalcitrant seeds because their germination rate decreases rapidly under dry conditions, and their breeding is very difficult, what’s more, the seeds are the only reproductive materials (Wei et al. 2017). So it is important to understand seed germination to inform strategy for the propagation and conservation of recalcitrant species. Drought stress induces various physiological activities and changes to the expression of drought-responsive genes (Wei et al. 2017; Pammenter and Berjak 2014) . Regarding parasitic loranthus, the sensitivity of its seeds to dehydration is one of the major abiotic factors limiting the long-term conservation of germplasm by cryopreservation. Despite the increasing interest in seed dehydration sensitivity, there have been relatively few related studies involving Loranthaceae species. Previously, high-throughput RNA sequencing technology were performed to estimate the gene expression changes occurring during the dehydration process. The resulting global transcriptome profile provided insights into gene regulation in response to water loss (Wei et al. 2017).
Global proteomic analyses can generate substantial amounts of information at the protein level. Comparative proteomics involving tandem mass tag (TMT) labeling is a powerful approach for investigating protein variations due to environmental changes (Washburn et al. 2001). To date, proteome changes induced by dehydration have been investigated in some plant species (Balbuena et al. 2011; Kalemba and Pukacka 2012; James et al. 2015) . Previous studies have clarified the drought tolerance mechanism, including the regulatory effects of protein expression changes on signal transduction. In the current study, the proteomic changes in parasitic loranthus seeds in response to various dehydration treatments were analyzed. The objective of this investigation was to identify the differentially expressed proteins (DEPs) under different dehydration conditions to elucidate the molecular and physiological mechanisms underlying the sensitivity of parasitic loranthus seeds to dehydration. Our findings may be useful for improving the conservation of parasitic loranthus seeds and not only provides theory basis for the key technology in the preservation of Taxillus chinensis (DC.) Danser seeds, but also new knowledge and technology for other recalcitrant seed’s preservation.
Materials and methods
Seed dehydration, germination and processing
Parasitic loranthus plants were grown in the germplasm nursery of the Guangxi Botanical Garden of Medicinal Plants. The seeds were collected from trees as previously described, and all the treatments followed that of our previous research and used the same experimental design and seed samples (Wei et al. 2017). They were dehydrated for 4, 8, 12, 16, 20, 24, 28, 32, 36, and 40 h in the different desiccator which containing the same anhydrous silica gel as the desiccant. The dehydrated seeds were incubated at 25 °C for a germination treatment, ultrastructural imaging and other analyses. At each time-point, three replicates of 25 seeds were placed in dishes containing moistened filter paper to determine the germination rate and assess seed viability. Germination was considered to have occurred when a 5-mm radicle protruded from the seed. Seed viability was evaluated according to the tetrazolium chloride method (Egido et al. 2017). On the basis of previous studies (Wei et al. 2017), proteomic analysis was carried out with fresh seeds (0 h), 16 h and 36 h, respectively. The workflow for the proteomic analysis is presented in Fig. 1.
Transmission electron microscopy
Seeds were prepared for an examination by transmission electron microscopy (TEM) as previously described (Spurr 1969; Lechowska et al. 2019) . Briefly, parasitic loranthus seeds were fixed with 2% glutaraldehyde and then treated with 1% osmic acid (OsO4), after which they were embedded in low-viscosity epoxy resin. Ultrathin sections were prepared with an ultramicrotome (LKB-7800, Sweden) and immersed in a 2% aqueous solution of uranyl acetate. They were then examined with the HT7700 transmission electron microscope (Hitachi, Japan).
Antioxidant enzyme and phytohormone analyses
Parasitic loranthus seeds were ground to a powder. To measure the seed malondialdehyde (MDA) content, 50 mg ground seed material was treated with 5 mL 5% trichloroacetic acid. To quantify the superoxide anion (\({\text{O}}_{2}^{ - }\)) content, 50 mg ground seed material was treated with 0.1% trichloroacetic acid. After a 5-min centrifugation at 10,000 × g, the supernatant was collected and used to measure the hydrogen peroxide (H2O2) content. The MDA and \({\text{O}}_{2}^{ - }\) contents were measured as previously described (Sahitya et al. 2018). The peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) activities were determined with commercial assay kits and the Epoch microplate spectrophotometer (Biotek, Winooski, VT, USA). The indole-3-acetic acid (IAA), zeatin riboside (ZR), gibberellin (GA), and abscisic acid (ABA) phytohormone levels were quantified with a liquid chromatography and mass spectrometry (LC–MS) system (1120-6460, Agilent, USA). IAA, ZR, GA and ABA were all extracted from loranthus seeds based on previous method (Pan et al. 2010). The phytohormone were separated by C18 column (Hypersil Gold, 100 mm × 2.1 mm, 1.9 μm, Thermo Fisher Scientific) at flow rate of 0.3 mL/min with a 17 min gradient elution. For Multiple Reaction Monitoring (MRM), the phytohormone were analyzed with negative mode by ESI ion source.
