Abstract
The Arabidopsis calcineurin B-like protein-interacting protein kinase CIPK21 gene (AtCIPK21) plays important roles in cell metabolism, plant development, and abiotic stress responses. However, the function of the AtCIPK21 gene in cold stress tolerance in plant cells is not fully understood. In this study, we use cell cultures of three plant species including rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), and white pine (Pinus strobus L.), as well as Agrobacterium tumefaciens strain GV3101 harboring pBI-AtCIPK21 to generate transgenic cell lines. After confirmation of integration of the AtCIPK21 gene into the genome by polymerase chain reaction (PCR), southern blotting, and northern blotting analyses, the rice, cotton, and pine AtCIPK21 transgenic cell lines were used to examine cold stress tolerance. The experimental results demonstrated that overexpression of the AtCIPK21 gene enhanced cold stress tolerance of transgenic cells by increasing cell viability and cell growth rate, decreasing lipid peroxidation and ion leakage, increasing the content of polyamines, as well as elevating the activity of antioxidative enzymes. In rice cells, AtCIPK21 increases expression of Ca2+-dependent protein kinase (CPK) genes and mitogen-activated protein kinase (MAPK) genes under cold stress. These results indicated that overexpression of the AtCIPK21 gene in plant cells improved cold stress tolerance by elevating polyamines content, antioxidative enzyme activity, and expression of CPK genes and MAPK genes. Overexpression of the AtCIPK21 gene may be a valuable approach for engineering plant cold stress tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses such as low temperature, high NaCl, drought, and heavy metal exposure affect plant growth, development, and productivity (Kovacs et al. 2010; Zhang et al. 2010). Cold stress is one of these factors. Plants protect themselves from cold stress through different signal transduction pathways and molecular genetic regulation (Cui et al. 2005; Dekanski et al. 2009; Yabuta et al. 2002; Zur et al. 2009). To respond to cold stress, cells regulate their biological activities at transcriptomic and proteomic levels and genes related to ROS, transcription factors, hormones, and carbohydrate metabolisms play important roles in keeping cell membrane integrity (Chan and Shi 2015). Calcium-related signaling play an important role in abiotic stress tolerance of diverse plants. Calcineurin B-like protein-interacting protein kinases (CIPKs) are associated with calcium-signaling processes of plant stress responses. Plant calcineurin B-like protein-type Ca2+ sensors interact with and activate CIPKs that phosphorylate downstream components to transduce Ca2+ signals (Kurusu et al. 2010; Pandey et al. 2007; Yu et al. 2011). Multiple CIPKs have been identified in different plant species (Chen et al. 2014; Deng et al. 2013a, b; Deng et al. 2013a, b; Kimura et al. 2013; Liu et al. 2013; Piao et al. 2010; Ruan et al. 2013; Zhou et al. 2014). CIPKs may be involved in particular types of signal that are related to stimulus–response process (Gonzalez Bosc et al. 2016; Pandey et al. 2015; Rodriguez-Medina et al. 2015). Several CIPK genes have been reported to function in plant responses to drought, salinity, and osmotic stress in Arabidopsis (Arabidopsis thaliana) (Kimura et al. 2013; Liu et al. 2013; Pandey et al. 2007), rice (Oryza sativa) (Piao et al. 2010), pea (Pisum sativum), and maize (Zea mays) (Chen et al. 2014). The Arabidopsis calcineurin B-like protein-interacting protein kinase CIPK21 gene (AtCIPK21) has been reported to play important roles in cell metabolism and abiotic stress responses (Pandey et al. 2015).
Calcineurin B-like protein-interacting protein kinases (CIPKs) are featured with a group of typical Ser/Thr functional regions that mediate calcium signals (Chen et al. 2014; Pandey et al. 2015). Abiotic stresses including salt, cold, and drought stimulate CIPK21 expression and enhanced expression of CIPK21 contributes to stress tolerance in plants (Beier et al. 2018; Pandey et al. 2007). Among different members of CIPKs, CIPK1 and CIPK19 overexpression result in Ca2+ overaccumulation and affect the pollen tube growth in Arabidopsis. It has been reported that many calcium signatures activate CIPKs in Arabidopsis plants to protect plants from damage caused by abiotic stresses. In rice, shorter exposure to abiotic stress enhances OsCIPK31 expression and longer exposure to stresses reduces OsCIPK31 expression (Dirks-Hofmeister et al. 2013).
In this report, we examined the function of the AtCIPK21 gene in cold stress response in rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), and white pine (Pinus strobus L.) by examining the activity of antioxidative enzymes and the content of polyamines. In rice cells, the expression of Ca2+-dependent protein kinase (CPK) genes and mitogen-activated protein kinase (MAPK) genes was evaluated in AtCIPK21 transgenic cells under treatment of cold. Our findings suggest that AtCIPK21 mediates responses to cold stress condition in O. sativa, G. hirsutum, and P. strobus, at least in part, by regulating multiple cell signaling pathways across several plant species. Overexpression of the AtCIPK21 gene may be a valuable approach for engineering plant cold stress tolerance.
Materials and methods
Plasmid constructs
The full-length AtCIPK21 coding sequence (1251 bp) was cloned into expression vector pBI121 according to the method previously described (Guan and Nikolau 2016). The vector of pBI121 and the cDNA of AtCIPK21 were digested by restriction enzymes KpnI and BamHI (Promega, Madison, WI, USA) at 37 °C. The digested DNA was purified using QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA) and ligated to generate the expression vector (Tang and Newton 2005). The resulting expression vector was designated as pBI-AtCIPK21 and the vector pBI-AtCIPK21 was introduced into Agrobacterium tumefaciens strain LBA4404 using electroporation.
Transformation of suspension cells
AtCIPK21 transgenic cell lines of rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), white pine (Pinus strobus L.), and Arabidopsis (Arabidopsis thaliana) were generated as described before (Tang and Page 2013), using Agrobacterium tumefaciens strain LBA4404 carrying pBI-AtCIPK21 and cultured cells of O. sativa, G. hirsutum, and P. strobus (Tang, et al. 2007; Tang and Page 2013). After transformation, cell cultures of O. sativa, G. hirsutum, and P. strobus were grown for 7 weeks and then these cell cultures were used for further analysis.
Polymerase chain reaction analyses of transgenic cells
Polymerase chain reaction (PCR) analysis of transgenic cells was conducted as previously described (Tang, et al. 2007; Tang and Page 2013). Seven grams of control cells and transgenic cells were used to isolate genomic DNA, using a Genomic DNA Isolation Kit (Sigma). The primers used to amplify the neomycin phosphotransferase II gene (NPTII) are forward primer (nfp: 5′-GTCGACATGGCGGAGGAATTTGGAAGCATAG-3′) and the reverse primer (nrp: 5′-CCATGGTAGACTCCTGCTTCGACATCATGG-3′). The primers used to amplify the transcription factor AtCIPK21 DNA fragments are forward primer (cf: 5′-CTTTCAATGG CGGATTTGTT AAGAAAAGTG-3′) and the reverse primer (cr:5′-CTTCATTTTT CATCTTCCTA ATCAGTATCA GAAAG-3′). The PCR mixture, the PCR conditions, and gel electrophoresis were carried out as described previously (Tang and Page 2013). PCR was done in a PTC-100TM machine (MJ Research, San Francisco, CA, USA). A total of 285 ng of genomic DNA of rice, cotton, and pine was used as a template, respectively. Cell cultures of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) were used for further analysis.
