Abstract
Flowering time (Ft) is the most important characteristic of Chinese cabbage with high leaf yields and late-flowering are favorable traits, while little knowledge on genes involved in Ft and the flowering mechanism in this crop. In this study, we conducted genome-wide RNA-seq analysis using an inbred Chinese cabbage ‘4004’ line in response to vernalization and compared the Ft gene expression with radish crop. A number of Ft genes which play roles in flowering pathways were performed quantitative RT-PCR analysis to verify the regulatory flowering gene network in Chinese cabbage. We found that a total of 223 Ft genes in Chinese cabbage, and 50 of these genes responded to vernalization. The majority of flowering enhancers were upregulated, whereas most flowering repressors were downregulated in response to vernalization as confirmed by RT-qPCR. Among the major Ft genes, the expression of BrCOL1-2, BrFT1/2, BrSOC1/2/3, BrFLC1/2/3/5, and BrMAF was strongly affected by vernalization. In reference to comparative RNA-seq profiling of Ft genes, Chinese cabbage and radish revealed substantially different vernalization response in particular GA flowering pathway. Thus, this study provides new insight into functional divergence in flowering pathways and the regulatory mechanisms in Brassicaceae crops. Further analysis of the major integrator genes between early and late-flowering inbred lines facilitates understanding flowering trait variation and molecular basis of flowering in Chinese cabbage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land plants evolved mechanisms for the precise control of flowering time (Ft) to maximize vegetative growth and sexual reproduction, and ensure the development and dissemination of seeds in a range of environments (Putterill et al. 2004). Genetic, molecular, and physiological analyses have identified more than 180 genes involved in Ft control in Arabidopsis (Fornara et al. 2010). Most of these genes occur in major flowering pathways, which eventually converge onto the floral integrator genes LEAFY (LFY), FLOWERING LOCUS T (FT), and SUPRESSOR OF OVEREXPRESSION OF COSTANCE1 (SOC1) (Parcy 2005; Simpson and Dean 2002). Ft is regulated by a complex network of distinct genetic pathways; photoperiod, vernalization, ambient temperature, and gibberellic acid pathways mediate the response to environmental cues, whereas aging and autonomous pathways are regulated by internal programs (Wullschleger and Weston 2012; Srikanth and Schmid 2011; Mutasa-Gottgens and Hedden 2009; Kim et al. 2009). A prolonged cold period can induce the floral transition; this period is referred to as vernalization (Kim et al. 2009). The requirement for vernalization in Arabidopsis depends on two dominant genes; FRIGIDA (Kedersha et al.) and FLOWERING LOCUS C (FLC) (Koornneef et al. 1994; Lee et al. 1994). FRI activates the level of FLC mRNA via interaction with the mRNA cap-binding protein, whereas the MADS-box transcription factor FLC has a critical role as a repressor of flowering (Geraldo et al. 2009; Michaels and Amasino 1999). Exposure to a typical cold season (vernalization) represses FLC expression and enables the plant to flower during the favorable conditions of spring (Sheldon et al. 2000).
Chinese cabbage is one of the most significant Brassica vegetable crops in Asian countries and it is rapidly gaining appreciation and use as a substitute for white cabbage in Western Europe (Eniko et al. 2011). Brassica rapa diverged from the Arabidopsis lineage around 13–17 million years ago due to whole-genome triplication events (Mun et al. 2009; Park et al. 2005; Teutonico and Osborn 1994). The Chinese cabbage (Chiifu-401-42, 2n = 2x = 10) genome size is estimated as 485 Mb; it was sequenced and first assembled (Ver. 1.5) to cover 283.8 Mb (58.52%) of the genome in 2011 (Wang et al. 2011). Subsequent work used Illumina and PacBio sequencing and revised the assembly, with approximately 85% of the assembly (~ 330 Mb) assigned to the chromosomes (Brassica rapa Genome V2.0) (Cai et al. 2017). Although more complete assembly of the reference genome is still required, these current results provide a ready-to-use dataset for research on comparative genomics and gene function in Chinese cabbage.
Recent progress in next generation sequencing has substantially advanced genome sequencing and RNA-seq analysis (Weber et al. 2007). RNA-seq was performed recently in Chinese cabbage to enable identification of differentially expressed genes related to abiotic stress and heading (fold leaves) traits; subsequently, the reference genome has been assembled (Huang et al. 2015; Li et al. 2017; Wang et al. 2012, 2016). Despite, early bolting and flowering can adversely affect the total yield of Chinese cabbage, even reducing the yield by half, few studies have investigated the genes and signaling pathways associated with Ft in Chinese cabbage (Song et al. 2015; Sun et al. 2015).
In this study, we used RNA-seq analysis to identify genome-wide Ft genes in Chinese cabbage, and then characterized gene expression patterns in response to vernalization. We also investigated the major flowering gene regulatory networks in Chinese cabbage using RT-qPCR analysis. We compared genetic regulation of the major flowering pathway in Chinese cabbage and radish, and identified genes that show conserved or inverse expression profiles in response to vernalization. The combined results can facilitate our understanding of the molecular regulation of vernalization in Brassicaceae family.
Materials and methods
Plant materials and treatments
This study used a Chinese cabbage (Brassica rapa ssp. Pekinensis) inbred line ‘4004’ developed by NongHyup Seed in Korea (Gyeonggi-do, Anseong, Korea), which exhibits early flowering. Twenty seeds were sowed in sterilized soil and grown in a growth room (23 °C, 16-h light/8-h dark) for 2 weeks. Then, vernalization was initiated for 15, 20, and 35 days in a cold room (12-h light/12-h dark, 5 ± 1 °C). After the vernalization period, the plants were transferred to the growth room and allowed to grow for 30 days under the same growth room conditions described above. For RNA-seq analysis, we collected samples that were subjected to vernalization for 0 and 35 days. For each vernalization time point, shoot tissue samples were harvested at the same time point in the light/dark cycle; two independent biological replicates were sampled. A total of four samples were collected from the ‘4004’ inbred line, immediately frozen in liquid nitrogen, and stored at − 70 °C. Total RNA was isolated from shoot tissues as described previously (Jung et al. 2014).