Protein extraction
Briefly, 20 seeds collected at three time-points (0, 16, and 36 h) were ground to a powder in liquid nitrogen, after which 3–4 g ground seed material was suspended in 1 mL lysis solution (8 M urea, 2% CHAPS, 50 mM DTT, and protease inhibitor). The solution was then centrifuged at 20,000 × g for 30 min. The pellet was washed twice with chilled acetone and resuspended in 1 mL solution. After another centrifugation, the supernatant was collected and the protein concentration was determined with a 2-D Quant Kit (GE Healthcare, Little Chalfont, UK).
Protein digestion, labeling, and LC–MS/MS separation
In each replicate, proteins (100 μg) from the samples collected at 0 (control time-point), 16, and 36 h were alkylated and digested according to the published filter-assisted sample preparation (FASP) method (Wisniewski et al. 2009). The digested peptides were collected by centrifugation and labeled with TMT reagents (Pierce, Rockford, USA). The labeled peptides for the three time-points were combined and fractionated by by high-pH reversed phase LC involving an XBridge C18 column (4.6 mm internal diameter, 25 cm, 5 μm, 130 Å; Waters, Milford, USA) and an LC instrument (Waters e2695). For the LC and tandem MS (MS/MS) analysis, 10 peptide fractions were separated on an nLC-1000 LC system (Thermo Fisher, Odense, Denmark) at a flow rate of 300 nL/min with a 60 min gradient elution. The eluted peptides were analyzed with the LTQ-Orbitrap mass spectrometer (Thermo Fisher, Bremen, Germany). Survey scans in the 150–1800 m/z range were acquired, followed by MS/MS scans of the 10 most abundant precursor ions resulting from collision-induced dissociation fragmentation.
Protein identification and bioinformatic analysis
To identify and quantify proteins, the raw data were used to screen a custom database with the Proteome Discoverer software. Carbamidomethylation and TMT-6plex were set as fixed modifications. The mass tolerance of the precursor ions was ± 20 ppm and that of the fragment ions was ± 0.02 Da. Only peptides that were filtered with a 95% confidence level were accepted. All identified proteins were validated with a decoy database. Furthermore, gene ontology (GO) classifications were analyzed with the DAVID 6.8 program (Huang et al. 2009). The proteins were also examined with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to identify enriched pathways. A heat map was plotted with MeV 4.0 software.
Results
Microstructural changes
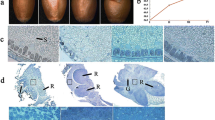
Seed microstructural changes due to dehydration were investigated by optical imaging and TEM. Parasitic loranthus seeds comprise a seed coat, seed hole, radicle, embryo, and endosperm (Fig. 2). The fresh parasitic loranthus seeds were intensely stained with a clear cellular border, the radicle cortex was covered by epidermal cells that were closely arranged in one layer and the epidermal cells were linked to intercortical cells with tight gap junctions. The 16-h dehydration resulted in partial cytoplasmic degradation that gradually increased the intercellular space of the radicle, embryo, and endosperm. The 36-h dehydration (i.e., moisture content less than 25%) led to lightly stained tissues and further increases in the intercellular space. The optical imaging results revealed distinct microstructural changes in the parasitic loranthus seeds due to dehydration.
An examination of the ultrastructural images (10,000 × and 30,000 × magnification) indicated the fresh seeds (0 h) had clear and intact cell structures (Fig. 3). The nucleus, nucleolus, and nuclear membrane were clearly visible and the border between the nucleus and cytoplasm was distinctly defined. Additionally, a regularly shaped lipid droplet and a few vacuoles were detected in the cytoplasm. Following the 16-h dehydration, the whole cellular structure was nearly intact, but the cellular space was larger than that of fresh seeds. The nuclear membrane was irregularly shaped because of the dehydration-associated extrusion. The 36-h dehydration caused the nuclear membrane to degrade, in some cases completely, and the nucleus was fragmented. Cell vacuolation increased and the vacuoles contained precipitated starch. The ultrastructural analysis indicated the dehydration treatments resulted in various degraded and deformed cellular organelles as well as the formation of cellular cavities.
