Abstract
The influence of light quality on the regeneration (i.e., formation, proliferation, growth, development, and rooting) and naphthoquinones’ accumulation of Arnebia euchroma was investigated in vitro. The cotyledons of sterile seedings were placed on modified LS medium supplemented with 0.8 mg/L thidiazuron to obtain callus, which was incubated on the corresponding medium at different culture stages under varying light conditions, including dark, fluorescent lamp (Fl), white light-emitting diodes (LEDs), blue LEDs, red LEDs, and a combination of 25% red, 25% blue, and 50% white LEDs [RBW (1:1:2)]. During the establishment of the A. euchroma regeneration system, we obtained the maximum fresh weight, dry weight, germination rate, shoot number, and proliferation rate under RBW (1:1:2) LEDs in the formation and multiplication phases; the growth index, shoot height, leaf number, and photosynthetic pigment concentration were promoted under red LEDs in the growth and development phase; and the maximum rooting rate (89.29% ± 2.06%) was observed under red LEDs in the rooting phase. To study the effect of light quality on naphthoquinones’ accumulation of A. euchroma, we used the euphylla to achieve adventitious roots, and the highest shikonin (121.77 ± 6.68 μg/g DW) and β′β-dimethylacrylalkannin (619.18 ± 15.71 μg/g DW) contents were detected in the dark culture. The lowest naphthoquinone contents were recorded under Fl in the adventitious roots. Overall, RBW (1:1:2) LEDs enhanced A. enchroma formation and proliferation, while red LEDs promoted the plant’s growth, development, and rooting. The dark condition was beneficial to naphthoquinones’ accumulation, whereas the light condition inhibited the naphthoquinones production of A. enchroma adventitious roots. Determining the suitable light quality for the regeneration and accumulation of naphthoquinones that is conducive to the protection and promotes resource utilization is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arnebia euchroma (Royle) Johnst, which belongs to family Boraginaceae, is a highly valued and critically endangered medicinal herb that grows on gravel slopes, alluvial fans, grasslands, and meadows in the regions with an altitude ranging from 2500 to 4200 m. A. euchroma is the national secondary protected plant in China, and used to treat various diseases, such as hepatitis, phlebitis, vascular purpura, psoriasis, and burns (Mohsenikia et al. 2017; Fan et al. 2012; Nasiri et al. 2016). The medicinal parts, that is, the roots of A. euchroma, contain naphthoquinones, phenols, benzoquinones, phenolic acids, alkaloids, and sterols (Xin et al. 2012). Meanwhile, the most prominent compound is naphthoquinones, which is not only the main active ingredient of A. euchroma with a variety of physiological activities [i.e., antibacterial (Singh et al. 2015), anti-inflammatory (Kaith et al. 1996) and anticancer (Jeziorek et al. 2018)], but is also widely used as natural pigments in pharmaceutical, cosmetic, and printing industries (Hou et al. 2018; Buchanan et al. 1997). Given its important medicinal efficacy and economic value, A. euchroma has been overexploited. A. euchroma is also difficult to cultivate because its growth environment is extremely strict, and it has a long growth cycle, high seed abortion rate, and low germination rate (Xiong et al. 2009). Thus, the narrowly distributed and sparsely populated wild resources of A. euchroma have been severely damaged, thereby resulting in a rapid decline in the distribution and quantity of its wild species and a serious resource shortage. Therefore, protecting the germplasm resources of A. euchroma and providing a stable and sustainable use of medicinal and industry materials are crucial (Seabrook 2005; Li et al. 2010; Dutta and Jatothu 2013). Plant tissue culture is an important way to solve the lack of resources via the acquisition regenerated plantlets and the produce medicinal active substances. Some studies have shown that plant tissue culture can be used for shoot organogenesis and secondary metabolite accumulation in A. euchroma (Jiang et al. 2005; Zakhlenjuk and Kunakh 1998).