RNA isolation and RNA-seq analysis
Total RNA was isolated from shoot tissue using RNAiso Plus reagent (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. The isolated total RNA was used for messenger RNA isolation and subsequent library construction, purified, end-repaired, polyA-tailed, and ligated to index adapters using the RNA sample preparation protocol from Illumina HiSeqTM2000 sequencer (Illumina, Inc., San Diego, CA, USA). A 101-bp paired-end sequencing protocol was employed. All raw read data generated in this study were deposited in the short read archive (SRA) of NCBI under study’s Accession Number SRP116747.
Filtering and Brassica reference mapping
Raw sequencing data were filtered using standard RNA-seq parameters (Illumina pipeline). Adapter contamination, low-quality regions, and N-base reads were trimmed from the raw reads. Reads with a Phred quality score of 31 (Q ≥ 20) or a minimum length at 25 base pairs (bp) were filtered out using the DynamicTrim and LengthSort programs of the SolexaQA (v.1.13) package (Cox et al. 2010). These datasets were pooled and mapped to the Brassica reference gene set (http://brassicadb.org/brad/, version 1.5, CDS). Mapping was performed using the Bowtie2 (v2.1.0) program (mismatch ≤ 2 bp; other options were set to default) (Langmead and Salzberg 2012). The expression levels in each sample were calculated with an in-house script (read count ≥ 200), and read counts for each gene were normalized against library size and rounded to the nearest whole number. The expression analysis results were deposited in the GeneBank Short Read Archive with the accession number of GSE106444.
Functional annotation
Annotation was based on that used in the Brassica database (http://brassicadb.org/, version 1.5). The annotated genes were validated by comparison with gene sequences in the Phytozome database (http://www.phytozome.net/) using BLASTP with E-values of at least 1E-10 (BLAST v.2.2.28+) (Altschul et al. 1997). For gene ontology (GO) analysis, the GO database (http://www.geneontology.org/) was downloaded, and the transcripts were annotated according to the GO database using BLASTP (E value ≤ 1E−06). GO term annotation was performed using GO classification results from the Map2Slim.pl script. Protein sequences with the highest sequence similarities and cutoffs were retrieved for analysis. Functional enrichment analysis was carried out using DAVID (http://david.abcc.ncifcrf.gov/) (Huang da et al. 2009). The gene lists were filtered according to default criteria (counts ≥ 2 and EASE score ≤ 0.1). Kyoto and Encyclopedia of Gene and Genome (KEGG) pathways were assigned to the sequences by the single-directional best-hit method using the KEGG Automatic Annotation Server (Moriya et al. 2007).
Differentially expressed gene (DEG) analysis
Gene expression data were generated from the four samples of the ‘4004’ inbred line. To identify DEGs during vernalization treatments of 0 and 35 days each time, raw counts were normalized and the DESeq library was analyzed using R (v3.2) (Anders and Huber 2010). For a gene to be considered as a DEG, we required |log2 (fold-change)| ≥ 1, and it was filtered by requiring the adjusted p value (FDR) to be ≤ 0.01. Comparisons of DEGs in Chinese cabbage ‘4004’ and radish ‘NH-JS2’ were performed to identify up- and downregulated genes on the basis of 0 days. We performed Venn diagram analysis in R for up and downregulated DEGs and Ft genes. Overrepresentation pathway analysis of Ft DEGs was performed using a hypergeometric test with a corrected p value < 0.05.
Identification of Ft genes in Chinese cabbage
To identify genes related to flowering pathways in our RNA-seq, a set of 174 Ft genes was selected as a reference set based on published literature and studies in Arabidopsis thaliana (Amasino and Michaels 2010; Nilsson et al. 1998) as described previously (Jung et al. 2016). Published sequences were obtained from the TAIR database (http://www.arabidopsis.org/index.jsp) based on Arabidopsis accession numbers for Ft genes. BLAST was used to query the 174 Ft genes against the mapped 36,698 expressed genes of Chinese cabbage. Top hits were filtered based on the highest percentage of hit coverage and sequence similarity. Cutoffs were E values ≤ 1E−25 and identity ≥ 65%. The Ft gene sequences in Arabidopsis were compared with Ft gene sequences in radish using BLASTn (E value ≤ 1E-25, identity ≥ 70%).
Reverse transcription-quantitative PCR (RT-qPCR) analysis
2 µg of total RNA was used for first-strand cDNA synthesis using a RevertAid First-Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada) according to the manufacturer’s instructions. The cDNA was resuspended in nuclease-free water and used for RT-qPCR analysis with a CFX Connect™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The BrActin gene was used as the internal control for normalizing mRNA levels. Two biological replicates were analyzed for each RT-qPCR assay to ensure the reliability of quantitative analysis with ± SE error bars. All primer sets used in this study are listed in Table S3.
Results
Vernalization response of the ‘4004’ inbred line
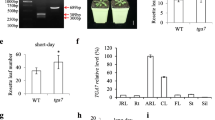
To obtain an overview of flowering phenotypic characteristics in Chinese cabbage, flowering parameters including bolting days and the number of leaves were evaluated. We planted eight inbred lines provided by Nong Hyup Seed in Korea (Gyeonggi-do, Anseong, Korea) into a growth room after at 4 °C for 35 days vernalization. Ft varied considerably among the inbred lines, ‘4004’ is the earliest inbred line compared with other seven lines, moreover, the number of leaves was quite a small number showed (Fig. S1). We examined the Ft of the ‘4004’ inbred line with or without vernalization. The ‘4004’ seedlings were grown in a growth room (16-h light/8-h dark, 23 °C) for 2 weeks, and then transferred to a cold room for vernalization treatments (0, 15, 20, or 35 days). After each vernalization test at each time point was completed, the plants were moved back to the growth room and allowed to grow in soil for an additional 30 days. The ‘4004’ plants did not flower at all (0%) without vernalization treatment, or with 15 or 20 days of vernalization treatment. Even after more than 40 days after the vernalization treatment, the plants treated with 0, 15, and 20 days of vernalization were stopped at the vegetative growth stage. By contrast, plants treated with 35 days of vernalization were flowering after 30 days of normal growth (100%) (Fig. 1). These combined results suggest that the vernalization period significantly affects the flowering phenotype in Chinese cabbage, and this crop requires more than 20 days of vernalization to induce flowering.