Southern blot analysis of transgenic cells
Southern blotting analysis of O. sativa, G. hirsutum, and P. strobus transgenic cells was conducted as previously described (Tang et al. 2007; Tang and Page 2013). One gram of control cells and transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) was used to isolate genomic DNA, using a Genomic DNA Isolation Kit (Sigma). Twenty-eight micrograms of DNA were digested within 16 h with the enzyme Xba I (Boehringer Mannheim) at 37 °C. The molecular probes (1251 pb fragment of AtCIPK21) were labeled using Digoxigenin (DIG) (Roche Diagnostics, Indianapolis, IN, USA).
RNA isolation and Northern blot analysis
Six grams of transgenic and control cells of rice, cotton, and pine were used to extract total RNA, using a RNeasy Mini Plant Kit (Germantown, MD, USA) by following the manual. Nine micrograms of total RNA was used for northern blotting which was performed as described before (Tang et al. 2007). The digoxigenin (DIG) labeling AtCIPK21 DNA (1251 pb) (Roche Diagnostics) was used as the hybridization probe. The rRNA was used as the loading control of RNA samples.
Cold treatment
Cold treatment was carried out by incubation of AtCIPK21 transgenic cells and control cells of Os1, Os2, and Os3 derived from O. sativa, Gh1, Gh2, and Gh3 were derived from G. hirsutum, and Ps1, Ps2, and Ps3 were derived from P. strobus at − 12, 0, 12, and 24 °C in the dark for 24 h in growth chambers. After 24 h of cold stress treatment, cells were moved to the normal growth environment. Cell images of O. sativa, G. hirsutum, and P. strobus were taken using an inverted microscope. Cell size was measured using ImageJ.
Determination of cell viability and growth rate
After the AtCIPK21 transgenic cells and control cells of rice, cotton, and pine were treated by cold, cells were moved to the normal growth environment. The cell growth rate and the cell viability of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) were examined, as previously described (Tang and Page 2013). The cell growth rate was expressed as mg/g FW/day. The rate of cell growth was measured 7 days after treatment. Samples from both cold treated and control groups were collected with three biological replicates.
Measurement of thiobarbituric acid reactive substances
The amount of thiobarbituric acid reactive substances (TBARS) was determined using thiobarbituric acid (TBA) reaction as described previously (Tang and Page 2013; Tang, et al. 2005). Three grams of transgenic cells from each of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) and control cells were used for sampling. The sample mixture was heated at 95 °C for 30 min and centrifuged at 10,000×g for 15 min before the absorbance was measured at 532 nm. The content of TBARS was calculated using the method previously described (Tang and Page 2013; Tang et al. 2005).
Measurement of ion leakage
The ion leakage was measured using transgenic and control cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3), as previously described. Afterwards, the samples of rice, cotton, and pine were incubated in 1000 μl distilled water for 1 h respectively. The conductivity of transgenic and control cells was examined using a B-173 conductivity meter (Horiba, Kyoto, Japan).
Determination of APOX, CAT, PPO, POD activity and ROS level
The activities of ascorbate peroxidase (APOX), catalase (CAT), polyphenol oxidase (PPO), and peroxidase (POD) were determined as described previously (Tang, et al. 2007; Tang and Page 2013). Two grams of control and transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) were homogenized in 3 ml of extraction buffer. Extraction buffer consists of 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP, and 0.5% (v/v) Triton X-100 at 4 °C. The extracts were centrifuged at 10,000×g for 20 min and the supernatant was used to determine the enzyme activity, as described previously (Tang et al. 2007; Tang and Page 2013). The activity of APOX was determined using ascorbate as substrate and was expressed as mmol ascorbate oxidized mg protein-1 min-1. The activity of POD was determined using 4-methylcatechol as substrate. The increase in the absorption caused by oxidation of 4-methylcatechol by H2O2 was measured at 420 nm spectrophotometrically. The activity of CAT was measured spectrophotometrically at room temperature by monitoring the decrease in absorbance at 240 nm resulting from the decomposition of H2O2. The activity assay of PPO was carried out by measuring the increase in absorbance at 420 nm for 4-methylcatechol spectrophotometrically. Determination of reactive oxygen species (ROS) production was performed as previously described (Smith and Heese 2014).
Determination of polyamines
The concentration of putrescine (Put), spermidine (Spd), and spermine (Spm) in the control and transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) was determined as described previously (Tang and Newton 2005). The control samples and transgenic cells were characterized using a HPLC instrument and the Spector Monitor 3200 Detector by following the instructions. The measured polyamines (PAs) are total PAs.
Expression of Ca2+-dependent protein kinase genes
Expression of CPK genes in the control and the transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) was determined using qPCR as previously described (Wan et al. 2007). Total RNA was extracted from frozen sample cells using TRIzol reagent by following the description in the manufacturer’s protocol (Invitrogen). To synthesize the first-strand cDNA in a 50-mL reaction containing 2.5 mM oligo(dT) primers, 2.5 mM random hexamer, and 2.5 µg of total RNA, the PrimeScript™ RT reagent kit (TaKaRa Co., Ltd, Ohtsu, Japan) was used by following the instructions. Samples were analyzed in triplicate on the Applied Biosystems 7900HT System by following the description in the manufacturer’s manual. The primers for qPCR are listed in Table 1. The U6 gene was used as an internal control. Gene expression was measured after transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) were treated at − 12, 0, 12, and 24 °C. The data were normalized to the internal control. The delta–delta Ct method was used to obtain the expression value. The U6 gene was used as internal reference.
Expression of mitogen-activated protein kinase genes, ICE genes, and CBF genes
Expression of mitogen-activated protein kinase (MAPK) genes in the control and the transgenic cells of O. sativa (Os1, Os2, and Os3), G. hirsutum (Gh1, Gh2, and Gh3), and P. strobus (Ps1, Ps2, and Ps3) was examined using qPCR as previously described (Wan, et al. 2007). Total RNA was extracted from frozen sample cells using TRIzol reagent by following the description in the manufacturer’s protocol (Invitrogen). To synthesize the first-strand cDNA in a 50-mL reaction containing 2.5 mM oligo(dT) primers, 2.5 mM random hexamer, and 2.5 µg of total RNA, the PrimeScript™ RT reagent kit (TaKaRa Co., Ltd, Ohtsu, Japan) was used by following the instructions. Samples were analyzed in triplicate on the Applied Biosystems 7900HT System by following the description in the manufacturer’s manual. The primers for qPCR are listed in Table 1. The U6 gene was used as an internal control. Gene expression was measured after treatment with 10 µl Pseudomonas syringae (OD600 = 0.5) per 1 million transgenic cells at different time points (0, 1, 3, 5, and 7 days). The data were normalized to the internal control. The regular qPCR was used. The delta–delta Ct method was used to obtain expression value. The U6 gene was used as internal reference.
Statistical analyses
Statistical analysis of the control and transgenic cells of rice, cotton, and pine was performed using the General Linear Model procedure of SAS (Cary, NC, USA), employing ANOVA models. The significant differences were made at 5% level of probability. Each value was presented as means and standard errors of the mean.