Oxidative damage and antioxidant enzyme activities
To investigate the dehydration-induced oxidative damage in parasitic loranthus seeds, the MDA and \({\text{O}}_{2}^{ - }\) concentrations were measured. The MDA content in parasitic loranthus seeds started to increase at 12 h after initiating the dehydration treatments (Fig. 4). The \({\text{O}}_{2}^{ - }\) level gradually increased from 12 to 28 h and then remained relatively stable until the 36-h time-point. The results indicated that excessive amounts of reactive oxygen species (ROS) accumulated starting at the 12-h time-point, resulting in a constant increase in the oxidative damage to parasitic loranthus seeds.
The SOD and POD activities of parasitic loranthus seeds increased slowly for the first 16 h of the dehydration treatments (Fig. 5). However, at the subsequent time-points, the SOD activities decreased slightly to 15 U/mg. In contrast, the POD activities increased after the 20 h time-point, peaking at 36 h (250 U/mg). The CAT activities gradually decreased throughout the dehydration treatments, but was relatively stable at the later time-points.
Phytohormone concentrations
Four important phytohormones, ABA, IAA, ZR, and GA, were analyzed by LC–MS. The ABA and GA contents were rapidly affected by the dehydration stress, with significant increases detected starting at the 4-h time-point (Fig. 6). In contrast, IAA and ZR levels started to increase at 8 h after initiating the dehydration treatments. The overall changes in the IAA, ZR, and GA contents were similar. Specifically, the IAA, ZR, and GA contents peaked at the 16-h time-point, with values that were more than twofold greater than the corresponding control levels. The contents of all three of these hormones subsequently gradually decreased. Interestingly, the ABA content increased and initially peaked at 16 h. After a transient decrease, the ABA level slowly increased, peaking again at the 36-h time-point.
Proteomic profiles and analyses
A proteome-level investigation produced 21,987 peptide-to-spectrum matches, and 8,500 peptides were acquired. Ultimately, 1,479 overlapping proteins in three replicates were identified (Fig. 7). All identified proteins had at least one unique peptide (P < 0.01). A complete proteome list with protein names and accession numbers is presented in Table S1. Moreover, most protein molecular weights ranged from 20 to 60 kDa, whereas the isoelectric point mainly ranged from 5 to 7.
Comparative proteomic analyses of dehydration treatment times (16 h vs. 0 h, 36 h vs. 0 h, and 36 h vs. 16 h) were completed to identify dehydration stress-related proteins in parasitic loranthus seeds. The proteins whose abundance significantly differed by at least 1.5-fold between the analyzed time-points (P < 0.05) were defined as differentially expressed proteins (DEPs). 40 upregulated and 40 downregulated DEPs following the 16-h dehydration were detected, whereas 48 upregulated and 38 downregulated DEPs were revealed in response to the 36-h dehydration. A total of 141 DEPs were responsive to the 16- and 36-h dehydration treatments. A Venn analysis revealed 25 overlapping proteins (17.7%) that were affected at both time-points (i.e., 16 and 36 h).
The annotated proteins were classified into the three main GO categories (cellular component, biological process, and molecular function). Details regarding the bioinformatic analysis are provided in Table S2. Additionally, the main subcellular locations of the DEPs were as follows: chloroplast, cytosol, nucleus, mitochondria, endoplasmic reticulum, and extracellular. Several GO terms for crucial biological processes were significantly enriched, including response to stimulus, regulation of biological process, cellular component organization, and multi-organism process (Fig. 8).
The DEPs resulting from seed dehydration treatments were further characterized by identifying the associated enriched KEGG pathways. During the early dehydration stage, several key pathways were responsive to the treatments. The proteins with increased abundance were mainly involved in pathways related to RNA degradation, sphingolipid metabolism, glutamine and glutamate metabolism, amino acid biosynthesis, endocytosis, nitrogen metabolism, and the spliceosome. The proteins with decreased abundance were associated with starch and sucrose metabolism, galactose metabolism, and photosynthesis (Fig. 9). During the later dehydration stage, the proteins with increased abundance were involved in only three pathways, namely fructose and mannose metabolism, the pentose phosphate pathway, and glycolysis/gluconeogenesis, implying that energy metabolism was affected, which similar to the transcriptome level we studied before, It was also during the late stage of dehydration that its energy metabolism was affected. In contrast, the proteins whose abundance decreased during the later dehydration stage were related to hormone synthesis, protein processing in the endoplasmic reticulum, and the galactose metabolism (Fig. 9).