Phenotypes of the Chinese cabbage ‘4004’ inbred line after vernalization. Bolting phenotypes of the ‘4004’ inbred line following a vernalization period (0, 15, 20, and 35 days). The 20 seedlings were germinated in a 23 °C growth room for 2 weeks. The 0 day vernalization time point indicates that the ‘4004’ inbred line was grown in the 23 °C growth room for 30 days without vernalization. For the vernalization treatment, germinated seedlings were grown in a cold room (5 ± 1 °C, 12-h light/12-h dark) for 10, 15, 20 and 35 days, and then transferred to a 23 °C growth room for 30 days
Global analysis of the ‘4004’ transcriptome
To analyze DEGs during vernalization, we isolated total RNAs from the shoots of plants subjected to 0 or 35 days of vernalization, and used them to construct cDNA libraries. The libraries were sequenced using an Illumina HiSeq 2000 sequencer. An average of 49.5 and 48.5 million reads were obtained from the 0 and 35 days of vernalization libraries, respectively. Clean reads were obtained by filtering out adaptor sequences, contaminating sequences, and low-quality reads, which accounted for 72.52 (0 days_1st), 62 (0 days_2nd), 72.3 (35 days_1st) and 76.45% (35 days_2nd) of the total clean reads (Table S1). To identify expressed genes, high-quality reads from the four libraries were aligned to the Brassica CDS sequence (http://brassicadb.org/brad/) as the reference. A total of 76.39 (0 days_1st), 70.28 (0 days_2nd), 77.9 (35 days_1st), and 77.17% (35 days_2nd) of the clean reads were mapped to the reference sequence, including uniquely mapped reads and multiply mapped reads.
The number of mapped reads was only slightly higher at 35 days than at 0 days. Reproducibility of the expression datasets was tested by evaluating two samples between biological replicates by pairs plot. The correlation coefficient of the replicate pairs was more than 0.99 for all four samples (Fig. S2). To evaluate the transcript levels, we used transcripts per million kilobase (TPM) and trimmed means of M values (TMM) methods for calculation and normalization, respectively. A total of 36,698 genes were identified from the four transcriptomes. More than 89% of the genes were expressed for the Brassica references. Therefore, the sequences and reference were suitable for further studies as DEGs and expression profiling analysis.
Identification of DEGs by vernalization
To identify vernalization-responsive genes, DEG analysis was conducted for the unique reads that were mapped to the Brassica reference genome in libraries with and without vernalization treatment using the DESeq package. The DEGs were selected using the following criteria: FDR ≤ 0.01 and |log2 (fold-change)| ≥ 1). A total of 1657 DEGs were identified; 729 genes were upregulated and 928 genes were downregulated by vernalization treatment for 35 days compared with no vernalization treatments (Fig. 2a). A previous transcriptome analysis examined radish response to vernalization (Jung et al. 2016). Therefore, we compared the gene expression changes in response to vernalization in Chinese cabbage and radish plants by comparing all DEGs of the early flowering radish ‘NH-JS2’ inbred line with those of the Chinese cabbage ‘4004’ inbred line. The gene expression values were calculated for each sample to normalize gene expression profiles under different conditions. For pairwise comparisons, there were more DEGs in ‘NH-JS2’ than in the ‘4004’ transcriptome. However, the ratios of downregulated and upregulated DEGs was similar in Chinese cabbage and radish, 1.27-fold and 1.47-fold, respectively. These combined results indicate that the number of downregulated DEGs increased in response to vernalization in both Chinese cabbage and radish plants (Fig. 2a).
Comparison of differentially expressed genes response to vernalization between ‘4004’ and ‘NH-JS2’ inbred lines. a A number of differentially expressed genes (DEGs) under normal and vernalization conditions (0 and 35 days) of ‘4004’ and ‘NH-JS2’, respectively. b Venn diagram showing the overlap of the up- and downregulated DEGs in Chinese cabbage and radish. DEGs were identified with 743 and 864 upregulated and 935 and 1271 downregulated by the vernalization, respectively. The Venn diagrams depict the overlaps between each pairwise comparison. The blue and red diagrams indicate ‘4004’ and ‘NH-JS2’ genes, respectively. The gene numbers are indicated in each region of the diagrams. The parentheses are indicated the number of the duplicate genes (Chinese cabbage genes versus radish genes)
To determine the number of genes uniquely expressed in each plant and genes that were co-expressed between the plants, we analyzed Venn diagrams for the DEGs. Most DEGs were uniquely up and downexpressed in Chinese cabbage and radish, about 81% (1334 genes of 1657 DEGs) and 82.6% (1764 genes of 2135 DEGs), respectively, whereas a small number of DEGs were shared between the two plants, 19% (314 genes of 1657 DEGs) in Chinese cabbage and 17.4% (371 genes of 2135 DEGs) in radish. There were 179 and 210 overlapping upregulated DEGs in Chinese cabbage and radish, respectively, whereas 135 and 161 genes were downregulated in Chinese cabbage and radish, respectively (Fig. 2b). These results indicate that more DEGs were downregulated in response to vernalization than were upregulated, and the vernalization-responsive gene expression patterns differed significantly between Chinese cabbage and radish plants.