Results
Expression and integration of transgenes
Cold stress is one of the abiotic stresses that negatively affect plant growth, development, and yield. In rice, cold stress affects morphological development, decreases seed production, causes poor germination, and increases seedling injury, and reduces rice yield in the world (Shakiba et al. 2017). In cotton, cold stress impairs plant growth and restricts the productivity and spatial distribution of plants (Cai et al. 2019). In white pine, cold stress changes carbohydrate and compatible solute concentrations, membrane lipid composition, and proteins, causes proteomic and metabolomics changes (Strimbeck et al. 2015). To respond to cold stress, plants have developed capabilities to activate hormone signaling, enhance cold stress-related gene expression, and increase the activity of transcription factors to adapt to this extreme condition. Transcription factors are essential for plant cells to respond to stress signals.
To evaluate the effect of the AtCIPK21 gene on cold stress tolerance, cell cultures of O. sativa, G. hirsutum, and P. strobus were transformed using Agrobacterium tumefaciens strain GV3101 carrying pBI-AtCIPK21 (Fig. 1a) to produce AtCIPK21 overexpression cell lines. One hundred and thirty-one AtCIPK21 transgenic cell lines have been produced including 42 O. sativa lines, 51 G. hirsutum lines, and 38 P. strobus lines. Integration of transgenes in the genome of O. sativa, G. hirsutum, and P. strobus was confirmed by PCR amplification of the NPTII gene (Fig. 1b) and the AtCIPK21 gene (Fig. 1c) in O. sativa, G. hirsutum, and P. strobus. Integration of transgenes into the genome of rice, cotton, and pine was also confirmed by southern blotting (Fig. 1d) and northern blotting (Fig. 1e) using the AAE13.1 fragments as a probe in O. sativa, G. hirsutum, and P . strobus. After confirmation of the integration of transgene into the genome of rice, cotton, and pine, rice, cotton, and pine, cell lines Os1, Os2, and Os3 from O. sativa, from G. hirsutum, and Ps1, Ps2, and Ps3 from P. strobus were used for cold stress tolerance experiment.
Expression and integration of transgenes. a The T-DNA region that shows the right border (RB), left border (LB), the nos promoter (nos Pro), the NPTII gene (NPTII), the nos terminator (nos Ter), the 35 S promoter (35S Pro), and the AtCIPK21 gene (AtCIPK21). b PCR amplification of the NPTII gene (717 bp) in O. sativa, G. hirsutum, and P. strobus. c PCR amplification of the AtCIPK21 gene (1251 bp) in O. sativa, G. hirsutum, and P. strobus. d Southern blotting using the AtCIPK21 fragment as a probe in O. sativa, G. hirsutum, and P. strobus. e Northern blotting using the AtCIPK21 fragment as a probe in O. sativa, G. hirsutum, and P. strobus. The loading control is the tobacco 25S rRNA
Overexpression of AtCIPK21 increases the cell viability, growth rate, and decreases TBARS and ion leakage under cold stress
After confirmation of the AtCIPK21 gene integration into the genome of transgenic cell lines by PCR, southern blotting, and northern blotting analysis in O. sativa, G. hirsutum, and P. strobus (Fig. 1d, e), respectively, cell viability, cell growth rate, TBARS, and ion leakage of transgenic cells under cold stress at − 12 °C, 0 °C, 12 °C, and 24 °C were determined. The negative control is the transgenic cells that are transformed with the expression vector, which does not have the AtCIPK21 gene. Overexpression of the AtCIPK21 gene increases cell viability, cell growth rate, and reduces TBARS and ion leakage in transgenic cells of O. sativa (Fig. 2a, d, g, j), G. hirsutum (Fig. 2b, e, h, k), and P. strobus (Fig. 2c, f, i, l) after treatment with cold at − 12 °C and 0 °C, but does not change them at 12 °C and 24 °C, respectively, compared to the controls. Overexpression of the AtCIPK21 gene protects cells from cold stress in monocotyledonous (O. sativa), dicotyledonous (G. hirsutum), and gymnosperm (P. strobus) plant cells by decreasing TBARS and ion leakage. Overexpression of the AtCIPK21 gene cause phenotype changes (Fig. 2m) including cell death.
Overexpression of AtCIPK21 increases the cell viability, growth rate, and decreases TBARS and ion leakage under cold stress. Overexpression of AtCIPK21 increases the cell viability, the cell growth rate, and decreases TBARS and ion leakage in O. sativa (a, d, g, and j), G. hirsutum (b, e, h, and k), and P. strobus (c, f, i, and l), respectively. Overexpression of AtCIPK21 changes phenotype of transgenic cells (m). The asterisk shows the significant differences, as assessed by a t test. *P < 0.05, **P < 0.01, ***P < 0.001, significant relative to control. N.S. no statistical significance
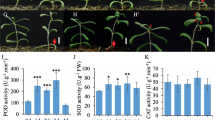
The activity of ascorbate peroxidase (APOX), catalase (CAT), polyphenol oxidase (PPO), peroxidase (POD), and ROS level
The activity of APOX, CAT, PPO, and POD was examined in AtCIPK21 transgenic cell lines of O. sativa, G. hirsutum, and P. strobus, treated with different temperatures at − 12 °C, 0 °C, 12 °C, and 24 °C, respectively. Overexpression of the AtCIPK21 gene significantly increases the activity of APOX, CAT, PPO, and POD in transgenic cell lines of O. sativa (Fig. 3a, d, g, j), G. hirsutum (Fig. 3b, e, h, k), and P. strobus (Fig. 3c, f, i, l), treated at − 12 °C and 0 °C, respectively. The activity of APOX, CAT, PPO, and POD in transgenic cell lines of O. sativa (Fig. 3a, d, g, j), G. hirsutum (Fig. 3b, e, h, k), and P. strobus (Fig. 3c, f, i, l) was not significantly changed in the treatment at 12 °C and 24 °C. These results indicated that overexpression of the AtCIPK21 gene increased cold stress tolerance which may be related to the increased activity of APOX, CAT, PPO, and POD in O. sativa, G. hirsutum, and P. strobus.
Effect of the AtCIPK21 gene on the activities of APOX, CAT, PPO, and POD in transgenic cells. Overexpression of the AtCIPK21 gene increases the activities of APOX, CAT, PPO, and POD in transgenic cells of O. sativa (a, d, g, and j), G. hirsutum (b, e, h, and k), and P. strobus (c, f, i, and l), respectively. Overexpression of the AtCIPK21 gene decreases the level of ROS in transgenic cells of O. sativa (m), G. hirsutum (n), and P. strobus (o), respectively. The asterisk indicates the significant differences, as assessed by a t test. *P < 0.05, significant relative to control
Overexpression of AtCIPK21 elevates the levels of polyamines
To examine if overexpression of the AtCIPK21 gene-enhanced cold stress tolerance is related to the biosynthesis of putrescine (Put), spermidine (Spd), and spermine (Spm), the contents of Put, Spd, and Spm were analyzed in transgenic cell lines of O. sativa, G. hirsutum, and P. strobus. Overexpression of the AtCIPK21 gene significantly increases the levels of Put, Spd, and Spm in transgenic cells of O. sativa (Fig. 4a–c), G. hirsutum (Fig. 4d–f), and P. strobus (Fig. 4g–i), respectively, under stress at − 12 °C and 0 °C, respectively. Overexpression of the AtCIPK21 gene does not significantly increase the levels of Put, Spd, and Spm in transgenic cells of O. sativa (Fig. 4a–c), G. hirsutum (Fig. 4d–f), and P. strobus (Fig. 4g–i), respectively, under stress at 12 °C and 24 °C.