The x axis presents the number of DEPs in each pathway, whereas the y-axis presents the KEGG pathway categories.
Identification of dehydration stress-related proteins
A heat map clustering analysis was performed to reveal the dynamic expression patterns of DEPs and to identify the dehydration stress-related proteins. The DEPs were grouped in three main clusters based on their expression profiles at 16 and 36 h after starting the dehydration treatments. The abundance of the proteins in the first cluster continued to increase in response to dehydration stress. These proteins were mainly metabolic enzymes, including crucial ones (e.g., glutathione S-transferase), and heat shock proteins (HSPs), which have key roles in plant stress responses, such as the heat shock proteins were up-regulated in the early stage of dehydration (16-h) and down-regulated in the late stage of dehydration (36-h), it’s worth mentioning that the same trend occurred in the previous transcriptome analysis. The second cluster comprised POD and glucan endo-1,3-beta glucosidase (GLU), whose abundance decreased during the early dehydration stage and then subsequently increased. The proteins in the third cluster were negative effectors of dehydration stress responses, and their contents decreased throughout the dehydration treatments. These proteins may contribute to chitinase, amylase, G3PDH, and protein kinase activities (Fig. 10). The proteins related to dehydration stress responses are listed in Table S3.
Discussion
Parasitic loranthus is often included in traditional Chinese medicines. Long-term analyses confirmed that parasitic loranthus seeds are always dispersed as fresh seeds under natural conditions, but the parasitic loranthus seeds were typical recalcitrant which were fast inactivation and very sensitive to dehydration, which affected their preservation and breeding seriously. A seed germination treatment indicated that the germination rate decreases from 86 to 40% after a 3-day storage period, but decreases to 5% following a 6-day storage. In the current study, the viability and germination rate decreased quickly when parasitic loranthus seeds were dehydrated. Thus, the viability and germination rate of these seeds are associated with seed moisture contents. Regarding the 16-h dehydration, the germination rate decreased from 86 to 66%, whereas the moisture content decreased from 50.7% to 37.2%. For the 36-h dehydration, the seed moisture content decreased to 24.9%, the seed viability decreased to 15%, and the germination rate was only 6%. Earlier investigations proved that the seed moisture content influences several biochemical reactions related to germination and is a key factor in the early seed germination stage (Weitbrecht et al. 2011; Dekkers et al. 2013). Subsequent studies indicated that an exposure to dehydration stress leads to high \({\text{O}}_{2}^{ - }\) concentrations and oxidative damage in seeds. The dehydration-induced germination rates and the accumulation of ROS exhibited the opposite trends. Increases in ROS were accompanied by changes to the activities of antioxidant enzymes, including SOD, POD, and CAT. However, the parasitic loranthus seed responses to dehydration stress were relatively unknown at the molecular level. Recently, the gene expression profiles changes in parasitic loranthus seeds due to water loss for the first time (Wei et al. 2017). To further investigate the dehydration stress-related proteins in recalcitrant parasitic loranthus seeds, we completed microstructural and proteomic analyses of the seeds that underwent 16 and 36 h dehydration treatments.
Proteomic experimental techniques are useful for global analyses of protein abundance, and may be more effective than transcriptomic methods for inferring the roles of dehydration stress-related proteins. In this study, the peptides generated from the total proteins extracted from seeds after 0-h (control), 16-h, and 36-h dehydration treatments were labeled with TMTs and analyzed by LC–MS/MS (Unwin et al. 2010). An evaluation of the resulting data revealed the mass error was less than 10 ppm, confirming the accuracy of the MS data. The peptides were mainly 7–20 amino acids long, indicative of a highly efficient enzymatic digestion (Fig. 11). Our experimental approach resulted in 1479 overlapping proteins in three biological replicates. The sequence coverages of the identified proteins are presented in Table S1. Because the parasitic loranthus genome has not been sequenced, the protein data were used to screen databases assembled with RNA sequencing data, which may have limited the number of identified proteins.