Numerous studies have indicated that different physicochemical factors influence the establishment of regeneration system and accumulation of secondary metabolite of A. euchroma (Manjkhola et al. 2005; Malik et al. 2013). While, among various physicochemical factors, light plays a significant role in regulating the growth, development, and secondary metabolic accumulation of many plants in vitro, such as blue LEDs promoted bigger biomass of Myrtus communis L. (Cioć et al. 2017), red LEDs stimulated the maximum biomass of Rhodiola imbricata Edgew (Kapoor et al. 2018), red LEDs or RB (1:2) stimulated the maximum shoots production of Dendrobium officinal (Lin et al. 2011), B:R (1:1) LEDs stimulated the elongation of shoots and chlorophyll synthesis of Vanilla planifolia (Ramírez-Mosqueda et al. 2017), and the highest Ginsenoside-Rg1 and Ginsenoside-Rb1 contents of Panax vietnamensis were found under fluorescent light (Nhut et al. 2015). light is considered as an important abiotic which can affect the metabolite production (Akula and Ravishankar 2011), and there has research that naphthoquinones, compound of A. euchroma, its derivative can be sensitive to changed light conditions and can induced chemical reactions by light (Tasdelen and Yagci 2013). At present, although some factors, such as plant growth regulator temperature, sucrose, and pH, affect A. euchroma tissue culture, studies on the regeneration system and accumulation of the secondary metabolite of A. euchroma under different light conditions are lacking.
Therefore, the objectives of this work were to examine how the regeneration (including shoot formation, proliferation, growth, and development) and naphthoquinone accumulation of A. euchroma were affected under different light conditions, including, darkness, fluorescent lamp (Fl), white LED, blue LEDs, red LEDs, and a combination of 25% red, 25% blue, and 50% white LEDs [RWB (1:1:2)]. Then, suitable light quality for plantlet formation and naphthoquinones accumulation, which can contribute to the propagation and commercial production of this overexploited and valuable medicinal plant, can be obtained.
Materials and methods
Plant material and light conditions
Arnebia euchroma seeds were collected from Wenquan County, Xinjiang. The seeds were washed under running tap water for 30 min, treated with 75% (v/v) ethanol for 15 s followed by 15% (v/v) H2O2 for 24 min, and then washed five times with sterile distilled water. The sterilized seeds were inoculated on 1/2 MS medium (Murashige and Skoog 1962) and supplemented with 0.05 g/L activated carbon and 7 g/L agar. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C for 25 min. The seeds were incubated for approximately 15 days in the culture room maintained at 25 ± 1 °C and under the dark for germination. The sterile seedling cotyledons were placed on modified LS medium (825 mg/L NH4NO3), supplemented with 40 g/L sucrose, 7 g/L agar, 0.05 g/L casein hydrolysate, 0.08 mg/L AgNO3, and 0.8 mg/L thidiazuron to attain callus, which was used for the next experiment.
The cultures were maintained under different lighting conditions, including (1) darkness, (2) Fl (with a wavelength of 420 nm), (3) white LEDs (with a wavelength of 410–416 nm), (4) blue LEDs (with a wavelength of 450–455 nm), (5) red LEDs (with a wavelength of 650–660 nm), and (6) the combination of red, blue, and white LEDs at the percentages of 25, 25, and 50%, respectively. All the treatments were cultured at 60 μmol m−2 s−1, 25 ± 1 °C temperature, 14/8 h photoperiod (day/night), and 50% relative humidity.
Formation and proliferation
Callus was transferred into the modified LS medium, supplemented with 40 g/L sucrose, 7 g/L agar, 0.05 g/L casein hydrolysate, 0.08 mg/L AgNO3, 0.01 mg/L naphthylacetic acid (NAA), 0.5 mg/L kinetin (KT), and 2 mg/L 6-benzylaminopurine (6-BA) to obtain cluster bud after 30 days of culture. After culturing, the germination rate, fresh weight (FW), dry weight (DW), and shoot number were recorded. The cluster bud was cut into individual shoots and then inoculated into the same medium as the induced buds for proliferation culture. The proliferation rate was recorded. The germination and the proliferation rate were calculated according to the method of Wang et al. (2016) as follows:
Growth and development
After proliferation, the buds were cut into single shoot, inoculated into the modified LS medium, and then supplemented with 40 g/L sucrose, 7 g/L agar, 0.05 g/L casein hydrolysate, and 0.08 mg/L AgNO3. After 30 days of culture, the growth index (Kokotkiewicz et al. 2014), plant height, and leaf number were recorded:
where GI is the growth index, G1 is the plant fresh weight at the end of cultivation, and G0 is the fresh weight of the inoculum.