AtCIPK21 elevates contents of Put, Spd, and Spm. Overexpression of the AtCIPK19 gene increases the levels of putrescine (Put), spermidine (Spd), and spermine (Spm) in transgenic cells of O. sativa (a–c), G. hirsutum (d–f), and P. strobus (G, H, and I), respectively. The asterisk indicates the significant differences, as assessed by a t test. *P < 0.05, significant relative to control. N.S. no statistical significance
Expression of CPK genes in AtCIPK21 transgenic rice cell
Ca2+-dependent protein kinase (CPK) genes protect cells from cold stress tolerance. To evaluate whether overexpression of the AtCIPK21 gene affects the expression of CPK genes in transgenic cells, expression of CPK protein kinase genes was examined by qRT-PCR in O. sativa transgenic cell lines under cold stress at − 12 °C, 0 °C, 12 °C, and 24 °C, respectively. Among 15 CPK genes examined, overexpression of the AtCIPK21 gene significantly increased expression of OsCPK1 (Fig. 5a), OsCPK5 (Fig. 5e). OsCPK6 (Fig. 5f), OsCPK7 (Fig. 5g), OsCPK8 (Fig. 5h), OsCPK9 (Fig. 5i), and OsCPK11 (Fig. 5k) in transgenic cells treated at − 12 °C and 0 °C, but did not significantly affect expression of OsCPK2 (Fig. 5b), OsCPK3 (Fig. 5c), OsCPK4 (Fig. 5d), OsCPK10 (Fig. 5j), and OsCPK12 (Fig. 5l) in AtCIPK21 transgenic rice cell under treatment at 12 °C and 24 °C, respectively. These results indicated that overexpression of the AtCIPK21 gene increased cold stress tolerance may be related to expression of CPK genes including OsCPK1 (Fig. 5a), OsCPK5 (Fig. 5e). OsCPK6 (Fig. 5f), OsCPK7 (Fig. 5g), OsCPK8 (Fig. 5h), OsCPK9 (Fig. 5i), and OsCPK11 (Fig. 5k), and may be not related to expression of CPK genes including OsCPK2 (Fig. 5B), OsCPK3 (Fig. 5c), OsCPK4 (Fig. 5d), OsCPK10 (Fig. 5j), and OsCPK12 (Fig. 5l) in AtCIPK21 transgenic rice cell.
Expression of CPK genes in AtCIPK21 transgenic rice cell. Expression of OsCPK1 (a), OsCPK2 (b), OsCPK3 (c), OsCPK4 (d), OsCPK5 (e), OsCPK6 (f), OsCPK7 (g), OsCPK8 (h), OsCPK9 (i), OsCPK10 (j), OsCPK11 (k), OsCPK12 (l), OsCPK13 (m), OsCPK14 (n), and OsCPK15 (o) in AtCIPK21 transgenic rice cell. The asterisk indicates significant differences, as assessed by a t test. *P < 0.05, **P < 0.01, ***P < 0.001, significant relative to control. N.S. no statistical significance
Expression of mitogen-activated protein kinase genes in transgenic cells
To investigate if the AtCIPK21 gene has an effect on expression of mitogen-activated protein kinase (MAPK) genes in rice under treatment of low temperature, expression of MAPK genes including OsMAPK1, OsMAPK2, OsMAPK3, OsMAPK4, OsMAPK5, OsMAPK6, OsMAPK7, OsMAPK8, and OsMAPK9 was examined in three O. sativa transgenic cell lines Os1, Os2, and Os3 under cold stress at − 12 °C, 0 °C, 12 °C, and 24 °C. Compared to the control, the expression of MAPK genes OsMAPK1 (Fig. 6a), OsMAPK2 (Fig. 6b), OsMAPK3 (Fig. 6c), OsMAPK8 (Fig. 6h), and OsMAPK9 (Fig. 6i) was increased significantly in transgenic rice cells with the treatment at − 12 °C and 0 °C, respectively. Expression of MAPK genes OsMAPK1 (Fig. 6a), OsMAPK2 (Fig. 6b), OsMAPK3 (Fig. 6c), OsMAPK8 (Fig. 6h), and OsMAPK9 (Fig. 6i) was not significantly changed in transgenic rice with the treatment at 12 °C and 24 °C. Expression of MAPK genes OsMAPK4 (Fig. 6d), OsMAPK5 (Fig. 6e), OsMAPK6 (Fig. 6f), and OsMAPK7 (Fig. 6g) was not significantly changed in O. sativa transgenic cell lines Os1, Os2, and Os3 under cold stress at − 12 °C, 0 °C, 12 °C, and 24 °C.
Expression of MAPK genes in AtCIPK21 transgenic rice cells. Overexpression of the AtCIPK21 gene modulates expression of transcription factor genes OsMAPK1 (a), OsMAPK2 (b), OsMAPK3 (c), OsMAPK4 (d), OsMAPK5 (e), OsMAPK6 (f), OsMAPK7 (g), OsMAPK8 (h), and OsMAPK9 (i) in transgenic rice cell lines. The asterisk indicates significant differences, as assessed by a t test. *P < 0.05, significant relative to control
We choose the Arabidopsis CIPK21, not the CIPK21 from cotton, rice, and pine, for the work presented in the manuscript because: (1) we are interested in investigating the function of CIPK21 that is derived from the popular model plant Arabidopsis in different economically plant species to see if the AtCIPK21 functions in the heterologous expression systems; (2) the biology of the Arabidopsis CIPK21 in abiotic stress has been well documented in Arabidopsis, but its function in cold stress has not been documented in cotton, rice, and pine; and (3) we hope to obtain insights into the mechanism of AtCIPK21-associated cold stress tolerance.
Overexpression of AtCIPK21 changes the cell morphology under cold stress
To examine whether overexpression of the AtCIPK21 gene affects cell morphology of transgenic cell lines, images of cells were examined in O. sativa, G. hirsutum, and P. strobus transgenic cell lines under treatment at − 12 °C, 0 °C, 12 °C, and 24 °C, respectively. Overexpression of AtCIPK21 changes the cell morphology of O. sativa (Fig. 7a–h), G. hirsutum (Fig. 7i–p), and P. strobus (Fig. 7q–x) under treatment at − 12 °C and 0 °C, respectively. Compared to the control, size of O. sativa (Fig. 7y), G. hirsutum (Fig. 7z), and P. strobus (Fig. 7za) transgenic cells was significantly increased under treatment at − 12 °C and 0 °C, respectively. These results indicated that overexpression of the AtCIPK21 gene increased cell size under treatment at − 12 °C and 0 °C, respectively.
Overexpression of AtCIPK21 changes the cell morphology under cold stress. Overexpression of AtCIPK21 changes the cell morphology under cold stress in O. sativa (a–h), G. hirsutum (i–p), and P. strobus (q–x), and the cell size (y–za), respectively. The asterisk indicates significant differences, as assessed by a t test. *P < 0.05, significant relative to control. N.S. no statistical significance
Overexpression of AtCIPK21 enhances cold stress tolerance in Arabidopsis
The activity of APOX, CAT, PPO, POD, and ROS was examined in AtCIPK21 transgenic cell lines of A. thaliana, treated with different temperatures − 12 °C, 0 °C, 12 °C, and 24 °C, respectively. Overexpression of the AtCIPK21 gene significantly increases the activity of APOX, CAT, PPO, and POD, and decreases ROS level in transgenic cell lines of A. thaliana (Fig. 8), treated at − 12 °C and 0 °C, respectively. Overexpression of the AtCIPK21 gene significantly increases expression of ICE1 gene and expression of CBF genes (Cramer et al. 2011; Yuan et al. 2018) in rice (Fig. 9). The AtCIPK21 gene participates in the process of cold stress signaling pathway in plant cells (Fig. 10).