Energy metabolism related to the chloroplast response to dehydration stress
Energy metabolism mainly affected the seeds during the later dehydration stage, it was worth emphasizing that the similar changes were seen in previous transcriptome analyses, the genes related to energy metabolism also accumulated in the later dehydration stage (Wei et al. 2017), we concluded that the transcriptional changes were found at the translational level, so this study verified the effect of dehydration on energy metabolism from the proteomic perspective. Our subcellular localization analysis revealed that 40% and 35% of the DEPs identified at 16 and 36 h after starting the dehydration treatment were located in the chloroplast, respectively. Accordingly, the chloroplast appears to be one of the organelles in parasitic loranthus seeds affected by dehydration stress. Additionally, our finding that the DEPs were mainly related to chlorophyll proteins is consistent with the results of a previous study on the artificial aging of wheat seeds (Lv et al. 2016). Chloroplasts are involved in cell wall reinforcement and help generate energy via their roles in photosynthesis as well as the production of carbohydrates, plastids, proteins, and fatty acids. Generally, the biochemistry underlying photosynthesis in seeds is fundamentally different than the corresponding activity in leaves. For example, seed photosynthesis relies on sucrose derived from plant tissues as the carbon source and the CO2 produced is released during seed respiration (Fig. 12) (Berjak et al. 2014; Galili et al. 2014). In the seed cytoplasm, sucrose is cleaved by invertase to generate α-glucose and β-fructose, which then contribute to glycolysis. In our proteomic analysis, the abundance of some glycolysis-related proteins, including glucose pyrophosphorylase and fructose aldolases, increased during the dehydration treatments, suggesting that glycolysis is enhanced when parasitic loranthus seeds are exposed to dehydration stress. The increase in glycolysis may help satisfy energy requirements and enable the seeds to counteract the detrimental effects of dehydration stress. The accumulated pyruvate is decarboxylated, and the acetyl-CoA formed is involved in fatty acid biosynthesis. In this process, the increase in the acetyl-CoA content is conducive to fatty acid synthesis and CO2 production. Finally, the CO2 generated by pyruvate dehydrogenase is converted to 1, 3-diphosphoglyceric acid (i.e., primary product of the Calvin cycle) in a reaction catalyzed by ribulose-1, 5-bisphosphate carboxylase/oxygenase (Baud and Lepiniec 2010; Tschiersch et al. 2011) . The significant increase in the expression of Calvin cycle-related proteins and cytochrome complexes may enhance the efficiency with which chloroplast synthesizes NADPH and ATP, which are required for the conversion of pyruvate to acetyl-CoA and fatty acids. Simultaneously, the O2 resulting from chloroplast photosynthetic activities supports mitochondrial respiration during parasitic loranthus seed responses to dehydration-mediated damages. Thus, seed chloroplasts are likely the main organelles responsive to dehydration stress.
Changes to antioxidant enzyme activities induced by dehydration stress
Dehydration leads to oxidative stress in seeds, which adversely affects photosynthetic and respiratory processes, resulting in the accumulation of ROS. In dehydrated recalcitrant seeds, the plasma membrane is the primary site damaged by ROS, including H2O2 and \({\text{O}}_{2}^{ - }\) (Sershen et al. 2016; Chandra and Keshavkant 2018). An appropriate ROS level is beneficial for stress signaling and the activation of defense mechanisms, but excessive amounts of ROS can lead to abnormal cellular metabolism. Damages due to accumulated ROS lead to a loss of cell membrane structural integrity. The \({\text{O}}_{2}^{ - }\) contents determined in this study corroborate the findings of previous investigations (Bai et al. 2012; Chen et al. 2011; Son et al. 2016). The rapid accumulation of \({\text{O}}_{2}^{ - }\) in dehydrated seeds disrupts cellular homeostasis. Moreover, the MDA content is an indicator of cell membrane oxidation. In the current study, the dehydration treatments increased and decreased the MDA and seed moisture contents, respectively. The accumulation of MDA implied the cell membranes were increasingly being damaged and was negatively correlated with seed germination. The TEM analysis confirmed that the 36-h dehydration treatment damaged the cell membrane integrity in parasitic loranthus seeds. This time-point also coincided with the peak \({\text{O}}_{2}^{ - }\) and MDA contents.