Functional classification of DEGs
To functionally annotate the vernalization-responsive DEGs, we used GO functional classification analysis based on the overall analysis of gene expression profiles presented above. The functions of DEGs in the vernalization treatment were filtered by FDR < 0.05 and were classified by GO enrichment analysis. A total of 447 genes were identified only in the upregulated set, whose encoded proteins were located in an ‘intracellular membrane-bounded organelle region’ in the cellular component category. They participated in upregulation of biological processes including ‘nucleotide-containing compound metabolic process’, ‘response to hormone’, ‘post-embryonic development’, and ‘transmembrane transport’, whereas downregulated DEGs participated in biological processes including ‘protein metabolic process’, ‘lipid metabolic process’, ‘secondary metabolic process’ and ‘cellular amino acid metabolic process’ (Fig. S3).
To further investigate the biological pathways that are active under vernalization conditions, we assigned the DEGs to pathways in the KEGG database, which resulted in 1657 mapped genes grouped into 121 KEGG pathways, and classified by five major classifications and 19 subclassifications. The major classifications were involved in metabolism (M), ‘glycan biosynthesis and metabolism’, ‘metabolism of terpenoids and polyketides’, ‘biosynthesis of other secondary metabolites’, ‘amino acid metabolism’, ‘lipid metabolism’, and ‘metabolism of cofactors and vitamins’ (Fig. 3). The top five pathways, including ‘cutin, suberine and wax biosynthesis’, ‘ABC transporters’, ‘plant hormone signal transduction’, ‘plant–pathogen interaction’, and ‘metabolic pathways’, might be regulated by vernalization as observed in the classification results. Comparing between the up- and downregulated DEGs, 13 subclassifications of the KEGG pathways were significantly altered in the downregulated DEGs, whereas six subclassifications were upregulated. The following five subclassifications ‘glycan biosynthesis and metabolism’, ‘membrane transport’, ‘environmental adaptation’, ‘metabolism of terpenoids and polyketides’, and ‘amino acid metabolism’, revealed the most distinct differences between vernalization-responsive up- and downregulated DEGs (Fig. 3).
Significantly enriched KEGG pathways of up- and downregulated DEGs during vernalization. Kyoto Encyclopedia of Genes and Genomes (KEGG) classification of the DEGs by KEGG Automatic Annotation Server. Major classifications are cellular process, environmental information processing, genetic information processing, metabolism and organismal systems. Upregulated genes are shown in red and downregulated genes are shown in blue color. CP cellular processes, EIP environmental information processing, GIP genetic information processing, M metabolism, OS organismal systems
Validation of DEGs by RT-qPCR
To evaluate the RNA-seq expression data, ten genes were selected from the RNA-seq data set that had greater than threefold change during the vernalization treatment, and were then examined by RT-qPCR. The most upregulated transcript in response to vernalization was Bra000263 (COR15B), an apparent homologue of COR15A in predominant cold signaling pathway (Jung et al. 2014a, b). Five of these transcripts, Bra000263 (COR15B, cold-regulated 15B), Bra030496 (LHY, late elongated hypocotyl), Bra000876 (GSTF2, glutathione S-transferase PHI2), Bra001086 (no annotation), and Bra013123 (PRB1, basic pathogenesis-related protein 1) were upregulated, and the other five transcripts, Bra018969 (BGL1, beta glucosidase 1), Bra012702 (CSLA10, cellulose synthase-like A1), Bra016073 (KTI, Kunitz-protease inhibitor), Bra022535 (LOX2, lipoxygenase 2), and Bra000129 (JAL22, jacalin-related lectin 22) were downregulated in response to vernalization in the ‘4004’ inbred line (Fig. S4). The RT-qPCR analysis indicated that all genes exhibited the same expression trends as in the RNA-seq analyses. Therefore, our RNA-seq results are reliable, and we conducted the remaining experiments based on RNA-seq data.
Identification of genes and expression profiling involved in the flowering pathway
To identify Ft genes in the transcriptome datasets for Chinese cabbage, we used 174 genes identified in previous flowering studies in Arabidopsis for a BLAST search as reported previously in radish (Jung et al. 2016). We also used reference annotation of the Brassica database. The top hits of BLAST were filtered based on the highest percentage of hit coverage and sequence similarity (all hits below an E value of 1E−25 and over an identity of 65%). A total of 223 putative Chinese cabbage flowering genes were found and assigned according to 135 Ft genes in Arabidopsis. The flowering genes were classified into major flowering pathways as follows: ‘C’ (circadian clock pathway) (16%, 36 genes); ‘L’ (light signaling pathway) (3%, 6 genes); ‘P’ (photoperiod pathway) (30%, 67 genes); ‘V’ (vernalization pathway) (22%, 49 genes); ‘A’ (autonomous pathway) (11%, 24 genes); ‘G/M’ (gibberellin signaling and metabolism) (7%, 16 genes); ‘D/M’ (development and metabolism response) (8%, 18 genes); ‘I’ (integrator) (1%, 3 genes); and ‘A’ (aging) (2%, 4 genes) (Table 1). The expression of a number of Chinese cabbage Ft genes was approximately two times more than that of A. thaliana Ft genes, and this result is in agreement with a previous radish study, results from genome triplication.
To discover DEGs related to flowering pathways, we identified vernalization-responsive DEGs among 223 putative Ft genes. In total, 50 Ft genes were identified as DEGs in response to vernalization (Table 2). Based on RNA-seq data, BrLHY, BrCOL1-1 and BrSOC1-1 genes were the most upregulated flowering enhancers in response to vernalization, with more than threefold increases in expression. A total of 17 genes of 33 DEGs (57.6%) that play roles as enhancers in flowering pathways were upregulated by vernalization. BrFLCs and BrPRP1 were the most downregulated flowering repressors in response to vernalization, and 12 genes of 17 DEGs (71%) that function as repressors in flowering pathways were downregulated by vernalization. RNA-seq data indicated that Ft repressor DEGs were more appropriately expressed in accordance with their functions in the flowering pathway under vernalization than the enhancer DEGs in Chinese cabbage.