The chlorophyll (Chl) and carotenoids’ (Car) concentrations were measured via spectrophotometry by referring to the method of Wang and Huang (2015). Chl and Car were extracted from the leaves, and the leaf (0.2 g FW) samples were placed into a mortar with quartz sand, CaCO3, and 2–3 mL of 95% ethanol and then ground into homogenate. Then, the sample was ground in 10 mL of ethanol until the leaf tissue turned white. The absorbance was measured using UV-1600PC at the wavelengths of 470, 649, and 665 nm, as follows:
Rooting culture
The plantlets were transferred into the rooting medium-containing modified LS medium, 20 g/L sucrose, 7 g/L agar, 0.05 g/L casein hydrolysate, 0.05 mg/L NAA, and 0.05 mg/L indole-3-butyric acid to induce rooting. After 40 days of culture, the rooting rate was measured, as follows:
.
Naphthoquinone quantification using HPLC
The euphylla obtained via seed germination was inoculated in the modified LS medium and supplemented with 30 g/L sucrose, 7 g/L agar, 0.05 g/L casein hydrolysate, 0.5 mg/L NAA, and 0.1 mg/L KT for 20 days to achieve adventitious roots. Then, collect adventitious roots and dry it at 50 °C for 5 h. In addition, naphthoquinones were extracted from the adventitious roots (0.5 g DW), which were ultrasonicated with 20 mL of methanol (150 w, 40 kHz) for 50 min. After the extracted solution was cooled to room temperature, and the lost weight via volatilization was made up with methanol, the solution was filtered with 0.45 μm microporous membrane, and the filtrate for the next analysis. Naphthoquinones (shikonin and β′β-dimethylacrylalkannin) were quantified using HPLC (Agilent 1260) with InertSustain C18 column (250 × 4.6 mm, 5 μm). The mobile phases of solvents A (acetonitrile) and B (water:formic acid = 100:0.5) were used as gradient elution. The condition for the gradient is as follows (v/v): 0–10 min, 70% A; 10–20 min, 90% A; 20–25 min, 100% A; 25–27 min, 70% A. The flow rate was 1 mL/min at the temperature of 30 °C, and the detection wavelength was 516 nm.
Data collection and analysis
All treatments were in triplicates, and each replicate had 30 explants. Data were scored and analyzed for variance at the end of cultivation. All statistical data were analyzed using SPSS software (version 23.0) and subjected to one-way ANOVA. Means were compared using the LSD multiple test, and the difference was considered statistically significant at P < 0.05.
Results and discussion
Effect of light quality on A. euchroma regeneration
Effect of light quality on formation and proliferation
The shoot formation and proliferation of A. euchroma from callus were significantly affected by different lights (Table 1, Figs. 1 and 2). As shown in Table 1, the maximum FW and DW were obtained under RBW (1:1:2) LEDs, and the minimum FW and DW were obtained under dark conditions. These results were consistent with those reported by Kim et al. (Kim et al. 2004), who observed the high FW and DW of Chrysanthemum under mixed LEDs. The highest germination rate and number of shoots were also recorded under RBW (1:1:2) LEDs, and the minimum values were observed in the dark. This finding was consistent with those reported in the study of Nhut et al. (2015), who observed the highest germination rate and number of shoots on Panax vietnamensis under mixed LEDs, whereas the lowest number of shoots was obtained in the dark. Meanwhile, Fig. 1 shows that the bud was white and slender in the dark (Fig. 1a) but green and healthy under Fl (Fig. 1b) and LEDs (Fig. 1c–f). When the bud was cut into individual shoots and cultured for 30 days to proliferate, the maximum proliferation index (14.66 ± 0.84, P < 0.05) was observed under RBW (1:1:2) LEDs (Fig. 2). However, the minimum proliferation index (2.33 ± 0.33, P < 0.05) was obtained in the dark (Fig. 2).