Under optimal conditions, the balance between ROS generation and elimination is tightly controlled by components of the antioxidative system, including a series of antioxidant enzymes. Previous studies proved that antioxidants are important for scavenging excess ROS (Chandra and Keshavkant 2018; Chen et al. 2011). Antioxidant enzymes include CAT, glucan endo-1,3-beta glucosidase (GLU), glutathione reductase, glutathione peroxidase (GP), POD, and SOD. The observed antioxidant enzyme activities were consistent with the results of our proteomic analysis. Plant with high levels of antioxidants, either constitutive or induced, have been reported to have greater resistance to oxidative damage. Dehydration stress induced a rapid decrease in the activities of various antioxidant enzymes, including a GP, two PODs, and a GLU, were determined during the early dehydration stage. The decrease in GP activity inhibits the cell redox homeostasis and triggers defense-related signals. Moreover, SOD contributes to the initial defense responses by detoxifying \({\text{O}}_{2}^{ - }\) (Khan et al. 2017). Therefore, inhibited SOD activities may promote ROS accumulation and are negatively correlated with the \({\text{O}}_{2}^{ - }\) content. Simultaneously, the dismutation of \({\text{O}}_{2}^{ - }\) leads to the formation of H2O2, which is a substrate of CAT (i.e., another important defense system enzyme). The results of an earlier study indicated CAT is essential for eliminating H2O2 under abiotic stress conditions (Choudhury et al. 2017). The loss of SOD and CAT activities results in the inability to mitigate the oxidative stress induced by dehydration. Damages due to the excessive accumulation of ROS may contribute to the sensitivity of parasitic loranthus seeds to dehydration stress. The current relative lack of available information regarding how ROS are regulated in recalcitrant seeds should be addressed in future studies, which may be useful for improving parasitic loranthus seed germination via the regulation of antioxidant enzyme activities.
Phytohormone responses to dehydration stress
Abscisic acid is an important hormone that functions in adaptive responses to various environmental stresses (Hauser et al. 2011). Additionally, ABA is a vital factor for seed dehydration tolerance (Son et al. 2016; Jin et al. 2018). In the current study of parasitic loranthus seeds, the dehydration-induced changes to the ABA content differed from the changes to the other examined phytohormones. The ABA, IAA, ZR, and GA concentrations increased significantly from 4 to 16 h after initiating the dehydration treatments, with peak levels at 16 h. Thereafter, the IAA, ZR, and GA levels decreased quickly, whereas the ABA content increased, peaking at 40 h. In a previous study, 49 ABA-associated transcripts were detected, of which 14 were differentially expressed following a dehydration treatment (Wei et al. 2017). Interestingly, in the current study, we revealed the increased abundance of ABA stress-ripening protein 1 (ASR1), Pyrabactin resistance like proteins (PYLs) related to ABA synthesis, that are also included in the 14 above. Moreover, the ASR1 was more abundant in dehydrated parasitic loranthus seeds than in fresh seeds. Thus, comprehensive transcriptome and proteome analyses suggest that ABA synthesis and signaling likely influence the desiccation sensitivity of recalcitrant parasitic loranthus seeds. Among the ABA-dependent proteins, the production of HSPs is reportedly induced by dehydration stress in seeds (Kaur et al. 2015). In the current study, HSP70 and HSC70 contents increased following dehydration treatments, suggesting that increasing the abundance of HSPs in parasitic loranthus seeds may lead to enhanced dehydration stress tolerance.
Among the various phytohormones, ABA and GA are considered to be major regulators of dormancy and germination (Dussert et al. 2018). Under unstressed conditions, ABA positively regulates and maintains seed dormancy, whereas GA promotes seed germination (Vishal and Kumar 2018) . When seeds are exposed to dehydration stress, the defense responses activated depend on the crosstalk between the ABA and GA signaling pathways rather than on the individual contributions of each hormone (Finch and Footitt 2017; Verma et al. 2016). Therefore, detailed examinations of the crosstalk among phytohormone pathways may lead to a more thorough understanding of the complexity of hormone regulatory activities. Additional studies on the homeostasis of key hormones may provide new insights into seed germination and the regulation of seed responses to dehydration.
To the best of our knowledge, this is the first study to explore the ultrastructure and comparative proteomic profiles of recalcitrant parasitic loranthus seeds during dehydration treatments. Our data indicate that parasitic loranthus seed viability and germination are positively correlated with the seed moisture content. An ultrastructural analysis suggested that the dehydration of parasitic loranthus seeds degrades or deforms various cellular components, especially the nuclear membrane and cellular junctions. Antioxidant enzyme assays confirmed SOD and CAT activities decrease in response to dehydration, and the resulting excessive ROS contents may adversely affect the seeds. Our proteomic investigation combining TMT-labeling and LC–MS/MS identified 1,479 proteins, including 141 DEPs. The subsequent GO and KEGG functional analyses indicated that the DEPs were mostly localized to the chloroplast and were involved in diverse functions, including signal transductions, photosynthesis, and glycolysis/gluconeogenesis. The variation on proteins related to enzyme activities confirmed the result of antioxidant enzyme assays. Energy metabolism related to chloroplast response to dehydration stress was induced. The 27 candidate dehydration stress-related proteins identified in this study may be relevant for improving the dehydration tolerance of recalcitrant parasitic loranthus seeds. The data presented herein provide molecular insights into the mechanisms underlying the desiccation sensitivity of recalcitrant seeds. Furthermore, the study findings may provide insights into the mechanism regulating the desiccation sensitivity of recalcitrant seeds.