Overexpression of AtCIPK21 in Arabidopsis enhances cold stress tolerance. a Expression of AtCIPK21 in Arabidopsis under Cold stress. b Confirmation of AtCIPK21 overexpression in Arabidopsis suspension cells by northern blotting. Overexpression of AtCIPK21 in Arabidopsis increases growth rate (c), cell viability (d), PPO activity (g), POD activity (h), APOX activity (i), CAT activity (j), and decreases TBARS (e), ion leakage (f), and ROS level (k), respectively. The asterisk indicates significant differences, as assessed by a t test. *P < 0.05, significant relative to control. N.S. no statistical significance
Expression of ICE and CBF genes in AtCIPK21 transgenic rice cells. Overexpression of the AtCIPK21 gene modulates expression of OsICE1 (a), OsCBF1 (b), OsCBF2 (c), OsCBF3 (d), OsCBF8 (e), OsCBF9 (f), OsCBF10 (g), OsCBF11 (h), and OsCBF12 (i) in transgenic rice cell lines. The asterisk indicates significant differences, as assessed by a t test. *P < 0.05, significant relative to control
Discussion
CIPKs play important roles in abiotic stress responses in plants by forming complexes that regulate Ca2+ changes (Chen et al. 2014; Kurusu et al. 2010; Pandey et al. 2007, 2015; Wang et al. 2016). Although functions of some of CIPKs have been identified in Arabidopsis (Beier et al. 2018; Liu et al. 2019; Wang et al. 2016; Wu et al. 2019; Yin et al. 2017; Zhou et al. 2015), functions of many CIPKs have not been characterized. This investigation is focused on the function of AtCIPK21 gene in cold stress tolerance in cells of rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), and white pine (Pinus strobus L.). In our study, molecular analyses were performed to explore the effect of the AtCIPK21 gene in response to cold stress in rice, cotton, and pine. Overexpression of the AtCIPK21 gene increases cell viability and cell growth rate, as well as decreases TBARS and ion leakage in transgenic cells of O. sativa, G. hirsutum, and P. strobus after treatment at − 12, and 0 °C, respectively. The overexpression of the AtCIPK21 gene in different plant species demonstrated a similar pattern of decreasing TBARS and ion leakage in transgenic cells of O. sativa (Fig. 2a, d, g, j), G. hirsutum (Fig. 2b, e, h, k), and P. strobus (Fig. 2c, f, i, l) after treatment with cold at − 12 °C and 0 °C, respectively. These results showed that overexpression of the AtCIPK21 gene-enhanced cold stress tolerance could be achieved in different plant species.
Increased activity of antioxidant enzymes is associated with the increased tolerance stress (Nejadsadeghi et al. 2014). In this investigation, responses of cells to cold stress were comparatively studied in O. sativa, G. hirsutum, and P. strobus. Increasing evidence showed that reactive oxygen species (ROS) are controlled by enzymatic antioxidant defense systems that are related to abiotic stress and homeostasis regulation of ROS in plants is involved in essential proteins that are related to abiotic stress tolerance (Cui et al. 2005; Guo et al. 2006; Kazemi Shahandashti et al. 2013; Kazemi-Shahandashti et al. 2014; You and Chan 2015; Zur et al. 2009). It has been reported that the activity of APOX, CAT, PPO, and POD is related to cold stress tolerance in a large number of plant species (Adak and Datta 2005; Correa-Aragunde et al. 2015; Faltin et al. 2010; Gundinger and Spadiut 2017; Miller et al. 2007; Stajic et al. 2004; Yang et al. 2006). For example, to protect from enzymatic browning, a single polyphenol oxidase (PPO) can play a critical role and RP-UHPLC-MS analysis demonstrated that a mixture of phenolic compounds and the phenolic fraction was responsible for inhibition of PPO (Dirks-Hofmeister et al. 2013; Kampatsikas et al. 2017; Kuijpers et al. 2014; Molitor et al. 2016; Nautiyal et al. 2008; Poiatti et al. 2009). Our results demonstrated that AtCIPK21 enhances cold stress tolerance by increasing the activity of APOX, CAT, PPO, and POD in different species including O. sativa, G. hirsutum, and P. strobus.
The involvement of polyamines as antioxidants against abiotic stress-derived oxidative damage has been reported (Basu et al. 2014; Groppa and Benavides 2008; Huang et al. 2015; Kasukabe et al. 2004; Kovacs et al. 2010; Sun et al. 2014). Polyamines have different effects that could help plants deal with abiotic stresses and they have also been proven to act as regulators of gene expression during the development of stress responses (Agurla et al. 2018; Romero et al. 2018; Zhao et al. 2017). In this investigation, the experimental results demonstrated that overexpression of the AtCIPK21 gene significantly increases the levels of Put, Spd, and Spm in transgenic cells of O. sativa (Fig. 4a–c), G. hirsutum (Fig. 4d–f), and P. strobus (Fig. 4g–i), respectively, under stress at − 12 °C and 0 °C, respectively. Overexpression of the AtCIPK21 gene does not significantly increase the levels of Put, Spd, and Spm in transgenic cells of O. sativa (Fig. 4a–c), G. hirsutum (Fig. 4d–f), and P. strobus (Fig. 4g–i), respectively, under stress at 12 °C and 24 °C.
Plant Ca2+-dependent protein kinase (CPK) signaling is important in protecting plant cells from abiotic stress including drought, salt and cold stress and has been demonstrated to function in mediating the signaling following Ca2+ influx after stress (Arimura and Sawasaki 2010). Calcium is an intracellular secondary messenger and CPKs are Ca2+ sensors that have both Ca2+ sensing function and kinase activity that play important roles in plant responses to various abiotic stresses. Characterization of CPK gene family members in plants provides an important foundation for crop improvement and improved understanding of signal transduction in plants (Geng et al. 2013; Hu et al. 2015; Zhang et al. 2014). In this study, our results indicated that overexpression of the AtCIPK21 gene increased cold stress tolerance may be related to expression of CPK genes including OsCPK1 (Fig. 6a), OsCPK5 (Fig. 6e), OsCPK6 (Fig. 6f), OsCPK7 (Fig. 6g), OsCPK8 (Fig. 6h), OsCPK9 (Fig. 6i), and OsCPK11 (Fig. 6k), and may be not related to expression of CPK genes including OsCPK2 (Fig. 6b), OsCPK3 (Fig. 6c), OsCPK4 (Fig. 6d), OsCPK10 (Fig. 5j), and OsCPK12 (Fig. 6l) in AtCIPK21 transgenic rice cell.