We conducted a comparative analysis of the vernalization-responsive Ft DEGs in Chinese cabbage and radish plants using a Venn diagram. The results indicated that 33 and 41 Ft DEGs were uniquely upregulated in ‘4004’ and ‘NH-JS2’ plants, respectively, whereas 17 and 12 Ft DEGs were uniquely downregulated in response to vernalization in ‘4004’ and ‘NH-JS2’, respectively. A proportion of DEGs overlapped between the two plants, with 14 and 16 Ft DEGs upregulated and 4 and 2 Ft DEGs downregulated in Chinese cabbage and radish, respectively. In particular, there were more than three times the number of upregulated Ft DEGs (77%) than the number of downregulated genes (23%) in the radish ‘NH-JS2’ line. Approximately 70% of Ft DEGs in Chinese cabbage and radish were uniquely up- and downregulated in each plant, whereas relatively few DEGs were shared between the two plants (30%) (Fig. 4). A total of 14 Ft genes (16 genes) were upregulated by vernalization in both plants, as common genes including many floral enhancer genes such as CCA1, COL5 (CONSTANS-LIKE 5), GI, LHY, PHYA, PRR5, SOC1, TOC1, and VIN3. By contrast, four Ft genes (two genes) were downregulated, including FLCs repressors in both plants. We performed heatmap analysis to identify the difference between vernalization-responsive Ft DEGs in Chinese cabbage and radish (Fig. S5). There were 24 and 22 uniquely expressed Ft genes in Chinese cabbage and radish, respectively (Fig. S6). These results revealed the diverse expression responses of Ft genes in response to vernalization, indicating that different regulatory pathways may control flowering in each plant.
Comparison of flowering time (Ft) genes in DEGs by vernalization in Chinese cabbage and radish. Venn diagram showing the overlap of the up- and downregulated Ft DEGs in the two plants. Thirty-three genes of ‘4004’ DEGs and 41 genes of ‘NH-JS2’ DEGs were upregulated, and 20 and 12 were downregulated by vernalization in ‘4004’ and ‘NH-JS2’, respectively. The Venn diagrams depict the overlaps between each pairwise comparison and overlaps is among common genes; 14 and 2 genes were up and downregulated, respectively. The ‘4004’ and ‘NH-JS2’ genes are shown in the blue and red diagrams, respectively. The numbers of genes are indicated in each region of the diagrams
Enriched flowering pathways under vernalization
To determine which flowering pathways had relevant responses to vernalization in Chinese cabbage and radish, we performed overrepresentation analysis. A hypergeometric distribution test was applied to identify which flowering pathways were enriched in the Ft DEGs. Ft DEGs were primarily associated with the vernalization pathway (V), gibberellin signaling/metabolism pathway (G/M), and integrator pathway (I) in Chinese cabbage, and the clock/light/photoperiod pathway (C/L/P), gibberellin signaling/metabolism pathway (G/M), and integrator pathway (I) in radish (Table 2). These results indicate that Chinese cabbage and radish have different flowering pathways controlling Ft under vernalization. Next, we analyzed up- and downregulated Ft DEGs separately for the hypergeometric distribution test (Table S2). For upregulated Ft DEGs, the integrator pathway (I) was the most enriched in Chinese cabbage, whereas the ‘C/L/P’ was in radish. For downregulated Ft DEGs, Aging pathway was the unique over representative flowering pathways in radish, whereas the ‘V’ and ‘G/M’ pathways were enriched in Chinese cabbage. These results indicate that up- and downregulated Ft DEGs were enriched in flowering pathways in Chinese cabbage, whereas the upregulated Ft DEGs were primarily involved in Ft control in radish under vernalization.
RT-qPCR analysis of flowering pathway genes under vernalization
To quantitate the expression levels of major flowering pathway genes, we conducted RT-qPCR analysis using the same RNA samples prepared for ‘4004’ RNA-sEq. As shown in Fig. 5a, flowering enhancer genes exhibited various expression patterns in response to vernalization. The major Ft genes, BrCO, BrSOC1, and BrFT were expensed multiple genes in the triplicated Chinese cabbage genome (Song et al. 2016). We designed primers that were specific for each paralog for RT-qPCR analysis (Table S3). Three BrCOLs were upregulated by vernalization, whereas BrCO essentially did not respond to vernalization. The flowering integrator genes BrFT1 and BrSOC1-1 were the most highly upregulated (more than 15-fold) in response to vernalization. The expression of other SOC1, BrSOC1-2, and BrSOC1-3 transcripts also increased in response to vernalization. BrFT2 expression also increased in response to vernalization, whereas BrFT3 and BrFT4 were slightly reduced. BrVRN1, BrAP1, and BrGID1A, which are enhancers of flowering pathways, were also slightly downregulated in response to vernalization, whereas the enhancers BrVRN2, BrVIN3, BrCCA1, BrGI, and BrNF-YA4 were upregulated by vernalization. The expression of the BrLFY, BrVRN2, and BrAGL19 enhancer genes were not significantly affected by vernalization.
We also analyzed quantitative changes in the expression of flowering repressor genes. FLC is the most important factor in regulating Ft as a repressor, and the transcript level declines dramatically when a plant is exposed to low temperature. Previous studies estimate that there are 4–5 FLC genes in Chinese cabbage (Song et al. 2016). Among them, four FLCs were expressed in our RNA-seq dataset, and we demonstrated that the expression levels of BrFLC1, BrFLC2, BrFLC3, and BrFLC5 were remarkably reduced after vernalization. Expression of the FLC regulator, BrFRI also decreased, but the difference was not significant. The expression level of BrELF3 did not change, but the expression level of BrELF4 was reduced by half in response to vernalization. The expression of BrMAF3, the MADS flowering gene, was strongly reduced in response to vernalization. BrSPA3 and BrSVP1 expression levels slightly decreased, but BrGAI and BrGA2ox2 were not (Fig. 5b). The RT-qPCR results revealed that major flowering enhancers were upregulated in response to vernalization, whereas essential repressors were downregulated in response to vernalization in Chinese cabbage.