Effects of light quality on plant formation of A. euchroma after cultivating callus on bud induction medium for 30 days. a Buds incubated in the dark. b Buds incubated under fluorescent lamp. c Buds incubated under white LEDs. d Buds incubated under blue LEDs. e Buds incubated under red LEDs. f Buds incubated under RBW (1:1:2) LEDs
In addition to providing energy source, light also plays an important role in the in vitro culture as a signal received by photoreceptors, thereby regulating growth, differentiation, and metabolism and affecting plant photomorphogenesis (Seabrook 2005; Ouyang et al. 2003). The results showed that the higher FW and DW levels, germination rate, shoot number, and proliferation index were observed under all LED treatments than those in the dark and Fl treatments. Specifically, RBW (1:1:2) LEDs were highly advantageous in promoting A. euchroma shoot formation and proliferation. Combined with previous evidence, the reason why RBW (1:1:2) LEDs are more advantageous in promoting the shoot formation and proliferation of A. enchroma may be attributed to the increase in the amount of Pfr, which can regulate the expression of photoreactive genes (Zhu et al. 2000).
Effect of light quality on growth and development
The growth and development of A. euchroma were significantly affected by different light conditions (Table 2 and Fig. 3). As shown in Table 2, the A. euchroma growth index, shoot height, leaf number, and photosynthetic pigment concentration, including those of Chl a, Chl b, Chl (a + b), and Car, were the highest under red LEDs. The growth index, shoot height, and leaf number showed significant difference with other treatments (P < 0.05). The contents of photosynthetic pigments are an important indicator of many processes taking place in the plant body (Münzbergová and Haisel 2019). The Chl (a + b) and Car concentration had insignificant differences among blue, red, and RBW (1:1:2) LED treatments. Meanwhile the minimum values of the growth index, shoot height, leaf number, and photosynthetic pigment concentration were observed in the dark, and the leaves became yellow and thin (Fig. 3a). The growth was slow and deformed compared with those under other conditions. These results showed that red LEDs significantly promoted the growth index and shoot height and increased the leaf number of A. euchroma and the photosynthetic pigment content. These results confirmed those of the previous studies on Oncidium, wherein that red light spectrum enhances induction, proliferation, and the carbohydrate contents of PLBs, as well as subsequent plantlet lengths (Liu et al. 2011).
Light can affect plant cell division, growth, and differentiation (Walter 2008). Schuerger et al. (1997) reported that red light is important for the development of the plant photosynthetic apparatus, and light increases starch accumulation by increasing net photosynthetic rate. Kim et al. (2004) obtained greatest stem elongation and internode length under R and RFr in Chrysanthemum. However, Cybularzurban et al. (2007) reported that red light promotes leaf growth, but decreased the Chl and Car contents of plantlets in vitro. Hahn et al. (2000) reported a reverse effect of red light, thereby suggesting that red light inhibits shoot growth, because monochromic red light causes the imbalance of light energy distribution available for photosystems I and II. The growth, stem elongation, leaf number, and photosynthetic pigment concentration may be promoted or inhibited using red light according to species. Meanwhile, our results showed that red LEDs were advantageous in enhancing the growth and development of A. euchroma.
Effect of light quality on rooting
The effect of light quality on A. euchroma rooting rate is shown in Fig. 4. The highest rooting rate in A. euchroma plantlets was obtained under red LEDs (89.29% ± 2.06%), and the difference in the rooting rate under blue LEDs (84.74 ± 0.26%, P > 0.05) was insignificant. Given that the plantlets cannot normally grow and develop and even die in the dark, the rooting rate cannot be recorded and absent under the dark condition. For other treatments, the lowest rooting rate was observed under Fl treatment (65.43% ± 1.56%) and was significantly lower than that under LED treatments (P < 0.05).