Mitogen-activated protein kinase (MAPK) signaling plays critical roles in protecting plant cells from abiotic stresses and MAPK signaling regulates the response to cold stress through the Inducer of CBF expression 1 (ICE1)–CBF-cold-responsive (COR) transcriptional pathway in Arabidopsis thaliana (Liu and Zhou 2018). The involvement of MAP kinases in abiotic stresses including drought, cold, and salt stress is related to the complexity of signal transduction pathways, as well as the specificity of MAP kinase signaling modules (Li et al. 2014; Moustafa et al. 2014; Wang et al. 2015). In this study, our results demonstrated that mitogen-activated protein kinase genes play an important role in cold stress tolerance. Our results showed that the expression of mitogen-activated protein kinase genes OsMAPK1 (Fig. 5a), OsMAPK2 (Fig. 6b), OsMAPK3 (Fig. 6c), OsMAPK4 (Fig. 6d), OsMAPK5 (Fig. 6e), and OsMAPK6 (Fig. 6f) was increased significantly in transgenic rice cells with the treatment at − 12 °C and 0 °C.
Cell growth and proliferation are dependent on the environmental conditions and are affected by different stresses. Changes in cell growth environment could result in alteration in the cell cycle regulation, because stresses regulate cell cycle progression, leading to a shortened G2 period (de Marcia et al. 2017; Herranz and Medina 2014; Lee et al. 2017; Majda et al. 2017; Matia et al. 2010). Embryogenic cell suspensions of wheat (Triticum aestivum L.) have been used as sources to study their morphogenic capacity under stress and changed culture conditions. Stress caused an abnormal chromosome number and low viability (Ahmed and Sagi 1993). Cell viability and cell size are important for increased cold stress tolerance. The cell viability determined by the trypan blue is valid after 7 days of culture. Growth rate was significantly increased in high cell viability cultures. Increased sensitivity of the cells to stresses could result in a 35% increase in the cell size (Steward et al. 1999). In Arabidopsis, physiologically homogenous cell cultures could be subcultured over several months under cold stress and low culture temperature is robust for heterotrophic and semi-autotrophic cells (Boisson et al. 2012; Trexler et al. 2005; Wucherpfennig et al. 2014). In our study, overexpression of the AtCIPK21 gene decreases the size of transgenic cells in rice (Fig. 5a–d), tobacco (Fig. 5e–h), and pine (Fig. 5i–l), respectively, under treatment of low temperature (− 10 °C and − 4 °C). These results will increase our understanding of the AtCIPK21 gene in cold stress tolerance in monocotyledonous (rice), dicotyledonous (cotton), and gymnosperm (pine) plants. The findings are also valuable in plant molecular biotechnology.
Conclusion
We report that overexpression of the AtCIPK21 gene increases cold stress tolerance by increasing both cell viability and growth rate and decreasing TBARS under cold stress in rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), and white pine (Pinus strobus L.). AtCIPK21 enhances cold stress tolerance by increasing the activity of APOX, CAT, PPO, and POD, as well as by elevating accumulation of Put, Spd, and Spm, respectively. The AtCIPK21 gene increased cold stress tolerance may be related to expression of OsCPK1, OsCPK5, OsCPK6, OsCPK7, OsCPK8, OsCPK9, and OsCPK11. Among nine mitogen-activated protein kinase genes examined, AtCIPK21 overexpression changed expression of MAPK transcription factors OsMAPK1, OsMAPK2, OsMAPK3, OsMAPK4, OsMAPK5, and OsMAPK6 under cold stress. AtCIPK21 counteracts cold stress by enhancing expression of stress response transcription factors. Our results indicate that overexpression of the AtCIPK21 gene in plant cells improved cold stress tolerance by elevating polyamines content, antioxidative enzyme activity, and expression of CPK genes and MAPK genes. Overexpression of the AtCIPK21 gene may be a valuable approach for engineering plant cold stress tolerance.
References
Adak S, Datta AK (2005) Leishmania major encodes an unusual peroxidase that is a close homologue of plant ascorbate peroxidase: a novel role of the transmembrane domain. Biochem J 390:465–474
Agurla S, Gahir S, Munemasa S, Murata Y, Raghavendra AS (2018) Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv Exp Med Biol 1081:215–232
Ahmed KZ, Sagi F (1993) Culture of and fertile plant regeneration from regenerable embryogenic suspension cell-derived protoplasts of wheat (Triticum aestivum L.). Plant Cell Rep 12:175–179
Arimura G, Sawasaki T (2010) Arabidopsis CPK3 plays extensive roles in various biological and environmental responses. Plant Signal Behav 5:1263–1265
Basu S, Roychoudhury A, Sengupta DN (2014) Deciphering the role of various cis-acting regulatory elements in controlling SamDC gene expression in rice. Plant Signal Behav 9:e28391
Beier MP, Obara M, Taniai A, Sawa Y, Ishizawa J, Yoshida H, Tomita N, Yamanaka T, Ishizuka Y, Kudo S, Yoshinari A, Takeuchi S, Kojima S, Yamaya T, Hayakawa T (2018) Lack of ACTPK1, an STY kinase, enhances ammonium uptake and use, and promotes growth of rice seedlings under sufficient external ammonium. Plant J 93:992–1006
Boisson AM, Gout E, Bligny R, Rivasseau C (2012) A simple and efficient method for the long-term preservation of plant cell suspension cultures. Plant Methods 8:4
Cai X, Magwanga RO, Xu Y, Zhou Z, Wang X, Hou Y, Wang Y, Zhang Y, Liu F, Wang K (2019) Comparative transcriptome, physiological and biochemical analyses reveal response mechanism mediated by CBF4 and ICE2 in enhancing cold stress tolerance in Gossypium thurberi. AoB Plant 1:17
Chan Z, Shi H (2015) Improved abiotic stress tolerance of bermudagrass by exogenous small molecules. Plant Signal Behav 10:e991577
Chen X, Huang Q, Zhang F, Wang B, Wang J, Zheng J (2014) ZmCIPK21, a maize CBL-interacting kinase, enhances salt stress tolerance in Arabidopsis thaliana. Int J Mol Sci 15:14819–14834
Correa-Aragunde N, Foresi N, Lamattina L (2015) Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: regulation of ascorbate peroxidase as a case study. J Exp Bot 66:2913–2921
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163
Cui S, Huang F, Wang J, Ma X, Cheng Y, Liu J (2005) A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5:3162–3172
de Marcia OBM, Iyer PR, Buanafina MF, Shearer EA (2017) Reducing cell wall feruloylation by expression of a fungal ferulic acid esterase in Festuca arundinacea modifies plant growth, leaf morphology and the turnover of cell wall arabinoxylans. PLoS ONE 12:e0185312
Dekanski D, Janicijevic-Hudomal S, Ristic S, Radonjic NV, Petronijevic ND, Piperski V, Mitrovic DM (2009) Attenuation of cold restraint stress-induced gastric lesions by an olive leaf extract. Gen Physiol Biophysics 28 Spec No:135–142