As part of a study to early flowering phenology for ‘4004’ inbred line, we evaluated three major flowering genes expression using a late-bolting inbred line ‘50’ under vernalization conditions (Fig. S1). A key repressor of flowering, BrFLC1 showed increased about 2.5 times and 5 times in the late-bolting line compared to ‘4004’ line under without and with vernalization conditions, respectively. On the other hand, enhancers of flowering, BrSOC1-1 and BrFT1 revealed that significantly increased expression levels in early-bolting ‘4004’ line (Fig. 6).
Differential expression of three major flowering-time (Ft) genes between ‘4004’ and’50′ inbred lines during vernalization. RT-qPCR analysis of BrFLC1, BrSOC1-1, and BrFT1 between ‘4004’ and’50′ inbred lines in response to vernalization. RT-qPCR expression level was normalized against the corresponding level of BrACT2. For each, the expression level from ‘4004’ on day 0 was defined as “1”. Error bars represent SE of two independent replicates
Comparative gene regulatory networks controlling flowering in Chinese cabbage and radish
To better understand the genetic regulatory networks of controlling Chinese cabbage flowering, we examined the major flowering gene networks based on RT-qPCR data (Fig. 7). The data revealed differences in flowering gene expression in response to vernalization in Chinese cabbage ‘4004’ radish ‘NH-JS2’ (Jung et al. 2016). We examined the three major flowering pathways, photoperiod/circadian, vernalization, and gibberellin. The key flowering genes FLC, CO, and SOC1, showed similar expression trends in ‘4004’ and ‘NH-JS2’ after vernalization. In response to vernalization, FLCs were negatively regulated, whereas COs and SOC1, which act as flowering enhancers, were positively regulated. The photoperiod/circadian pathway enhancer genes LHY, CCA1, GI, and NF-YA4 showed similar expression profiles in Chinese cabbage and radish, whereas repressor genes in this pathway (ELF4 and SPA3) were expressed in inverse: downregulated in Chinese cabbage, but upregulated in radish in response to vernalization. The enhancer BrAP1 was not essentially affected by vernalization. Most of the Chinese cabbage enhancer genes did not significantly respond to vernalization, whereas the expression of BrFLCs and BrMAF3 repressors were more significantly reduced than radish under vernalization. The expression of major GA pathway genes, BrGAI and BrGA2ox2 was negligible in response to cold in Chinese cabbage. In particular, the enhancer BrGID1A was downregulated under vernalization, unlike in radish. Integrator genes FTs were differentially expressed in Chinese cabbage and radish. BrFT1/2 was positively regulated by vernalization in Chinese cabbage ‘4004’, similarly as in most other plants. However, RsFT showed a tendency to be greatly reduced in response to vernalization in ‘NH-JS2’ (data not shown). Distinctively functional genes associated with the floral meristem identity, LFY, FLC positively regulated FRI genes in Chinese cabbage but not radish (Jung et al. 2016). RT-qPCR analysis confirmed most of the Ft gene expression data, and although several genes showed differences in gene expression values than between the RT-qPCR and RNA-seq data (BrELF4, BrSPA3, and BrGA20Ox2), most Ft gene expression was confirmed.
Comparative gene networks controlling flowering time (Ft) genes in Chinese cabbage and radish plants. The schematic represents regulatory network of Ft genes in the ‘4004’ and ‘NH-JS2’ lines after 35 days vernalization based on RT-qPCR. The red shows higher expression and blue shows lower expression compared to the 0 day sample. The arrows indicate transcriptional activation, whereas bars indicate transcriptional repression. N/A means not performed
Discussion
RNA-seq analysis of the vernalization response in Chinese cabbage
We investigated the early flowering phenotype of an inbred line of the Chinese cabbage ‘4004’ (Fig. S1) at different vernalization times (0, 15, 20, and 35 days) and showed that vernalization was necessary for controlling flowering in Chinese cabbage, and more than 20 days vernalization was needed for flowering (Fig. 1), which is in agreement with the results of Yang et al. (2005). RNA-seq was performed to identify genes involved in flowering regulation and determine the molecular network that regulates the flowering pathway response to vernalization (0 days and 35 days) in the ‘4004’ line. We generated 72 million reads from both 0 day and 35 day transcriptomes, representing 11 × coverage of the Chinese cabbage genome. Our transcriptome generated a total of 36,698 genes (89% of the genome based CDS) successfully, despite using only shoot tissue for the RNA-seq analysis (Table S1).
DEG responses to vernalization reveal candidate genes for flowering pathways
Genome-wide DEG analysis of the transcriptomes revealed that vernalization affected the transcription of a number of genes in Chinese cabbage and radish (Jung et al. 2016). A total of 729 DEGs were upregulated and 928 DEGs were downregulated by vernalization in the ‘4004’ line (Fig. 2a). Previous studies reported similar results showing that vernalization primarily downregulated gene expression (Huan et al. 2013; Li et al. 2016; Sun et al. 2015).
To identify candidate genes of Chinese cabbage flowering pathways, we searched homologs for 174 Arabidopsis Ft genes from our ‘4004’ transcriptomes. We identified 223 putative flowering genes among 36,698 transcripts and the genes were assigned to 135 Arabidopsis Ft genes (Table 1). The key flowering regulators, BrFT, BrAP1, and BrLFY were excluded from the list for the reason the expression values were below in-house script baseline, however, their dynamic expression were confirmed by RT-qPCR analysis (Fig. 5). Thus, almost the same number of flowering genes were identified in Chinese cabbage and radish plants (218 Ft genes were identified in radish under vernalization). Recent genome-level analyzes also have reported no significant differences in the numbers of Brassicaceae interspecific flowering genes and their associated flowering pathways (Wang et al. 2017). In addition, 50 of these Ft genes were differentially expressed in response to vernalization in Chinese cabbage. This number of Ft DEGs was very similar in radish (Figs. S5, 4; Table 2).