Understanding how plants respond to changes in light quality is difficult, because studies only compare specific ratios in many different species, and their responses are often contradictory (Długosz et al. 2017). A previous study validated that Tripterospermum japonicum rooting is promoted under red light (Moon et al. 2006), which is consistent with our results. However, a high number of Rehmannia glutinosa roots were obtained under blue LEDs (Manivannan et al. 2015). In the present study, A. euchroma rooting rate can be enhanced under red LEDs.
Effect of light quality on naphthoquinones (shikonin and β′β-dimethylacrylalkannin) accumulation
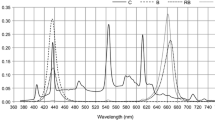
The previous studies on A. euchroma cell suspension cultures demonstrated that shikonin derivative production can be influenced by some physicochemical factors (Malik et al. 2008, 2013). Naphthoquinones (i.e., shikonin and β′β-dimethylacrylalkannin) are used as indicators of drug eligibility in the Chinese pharmacopeia due to their high biological and pharmacological activities. Therefore, the systematic investigation of the effect of different light qualities on naphthoquinone accumulation in A. euchroma is necessary. Considering that the roots are the medicinal parts of A. euchroma, we also obtained adventitious roots in the euphylla as explants. The naphthoquinone content of A. euchroma adventitious roots cultured for 20 days under different light conditions was analyzed using HPLC, and the results are shown in Fig. 5. The shikonin content ranged from 65.01 μg/g to 121.77 μg/g DW, and the maximum shikonin accumulation (121.77 ± 6.68 μg/g DW) was observed in the dark, which was significantly higher than that under blue (89.81 ± 4.93, P < 0.05), red (87.66 ± 7.29 μg/g DW, P < 0.05), RBW (86.06 ± 3.20), and white LEDs (65.01 ± 1.89 μg/g DW, P < 0.05). The minimum shikonin content (70.67 ± 3.71 μg/g DW) was observed under Fl. Similarly, the maximum β′β-dimethylacrylalkannin content was observed in the dark (619.18 ± 15.71 μg/g DW), and the minimum content was observed under Fl (224.31 ± 11.90 μg/g DW). These results were consistent with those of Gupta et al. (2014), who found that A. euchroma cell suspension cultures and napthoquinones’ production increased in the dark. Thus, the dark condition is beneficial to naphthoquinones accumulation, whereas the light condition was attributed to the inhibition of naphthoquinone accumulation. Specifically, shikonin and β′β-dimethylacrylalkannin contents that was cultured under LEDs was significantly lower than that under dark culture (P < 0.05), and the fluorescence light severely inhibited naphthoquinones accumulation. Some naphthoquinones’ production characterized specific light sensitivity, and the o-naphthoquinone methide/thiol click chemistry has a unique reversible and provides a light-directed release or replacement of the immobilized substances (Tasdelen and Yagci 2013). Thus, we suspect in this study that shikonin and β′β-dimethylacrylalkannin may be sensitive to light.
Conclusion
RBW (1:1:2) LEDs promoted plant formation and proliferation, and red LEDs stimulated the maximum growth, development, and rooting during the A. enchroma tissue culture. Dark condition was also beneficial to naphthoquinones (shikonin and β′β-dimethylacrylalkannin) accumulation in the roots when the euphylla was used to attain the adventitious roots in vitro. Therefore, this research may contribute to the protection of the overexploited medicinal plant. This work may also set a foundation for further studies on the efficient and large-scale commercial production of A. euchroma by searching for a suitable light quality to promote plant regeneration system and naphthoquinones (shikonin and β′β-dimethylacrylalkannin) accumulation.