Deng X, Hu W, Wei S, Zhou S, Zhang F, Han J, Chen L, Li Y, Feng J, Fang B, Luo Q, Li S, Liu Y, Yang G, He G (2013a) TaCIPK29, a CBL-interacting protein kinase gene from wheat, confers salt stress tolerance in transgenic tobacco. PLoS ONE 8:e69881
Deng X, Zhou S, Hu W, Feng J, Zhang F, Chen L, Huang C, Luo Q, He Y, Yang G, He G (2013b) Ectopic expression of wheat TaCIPK14, encoding a calcineurin B-like protein-interacting protein kinase, confers salinity and cold tolerance in tobacco. Physiol Plant 149:367–377
Dirks-Hofmeister ME, Kolkenbrock S, Moerschbacher BM (2013) Parameters that enhance the bacterial expression of active plant polyphenol oxidases. PLoS ONE 8:e77291
Faltin Z, Holland D, Velcheva M, Tsapovetsky M, Roeckel-Drevet P, Handa AK, Abu-Abied M, Friedman-Einat M, Eshdat Y, Perl A (2010) Glutathione peroxidase regulation of reactive oxygen species level is crucial for in vitro plant differentiation. Plant Cell Physiol 51:1151–1162
Geng S, Li A, Tang L, Yin L, Wu L, Lei C, Guo X, Zhang X, Jiang G, Zhai W, Wei Y, Zheng Y, Lan X, Mao L (2013) TaCPK2-A, a calcium-dependent protein kinase gene that is required for wheat powdery mildew resistance enhances bacterial blight resistance in transgenic rice. J Exp Bot 64:3125–3136
Gonzalez Bosc LV, Plomaritas DR, Herbert LM, Giermakowska W, Browning C, Jernigan NL (2016) ASIC1-mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial smooth muscle cells. Amer J Physiol Lung Cell Mol Physiol 311:L48–58
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Guan X, Nikolau BJ (2016) AAE13 encodes a dual-localized malonyl-CoA synthetase that is crucial for mitochondrial fatty acid biosynthesis. Plant J 85:581–593
Gundinger T, Spadiut O (2017) A comparative approach to recombinantly produce the plant enzyme horseradish peroxidase in Escherichia coli. J Biotech 248:15–24
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Herranz R, Medina FJ (2014) Cell proliferation and plant development under novel altered gravity environments. Plant Biol 16(Suppl 1):23–30
Hu W, Xia Z, Yan Y, Ding Z, Tie W, Wang L, Zou M, Wei Y, Lu C, Hou X, Wang W, Peng M (2015) Genome-wide gene phylogeny of CIPK family in cassava and expression analysis of partial drought-induced genes. Front Plant Sci 6:914
Huang XS, Zhang Q, Zhu D, Fu X, Wang M, Zhang Q, Moriguchi T, Liu JH (2015) ICE1 of Poncirus trifoliata functions in cold tolerance by modulating polyamine levels through interacting with arginine decarboxylase. J Exp Bot 66:3259–3274
Kampatsikas I, Bijelic A, Pretzler M, Rompel A (2017) Three recombinantly expressed apple tyrosinases suggest the amino acids responsible for mono- versus diphenolase activity in plant polyphenol oxidases. Sci Rep 7:8860
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Kazemi Shahandashti SS, Maali Amiri R, Zeinali H, Ramezanpour SS (2013) Change in membrane fatty acid compositions and cold-induced responses in chickpea. Mol Biol Rep 40:893–903
Kazemi-Shahandashti SS, Maali-Amiri R, Zeinali H, Khazaei M, Talei A, Ramezanpour SS (2014) Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J Plant Physiol 171:1106–1116
Kimura S, Kawarazaki T, Nibori H, Michikawa M, Imai A, Kaya H, Kuchitsu K (2013) The CBL-interacting protein kinase CIPK26 is a novel interactor of Arabidopsis NADPH oxidase AtRbohF that negatively modulates its ROS-producing activity in a heterologous expression system. J Biochem 153:191–195
Kovacs Z, Simon-Sarkadi L, Szucs A, Kocsy G (2010) Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 38:623–631
Kuijpers TF, van Herk T, Vincken JP, Janssen RH, Narh DL, van Berkel WJ, Gruppen H (2014) Potato and mushroom polyphenol oxidase activities are differently modulated by natural plant extracts. J Agri Food Chem 62:214–221
Kurusu T, Hamada J, Nokajima H, Kitagawa Y, Kiyoduka M, Takahashi A, Hanamata S, Ohno R, Hayashi T, Okada K, Koga J, Hirochika H, Yamane H, Kuchitsu K (2010) Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol 153:678–692
Lee DJ, Choi HJ, Moon ME, Chi YT, Ji KY, Choi D (2017) Superoxide serves as a putative signal molecule for plant cell division: overexpression of CaRLK1 promotes the plant cell cycle via accumulation of O2(−) and decrease in H2O2. Physiol Plant 159:228–243
Li C, Chang PP, Ghebremariam KM, Qin L, Liang Y (2014) Overexpression of tomato SpMPK3 gene in Arabidopsis enhances the osmotic tolerance. Biochem Biophys Res Commun 443:357–362
Liu Y, Zhou J (2018) MAPping kinase regulation of ICE1 in freezing tolerance. Trends Plant Sci 23:91–93
Liu LL, Ren HM, Chen LQ, Wang Y, Wu WH (2013) A protein kinase, calcineurin B-like protein-interacting protein Kinase9, interacts with calcium sensor calcineurin B-like Protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis. Plant Physiol 161:266–277
Liu H, Wang YX, Li H, Teng RM, Wang Y, Zhuang J (2019) Genome-wide identification and expression analysis of calcineurin B-Like protein and calcineurin B-Like protein-interacting protein kinase family genes in tea plant. DNA Cell Biol 38:824–839
Majda M, Grones P, Sintorn IM, Vain T, Milani P, Krupinski P, Zagorska-Marek B, Viotti C, Jonsson H, Mellerowicz EJ, Hamant O, Robert S (2017) Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Dev Cell 43(290–304):e294
Matia I, Gonzalez-Camacho F, Herranz R, Kiss JZ, Gasset G, van Loon JJ, Marco R, Javier Medina F (2010) Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J Plant Physiol 167:184–193
Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144:1777–1785
Molitor C, Mauracher SG, Rompel A (2016) Aurone synthase is a catechol oxidase with hydroxylase activity and provides insights into the mechanism of plant polyphenol oxidases. Proceed Natl Acad Sci USA 113:E1806–1815
Moustafa K, AbuQamar S, Jarrar M, Al-Rajab AJ, Tremouillaux-Guiller J (2014) MAPK cascades and major abiotic stresses. Plant Cell Rep 33:1217–1225
Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P (2008) Novel mechanism of modulating natural antioxidants in functional foods: involvement of plant growth promoting Rhizobacteria NRRL B-30488. J Agri Food Chem 56:4474–4481
Nejadsadeghi L, Maali-Amiri R, Zeinali H, Ramezanpour S, Sadeghzade B (2014) Comparative analysis of physio-biochemical responses to cold stress in tetraploid and hexaploid wheat. Cell Biochem Biophys 70:399–408
Pandey GK, Cheong YH, Kim BG, Grant JJ, Li L, Luan S (2007) CIPK9: a calcium sensor-interacting protein kinase required for low-potassium tolerance in Arabidopsis. Cell Res 17:411–421
Pandey GK, Kanwar P, Singh A, Steinhorst L, Pandey A, Yadav AK, Tokas I, Sanyal SK, Kim BG, Lee SC, Cheong YH, Kudla J, Luan S (2015) Calcineurin B-Like protein-interacting protein kinase CIPK21 regulates osmotic and salt stress responses in Arabidopsis. Plant Physiol 169:780–792