Abbreviations
- DEPs:
-
Differential expressed proteins
- ROS:
-
Reactive oxygen species
- POD:
-
Peroxidase
- CAT:
-
Catalase
- ZR:
-
Zeatin riboside
- ABA:
-
Abscisic acid
- FASP:
-
Filter-assisted sample preparation
- GP:
-
Glutathione peroxidase
- TCA:
-
Tricarboxylic acid cycle
- LC–MS:
-
Liquid chromatography and mass spectrometry
- TMT:
-
Tandem mass tag
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- IAA:
-
Indole-3-acetic acid
- GA:
-
Gibberellin
- TEM:
-
Transmission electron microscopy
- GLU:
-
Glucan endo-1,3-beta glucosidase
- MAPK:
-
Mitogen-activated protein kinase
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Bai XG, Chen JH, Kong XX, Toddd CD, Yang YP, Hu XY, De ZL (2012) Carbon monoxide enhances the chilling tolerance of recalcitrant Baccaurea ramiflora seeds via nitric oxide-mediated glutathione homeostasis. Free Radic Biol Med 53:710–720
Balbuena S, Jo L, Pieruzzi P (2011) Differential proteome analysis of mature and germinated embryos of Araucaria angustifolia. Phytochemistry 72:302–311
Baud S, Lepiniec L (2010) Physiological and developmental regulation of seed oil production. Prog Lipid Res 49:235–249
Berjak N, Pammenter W (2013) Implications of the lack of desiccation tolerance in recalcitrant seeds. Front Plant Sci 4:478
Berjak P, Cherian J, Makhathini P, Sershen PW (2014) Embryonic axes of a tropical recalcitrant-seeded species: successful elimination of micro-organisms and potential for zygotic synthetic seed (synseed) production. Seed Sci Technol 42:150–160
Chandra J, Keshavkant S (2018) Desiccation-induced ROS accumulation and lipid catabolism in recalcitrant Madhuca latifolia seeds. Physiol Mol Biol Plants 24(1):75–87
Chen Q, Yang L, Ahmad P, Wan X, Hu X (2011) Proteomic profiling and redox status alteration of recalcitrant tea (Camellia sinensis) seed in response to desiccation. Planta 233:583–592
Choudhury F, Rivero R, Blumwald MR (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Dekkers BJ, Pearce S, Veldkamp RP, Marshall A, Widera P, Gilbert J, Drost HG, Bassel GW, Muller K, King JR, Wood AT, Grosse I, Quint M, Krasnogor N, Leubner-Metzger G, Holdsworth MJ, Bentsink L (2013) Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol 163:205–215
Ding B, Dai Y, Hou Y, Wu XM, Chen X, Yao XS (2013) Four new hemiterpenoid derivatives from Taxillus chinensis. Fitoterapia 86:1–5
Dussert S, Serret J, Bastos-Siqueira A, Morcillo F, Dechamp E, Rofidal V, Lashermes P, Etienne H, Thierry JO (2018) Integrative analysis of the late maturation programme and desiccation tolerance mechanisms in intermediate coffee seeds. J Exp Bot 69:1583–1597
Egido L, Navarro D, Martinez V, Toorop E, Iannetta P (2017) A spectrophotometric assay for robust viability testing of seed batches using 2,3,5-Triphenyl Tetrazolium chloride: using Hordeum vulgare L. as a model. Front Plant Sci 8:747
Finch W, Footitt S (2017) Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J Exp Bot 68(4):843–856
Galili G, Wittenberg T, Angelovici R, Fernie AR (2014) The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front Plant Sci 5:1–6
Hauser F, Waadt R, Schroeder J (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21:346–355
Huang W, Sherman B, Lempicki R (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
James S, Christina W, Pammenter W, Berjak P (2015) Why is intracellular ice lethal A microscopical study showing evidence of programmed cell death in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum. Ann Bot 115(6):991–1000
Jin XF, Liu DD, Ma LL, Gong ZM, Cao D, Liu YL, Li YY, Jiang CJ (2018) Transcriptome and expression profiling analysis of recalcitrant tea (Camellia sinensis L.) seeds sensitive to dehydration. Int J Genomics 2018:1–11
Kalemba M, Pukacka S (2012) Association of protective proteins with dehydration and desiccation of orthodox and recalcitrant category seeds of three Acer genus species. J Plant Growth Regul 31(3):351–362
Kaur H, Petla BP, Kamble NU, Singh A, Rao V, Salvi P, Ghosh S, Majee M (2015) Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front Plant Sci 6:713
Khan M, Mobin M, Abbas Z, AlMutairi K, Siddiqui Z (2017) Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem 110:194–209