The Chinese cabbage genome is triplicated (Wang et al. 2011). Therefore, it is expected to contain several Ft gene homologs. In the RNA-seq results, a number of the Ft genes had multiple homologs. For example, FLC has four homologs, SOC1 has three, and FVE has five homologs (Table 1). By contrast, only one homolog was identified for FRI, GI, and FLK. It is unclear whether all homologous Ft genes are actually involved in flowering. Most multiple homologs of major Ft genes were similarly expressed in response to vernalization, but some homologs had different expression under vernalization (Fig. 5), so we anticipate that homologs may have different functions in vernalization.
Comprehensive understanding of flowering gene regulatory networks in Chinese cabbage
Three major pathways are involved in the transition to flowering in Arabidopsis: photoperiod, autonomous/vernalization, and GA pathways (Sugiyama et al. 2014). To understand flowering mechanisms and pathways in Chinese cabbage, we performed RT-qPCR analysis to measure the expression of major genes involved in the three pathways (Fig. 5). The upregulation of floral enhancers such as BrSOC1-1, BrSOC1-2, BrSOC1-3, and BrFT1 by vernalization revealed a conserved mechanism of other flowering pathways (Michaels et al. 2003; Oliver et al. 2009; Wang et al. 2017), whereas the expression of BrVRN1, BrAP1, and BrGID1A enhancers were not significantly changed. Our data indicate that SOC1 and FT flowering integrators may be primarily involved in Chinese cabbage vernalization. Vernalization significantly downregulated the repressors BrFLCs and BrMAF3, however, other repressors were not significantly changed. FLCs may have an essential role in Chinese cabbage flowering response to vernalization (Oliver et al. 2009; Wang et al. 2017). The central flowering repressor, BrFLC1-1 was relatively high expressed in the late-bolting line, whereas two enhancer integrators, BrSOC1-1 and BrFT1, were highly expressed in early-bolting ‘4004’ line confirmed by biological validation (Fig. 6). The expression pattern of the three major flowering genes was well correlated with the ‘4004’ phenotype, indicating that the Chinese cabbage major Ft genes are similar to Arabidopsis plant. RNA-seq classification of DEGs according to the flowering pathway can be used to correlate vernalization with each pathway. Among the DEGs with increased expression levels, genes belonging to C/L/P (clock/light signaling/photoperiod) pathway were the most common. Of the 33 upregulated DEGs, 21 were in the C/L/P pathway. The hypergeometric test showed that it was overrepresented. However, only three of 17 downregulated DEGs belonged to the C/L/P pathway. The reverse was true for genes in the vernalization pathway, which were overrepresented only in downregulated DEGs (Table S2). The autonomous pathway and the floral development pathway seem to be largely unrelated to the vernalization response. Only one gene in each pathway was included in the DEGs. The GA pathway was significantly altered during vernalization; it is overrepresented only in the downregulated DEGs (Table S2). This suggests that GA may be involved in the vernalization response. In Brassica oleracea, GA does not significantly affect flowering induction (Hamano et al. 2002). In the case of Brassica napus, flowering was accelerated after GA treatment, suggesting the possibility of modulating the photoperiod and vernalization response (Dahanayake and Galwey 1999). Further experiments with GA treatment on Chinese cabbage are needed. Nonconserved overrepresented flowering pathways by vernalization support divergence of regulatory networks on flowering in the Brassicaceae family.
Chinese cabbage and radish have different vernalization responses at the gene expression level
When treated with vernalization, the frequency of downregulated genes in both crops (55% and 59% in Chinese cabbage and radish, respectively) was higher than the upregulated genes. However, a detailed comparison of DEGs showed that the vernalization response of both crops was significantly different. First, the number of genes common to both crops in DEG was small. About 75% and 85% of genes differed in the upregulated and downregulated DEGs, respectively (Fig. 2b). Second, overrepresented pathways in response to vernalization differed (Fig. 3). Third, Ft control pathways responding to vernalization differed. In Chinese cabbage vernalization, integrator (I) pathways changed significantly, whereas aging pathways was the most overrepresented in radish (Table 3). Fourth, the number of common genes among the Ft genes whose expression level changed after vernalization treatment was small (Fig. 4). As a result of Ft DEGs analysis between the two crops, co-upregulated with radish Ft DEGs among the Chinese cabbage were mainly enriched in C/L/P pathway, whereas only three Ft DEGs were co-downregulated with radish and the two DEGs were FLC genes. In addition, the Ft DEGs that reacts inversely to vernalization between the two crops showed 12% of the total Chinese cabbage Ft DEGs and half of the DEG genes were involved in GA pathway. Suggesting that the effect on the GA mechanism may be different between the two crops. Although, both crops as annual plants to flowering in response to vernalization, our genome-wide transcripts of Ft genes data suggest that the molecular mechanism regulated by vernalization differ markedly (Fig. 7). In contrast, other plant studies reported that the vernalization response was conserved between Barchypodium and barley interspecies using correlation analysis of vernalization-related genes (Huan et al. 2013). Our comparative DEG analysis provides insight into evolutionary conservation, diversity, and specificity of the vernalization regulating mechanism in Brassicaceae lineage.