Abbreviations
- Fl:
-
Fluorescent lamp
- LEDs:
-
Light-emitting diodes
- RBW (1:1:2):
-
25% red and 25% blue plus 50% white light-emitting diodes
- Modified LS:
-
Linsmaier and Skoog medium, while reducing the concentration of NH4NO3 to 825 mg/L, but the other components are unchanged
References
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731
Buchanan MS, Gill M, Yu J (1997) Pigments of fungi. part 43.1,2 cardinalins1–6, novel pyranonaphthoquinones from the fungusdermocybe cardinalis horak. J Chem Soc Perkin Trans 6(6):919–926
Cioć M, Szewczyk A, Żupnik M, Kalisz A, Pawłowska B (2017) LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ Cult 132:433–447
Cybularzurban T, Hanusfajerska E, Swiderski A (2007) Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol Crac 49(1):113–118
Długosz GO, Wojciechowska R, Kruczek M, Habela A (2017) Supplemental lighting with LEDs improves the biochemical composition of two Valerianella locusta (L.) cultivars. Hortic Environ Biotechnol 58(5):441–449
Dutta GS, Jatothu B (2013) Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol Rep 7(3):211–220
Fan H, Yang M, Che X, Zhang Z, Xu H, Liu K (2012) Activity study of a hydroxynaphthoquinone fraction from Arnebia euchroma, in experimental arthritis. Fitoterapia 83(7):1226–1237
Gupta K, Garg S, Singh J, Kumar M (2014) Enhanced production of napthoquinone metabolite (shikonin) from cell suspension culture of Arnebia sp. and its up-scaling through bioreactor. Biotech 4(3):263–273
Hahn EJ, Kozai T, Paek KY (2000) Blue and red light-emitting diodes with or without sucrose and ventilation affect in vitro growth of Rehmannia glutinosa plantlets. J Plant Biol 43(4):247–250
Hou Y, Vasileva EA, Carne A, Mcconnell M, Alaa EDB, Mishchenko NP (2018) Naphthoquinones of the spinochrome class: occurrence, isolation, biosynthesis and biomedical applications. RSC Adv 8(57):32637–32650
Jeziorek M, Sykłowskabaranek K, Pietrosiuk A (2018) Hairy root cultures for the production of anti-cancer naphthoquinone compounds. Curr Med Chem 25:4718–4739
Jiang B, Yang YG, Guo YM, Guo ZC, Chen YZ (2005) Thidiazuron-induced in vitro shoot organogenesis of the medicinal plant Arnebia euchroma (Royle) Johnst. Vitro Cell Dev Biol Plant 41(5):677–681
Kaith BS, Kaith NS, Chauhan NS (1996) Anti-inflammatory effect of Arnebia euchroma root extracts in rats. J Ethnopharmacol 55(1):77–80
Kapoor S, Raghuvanshi R, Bhardwaj P, Sood H, Chaurasia OP (2018) Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J Photochem Photobiol B 183:258
Kim SJ, Hahn EJ, Heo JW, Paek KY (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci Hortic 101(1):143–151
Kokotkiewicz A, Bucinski A, Luczkiewicz M (2014) Light and temperature conditions affect bioflavonoid accumulation in callus cultures of Cyclopia subternata, Vogel (honeybush). Plant Cell Tissue Organ Cult 118(3):589–593
Li HM, Xu ZG, Tang CM (2010) Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult 103(2):155–163
Lin Y, Li J, Li B, He T, Chun Z (2011) Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale, in vitro. Plant Cell Tissue Organ Cult 105(3):329–335
Liu M, Xu Z, Yang Y, Yijie F (2011) Effects of different spectral lights on Oncidium, PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult 106(1):1–10
Malik S, Bhushan S, Verma S, Sharma N, Sinha AK, Sharma M (2008) Production of naphthoquinone pigments in cell suspension cultures of Arnebia euchroma (Royale) Johnston: influence of pH on growth kinetics and acetylshikonin. J Med Aromat Plant Sci 2(1):43–49
Malik S, Bhushan S, Sharma M, Ahuja PS (2013) Physico-chemical factors influencing the shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnston, a medicinally important plant species. Cell Biol Int 35(2):153–158
Manivannan A, Soundararajan P, Halimah N, Ko CH, Jeong BR (2015) Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro[J]. Hortic Environ Biotechnol 56(1):105–113