Lechowska K, Kubala S, Wojtyla L, Nowaczyk G, Quinet M, Lutts S, Garnczarska M (2019) New insight on water status in germinating Brassica napus seeds in relation to priming-improved germination. Int J Mol Sci 20(3):540
Lv Y, Zhang S, Wang J, Hu Y (2016) Quantitative proteomic analysis of wheat seeds during artificial ageing and priming using the isobaric tandem mass tag labeling. PLoS ONE 11:e0162851
Moghadamtousi S, Kamarudin M, Chan K, Goh H, Kadir H (2014) Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med 42(1):23–35
Pammenter W, Berjak P (2014) Physiology of desiccation sensitive (Recalcitrant) seeds and the implications for cryopreservation. Int J Plant Sci 175(1):21–28
Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5:986–992
Roberts E (1973) Predicting the storage life of seeds. Seed Sci Technol 1:99–514
Sahitya U, Krishna M, Deepthi R, Prasad G, Kasim D (2018) Seed antioxidants interplay with drought stress tolerance indices in chilli (Capsicum annuum L) seedlings. BioMed Res Int. https://doi.org/10.1155/2018/1605096
Sershen VB, Naidoo C, Pammenter NW (2016) The use of plant stress biomarkers in assessing the effects of desiccation in zygotic embryos from recalcitrant seeds: challenges and considerations. Plant Biol 18(3):433–444
Son S, Chitnis V, Liu A, Gao F, Nguyen T, Ayele B (2016) Abscisic acid metabolic genes of wheat (Triticum aestivum L.): identification and insights into their functionality in seed dormancy and dehydration tolerance. Planta 244:429–447
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26(1–2):31–43
Tschiersch H, Borisjuk L, Rutten T, Rolletschek H (2011) Gradients of seed photosynthesis and its role for oxygen balancing. Biosystems 13:302–308
Unwin R, Griffiths J, Whetton A (2010) Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC-MS/MS. Nat Protoc 5:1574–1582
Verma V, Ravindran P, Kumar P (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86
Vishal B, Kumar P (2018) Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front Plant Sci 9:1–15
Wang Y, Deng M, Zhang S, Zhou Z, Tian W (2008) Parasitic loranthus from Loranthaceae rather than Viscaceae potently inhibits fatty acid synthase and reduces body weight in mice. J Ethnopharmacol 118(3):473–478
Washburn P, Wolters D, Yates R (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19:242–247
Wei SG, Ma XJ, Pan LM, Miao JH, Fu JE, Bai LH, Zhang Z, Guan Y, Mo CM, Huang H, Chen M (2017) Transcriptome analysis of Taxillusi chinensis (DC.) Danser seeds in response to water loss. PLoS ONE 12(1):e0169177
Wei SG, Wan LY, He LL, Wei Y, Long HR, Ji XW, Fu JE, Pan LM (2020) De novo transcriptome reveals gene changes in the development of the endosperm Chalazal Haustorium in Taxillus chinensis (DC.) Danser. BioMed Res Int. https://doi.org/10.1155/2020/7871918
Weitbrecht K, Muller K, Leubner G (2011) First off the mark: early seed germination. J Exp Bot 62:3289–3309
Wisniewski J, Zougman A, Mann M (2009) Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res 8:5674–5678
Wong Z, Kadir H, Ling S (2012) Bioassay-guided isolation of neuroprotective compounds from Loranthus parasiticus against H2O2-induced oxidative damage in NG108-15 cells. J Ethnopharmacol 139(1):256–264
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81660637, 81703649, 81860672, 81960695), the Guangxi Natural Science Foundation, China (2016GXNSFDA380012, 2017GXNSFDA198026, 2018GXNSFAA281089), the Guangxi Botanical Garden of Medicinal Plants Research and Innovation Team Building Project (GYCH2019008) and the Scientific research funding project of Guangxi Botanical Garden of Medicinal Plants (GYJ202012). We thanks the Core facility center of State Key Laboratory for Conservation and Utilization of Subtropical Agro-resources (SKLCUSA) for mass spectrometer support. We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, L., Wan, L., He, L. et al. Comparative proteomic analysis of parasitic loranthus seeds exposed to dehydration stress. Plant Biotechnol Rep 15, 95–108 (2021). https://doi.org/10.1007/s11816-020-00651-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-020-00651-4