Piao HL, Xuan YH, Park SH, Je BI, Park SJ, Park SH, Kim CM, Huang J, Wang GK, Kim MJ, Kang SM, Lee IJ, Kwon TR, Kim YH, Yeo US, Yi G, Son D, Han CD (2010) OsCIPK31, a CBL-interacting protein kinase is involved in germination and seedling growth under abiotic stress conditions in rice plants. Mol Cells 30:19–27
Poiatti VA, Dalmas FR, Astarita LV (2009) Defense mechanisms of Solanum tuberosum L. in response to attack by plant-pathogenic bacteria. Biol Res 42:205–215
Rodriguez-Medina C, Boissinot S, Chapuis S, Gereige D, Rastegar M, Erdinger M, Revers F, Ziegler-Graff V, Brault V (2015) A protein kinase binds the C-terminal domain of the readthrough protein of Turnip yellows virus and regulates virus accumulation. Virology 486:44–53
Romero FM, Maiale SJ, Rossi FR, Marina M, Ruiz OA, Garriz A (2018) Polyamine metabolism responses to biotic and abiotic stress. Methods Mol Biol 1694:37–49
Ruan L, Torres CM, Buffett RJ, Kennard S, Fulton D, Venema RC (2013) Calcineurin-mediated dephosphorylation of eNOS at serine 116 affects eNOS enzymatic activity indirectly by facilitating c-Src binding and tyrosine 83 phosphorylation. Vasc Pharmacol 59:27–35
Shakiba E, Edwards JD, Jodari F, Duke SE, Baldo AM, Korniliev P et al (2017) Genetic architecture of cold tolerance in rice (Oryza sativa) determined through high resolution genome-wide analysis. PLoS ONE 12:e0172133
Smith JM (2014) Heese A (2014) Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 10:6
Stajic M, Persky L, Cohen E, Hadar Y, Brceski I, Wasser SP, Nevo E (2004) Screening of laccase, manganese peroxidase, and versatile peroxidase activities of the genus Pleurotus in media with some raw plant materials as carbon sources. Appl Biochem Biotech 117:155–164
Steward N, Martin R, Engasser JM, Goergen JL (1999) Determination of growth and lysis kinetics in plant cell suspension cultures from the measurement of esterase release. Biotechnol Bioeng 66:114–121
Strimbeck GR, Schaberg PG, Fossdal CG, Schröder WP, Kjellsen TD (2015) Extreme low temperature tolerance in woody plants. Front Plant Sci 6:884
Sun P, Zhu X, Huang X, Liu JH (2014) Overexpression of a stress-responsive MYB transcription factor of Poncirus trifoliata confers enhanced dehydration tolerance and increases polyamine biosynthesis. Plant Physiol Biochem 78:71–79
Tang W, Newton RJ (2005) Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.) plantlets. Plant Cell Rep 24:581–589
Tang W, Page M (2013) Transcription factor AtbZIP60 regulates expression of Ca2+ -dependent protein kinase genes in transgenic cells. Molecular Biol Rep 40:2723–2732
Tang W, Peng X, Newton RJ (2005) Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes encoding mannitol-1-phosphate dehydrogenase and glucitol-6-phosphate dehydrogenase. Plant Physiol Biochem 43:139–146
Tang W, Newton RJ, Weidner DA (2007) Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J Exp Bot 58:545–554
Trexler MM, McDonald KA, Jackman AP (2005) A cyclical semicontinuous process for production of human alpha 1-antitrypsin using metabolically induced plant cell suspension cultures. Biotechnol Prog 21:321–328
Wan B, Lin Y, Mou T (2007) Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett 581:1179–1189
Wang J, Pan C, Wang Y, Ye L, Wu J, Chen L, Zou T, Lu G (2015) Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genomics 16:386
Wang JJ, Lu XK, Yin ZJ, Mu M, Zhao XJ, Wang DL, Wang S, Fan WL, Guo LX, Ye WW, Yu SX (2016) Genome-wide identification and expression analysis of CIPK genes in diploid cottons. Genet Mol Res 15:4
Wu L, Sadhukhan A, Kobayashi Y, Ogo N, Tokizawa M, Agrahari RK, Ito H, Iuchi S, Kobayashi M, Asai A, Koyama H (2019) Involvement of phosphatidylinositol metabolism in aluminum-induced malate secretion in Arabidopsis. J Exp Bot 70:3329–3342
Wucherpfennig T, Schulz A, Pimentel JA, Corkidi G, Sieblitz D, Pump M, Gorr G, Schutte K, Wittmann C, Krull R (2014) Viability characterization of Taxus chinensis plant cell suspension cultures by rapid colorimetric- and image analysis-based techniques. Bioprocess Biosyst Eng 37:1799–1808
Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32:915–925
Yang XD, Dong CJ, Liu JY (2006) A plant mitochondrial phospholipid hydroperoxide glutathione peroxidase: its precise localization and higher enzymatic activity. Plant Mol Biol 62:951–962
Yin X, Wang Q, Chen Q, Xiang N, Yang Y, Yang Y (2017) Genome-wide identification and functional analysis of the calcineurin b-like protein and calcineurin b-like protein-interacting protein kinase gene families in turnip (Brassica rapa var. rapa). Front Plant Sci 8:1191
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Yu DF, Wu PF, Fu H, Cheng J, Yang YJ, Chen T, Long LH, Chen JG, Wang F (2011) Aging-related alterations in the expression and distribution of GluR2 and PICK1 in the rat hippocampus. Neurosci Lett 497:42–45
Yuan P, Yang T, Poovaiah BW (2018) Calcium signaling-mediated plant response to cold stress. Int J Mol Sci 19:3896
Zhang X, Shen L, Li F, Zhang Y, Meng D, Sheng J (2010) Up-regulating arginase contributes to amelioration of chilling stress and the antioxidant system in cherry tomato fruits. J Sci Food Agri 90:2195–2202
Zhang H, Liu WZ, Zhang Y, Deng M, Niu F, Yang B, Wang X, Wang B, Liang W, Deyholos MK, Jiang YQ (2014) Identification, expression and interaction analyses of calcium-dependent protein kinase (CPK) genes in canola (Brassica napus L.). BMC Genomics 15:211
Zhao H, Zhang K, Zhou X, Xi L, Wang Y, Xu H, Pan T, Zou Z (2017) Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci Rep 7:4998
Zhou Q, Jia J, Huang X, Yan X, Cheng L, Chen S, Li X, Peng X, Liu G (2014) The large-scale investigation of gene expression in Leymus chinensis stigmas provides a valuable resource for understanding the mechanisms of poaceae self-incompatibility. BMC Genomics 15:399
Zhou L, Lan W, Chen B, Fang W, Luan S (2015) A calcium sensor-regulated protein kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, is required for pollen tube growth and polarity. Plant Physiol 167:1351–1360
Zur I, Dubas E, Golemiec E, Szechynska-Hebda M, Golebiowska G, Wedzony M (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Rep 28:1279–1287
Acknowledgements
We acknowledge the instrumentation facility of the University for providing necessary experiment setup. We appreciate the University Council of Scientific Research for support. We thank Dr. Prasad, Dr. Lischewski, and Dr. Page for their critical reading and suggestions during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
WT, MZ, and WAT conceived and designed the experiments. WT wrote the paper. WT, MZ, and WAT performed the experiment and analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, W., Thompson, W.A. Role of the Arabidopsis calcineurin B-like protein-interacting protein kinase CIPK21 in plant cold stress tolerance. Plant Biotechnol Rep 14, 275–291 (2020). https://doi.org/10.1007/s11816-020-00597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-020-00597-7