Manjkhola S, Dhar U, Joshi M (2005) Organogenesis, embryogenesis, and synthetic seed production in Arnebia euchroma—a critically endangered medicinal plant of the himalaya. Vitro Cell Dev Biol Plant 41(3):244–248
Mohsenikia M, Khakpour S, Azizian Z, Ashkani ES, Razavipour ST, Toghiani P (2017) Wound healing effect of Arnebia euchroma gel on excisional wounds in rats. Adv Biomed Res 6(1):2
Moon HK, Park SY, Yong WK, Kim CS (2006) Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro, under various sources of light-emitting diode (LED) irradiation. J Plant Biol 49(2):174–179
Münzbergová Zuzana, Haisel D (2019) Effects of polyploidization on the contents of photosynthetic pigments are largely population-specific. Photosynth Res 140(3):289–299
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nasiri E, Hosseinimehr SJ, Hosseinzadeh ZA, Azadbakht M, Akbari J, Azadbakht M (2016) The effects of Arnebia euchroma ointment on second-degree burn wounds: a randomized clinical trial. J Ethnopharmacol 189:107–116
Nhut DT, Huy NP, Tai NT, Nam NB, Luan VQ, Hien VT (2015) Light-emitting diodes and their potential in callus growth, plantlet development and saponin accumulation during somatic embryogenesis of Panax vietnamensis Ha et Grushv. Biotechnol Biotechnol Equip 29(2):299–308
Ouyang J, Wang X, Zhao B, Wang Y (2003) Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci 165(3):657–661
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Luna-Sánchez IJ (2017) Light quality affects growth and development of in vitro, plantlet of Vanilla planifolia, Jacks. S Afr J Bot 109:288–293
Schuerger AC, Brown CS, Stryjewski EC (1997) Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann Bot 79(3):273–282
Seabrook JEA (2005) Light effects on the growth and morphogenesis of potato (Solanum tuberosum) in vitro: a review. Am J Potato Res 82(5):353–367
Singh LK, Maheshwari DK, Shukla S (2015) Antibacterial effect of butyryl alkannin from Arnebia euchroma against vancomycin-resistant pathogens of Enterococcus faecalis causing urinary tract infections. Nat Prod Res 29(24):2299–2301
Tasdelen MA, Yagci Y (2013) Light-induced click reactions. Angew Chem Int Ed 52(23):5930–5938
Walter A (2008) Plant growth dynamics: analysis of basic spatial and temporal growth patterns on the background of photosynthetic energy gain and interactions with the environment. Springer, Berlin, Heidelberg
Wang XK, Huang JL (2015) Principles and techniques of plant physiological biochemical experiment, 3rd edn. Higher Education Press, Beijing, pp 131–132
Wang H, Huang L, Hu F (2016) An efficient tissue culture technique of Acacia mangium from 16-year old plant. Mol Plant Breed 14(4):986–996
Xin G, Zhi JL, GulnarDawuti Qi X, Aibai S (2012) A Preliminary Study on the Chemical Components of Arnebia euchroma (Royle) Johnst. Lishizhen Med Mater Med Res 23(4):814–815
Xiong W, Gang L, Zhou L, Zeng Y, Yang W (2009) In vitro, and in vivo, antitumor effects of acetylshikonin isolated from Arnebia euchroma (Royle) Johnst, (Ruanzicao) cell suspension cultures. Chin Med 4(1):14
Zakhlenjuk OV, Kunakh VA (1998) Arnebia euchroma: in vitro culture and the production of shikonin and other secondary Metabolites. Biotechnol Agric For 41:28–44
Zhu Y, Tepperman JM, Fairchild CD, Quail PH (2000) Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci 97(24):13419–13424
Acknowledgements
This work was supported by the Sichuan Agricultural University “211 Project” Academic Support Personnel of the Special Research Support Program project under Grant Nos. 03570848; 03571547; 03572098, and Innovation Fund of Postgraduate of Xihua University No. ycjj2017212.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, J., Leng, Y., Jiang, Y. et al. Effect of light quality on regeneration and naphthoquinones accumulation of Arnebia euchroma. Plant Biotechnol Rep 13, 353–360 (2019). https://doi.org/10.1007/s11816-019-00543-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-019-00543-2