In conclusion, to date, we know little about the function of Ft genes and what happens to gene expression at the time of flowering in Chinese cabbage. This study performed genome-wide identification of flowering genes and analyzed expression in response to vernalization in Chinese cabbage. We performed comparative analysis with the flowering genes of radish identified in our previous study, and suggest that flowering processes may be different between Chinese cabbage and radish under vernalization. The results of this study confirm the candidate Ft genes of Chinese cabbage, thus presenting new insights into the flowering process in Chinese cabbage. This knowledge could be incorporated into molecular breeding programs for developing late-flowering varieties of Chinese cabbage.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amasino RM, Michaels SD (2010) The timing of flowering. Plant Physiol 154:516–520
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Cai C, Wang X, Liu B, Wu J, Liang J, Cui Y, Cheng F, Wang X (2017) Brassica rapa GENOME 2.0: a reference upgrade through sequence re-assembly and gene re-annotation. Mol Plant 10:649–651
Cox MP, Peterson DA, Biggs PJ (2010) SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinf 11:485
Dahanayake SR, Galwey NW (1999) Effects of interactions between low-temperature treatments, gibberellin (GA3) and photoperiod on flowering and stem height of spring rape (Brassica napus var. annua). Ann Bot 84:321–327
Eniko L, Alexandru Silviu A, Jolan V, Alexandru Ioan A (2011) Research concerning the bolting of Chinese cabbage (Brassica campestris var. pekinensis (Lour.) Olson) in early crops in polyethylene tunnels. Acta Univ Sapientiae Agric Environ 3:152–160
Fornara F, de Montaigu A, Coupland G (2010) SnapShot: control of flowering in Arabidopsis. Cell 141:550:550 e551–e552
Geraldo N, Baurle I, Kidou S, Hu X, Dean C (2009) FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol 150:1611–1618
Hamano M, Yamato Y, Yamazaki H, Miura H (2002) Endogenous gibberellins and their effects on flowering and stem elongation in cabbage (Brassica oleracea var. capitata). J Hortic Sci Biotechnol 77:220–225
Huan Q, Mao Z, Zhang J, Xu Y, Chong K (2013) Transcriptome-wide analysis of vernalization reveals conserved and species-specific mechanisms in Brachypodium. J Integr Plant Biol 55:696–709
Huang XY, Tao P, Li BY, Wang WH, Yue ZC, Lei JL, Zhong XM (2015) Genome-wide identification, classification, and analysis of heat shock transcription factor family in Chinese cabbage (Brassica rapa pekinensis). Genet Mol Res 14:2189–2204
Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Jung H-J, Dong X, Park J-I, Thamilarasan SK, Lee SS, Kim Y-K, Lim Y-P, Nou I-S, Hur Y (2014a) Genome-wide transcriptome analysis of two contrasting Brassica rapa doubled haploid lines under cold-stresses using Br135K oligomeric chip. PLoS One 9:e106069
Jung WY, Lee SS, Kim CW, Kim HS, Min SR, Moon JS, Kwon SY, Jeon JH, Cho HS (2014b) RNA-seq analysis and de novo transcriptome assembly of Jerusalem artichoke (Helianthus tuberosus Linne). PLoS One 9:e111982
Jung WY, Park HJ, Lee A, Lee SS, Kim YS, Cho HS (2016) Identification of flowering-related genes responsible for differences in bolting time between two radish inbred lines. Front Plant Sci 7:1844
Kim DH, Doyle MR, Sung S, Amasino RM (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25:277–299
Koornneef M, Balnkestijn-de Vries H, Hanhart C, Soppe W, Peeters T (1994) The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J 6:911–919
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Lee I, Michaels AS, Amasino RM (1994) The late-flowering phenotype of FRIGIDA and mutations in LUMINIDEPENDENS is suppressed in the Landsberg erecta starin od arabidopsis. Plant J 6:903–909
Li W, Liu X, Lu Y (2016) Transcriptome comparison reveals key candidate genes in response to vernalization of Oriental lily. BMC Genom 17:664
Li J, Zhang Y, Ding Q, Li H, Liu L, Wang F, Gao J (2017) Transcriptome analysis of orange head Chinese cabbage (Brassica rapa L. ssp. pekinensis) and molecular marker development. Int J Genom 2017:6835810
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM (2003) AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J 33:867–874
Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M (2007) KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res 35:W182–W185
Mun JH, Kwon SJ, Yang TJ, Seol YJ, Jin M, Kim JA, Lim MH, Kim JS, Baek S, Choi BS et al (2009) Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol 10:R111
Mutasa-Gottgens E, Hedden P (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60:1979–1989
Nilsson O, Lee I, Blazquez MA, Weigel D (1998) Flowering-time genes modulate the response to LEAFY activity. Genetics 150:403–410
Oliver SN, Finnegan EJ, Dennis ES, Peacock WJ, Trevaskis B (2009) Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc Natl Acad Sci USA 106:8386–8391
Parcy F (2005) Flowering: a time for integration. Int J Dev Biol 49:585–593
Park JY, Koo DH, Hong CP, Lee SJ, Jeon JW, Lee SH, Yun PY, Park BS, Kim HR, Bang JW et al (2005) Physical mapping and microsynteny of Brassica rapa ssp. pekinensis genome corresponding to a 222 kbp gene-rich region of Arabidopsis chromosome 4 and partially duplicated on chromosome 5. Mol Genet Genomics 274:579–588
Putterill J, Laurie R, Macknight R (2004) It’s time to flower: the genetic control of flowering time. Bioessays 26:363–373
Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES (2000) The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc Natl Acad Sci USA 97:3753–3758
Simpson GG, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296:285–289
Song X, Duan W, Huang Z, Liu G, Wu P, Liu T, Li Y, Hou X (2015) Comprehensive analysis of the flowering genes in Chinese cabbage and examination of evolutionary pattern of CO-like genes in plant kingdom. Sci Rep 5:14631
Song H, Dong X, Yi H, Nou I-S, Hur Y (2016) Differential expression of flowering genes between rapid- and slow-cycling Brassica rapa. Plant Breed Biotechnol 4:145–157
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 68:2013–2037
Sugiyama H, Natsui Y, Hara M, Miyata K, Nefissi R, Mizoguchi T (2014) Late flowering phenotype under ultra-short photoperiod (USP) in Arabidopsis thaliana. Plant Biotechnol 31:29–34
Sun M, Qi X, Hou L, Xu X, Zhu Z, Li M (2015) Gene expression analysis of pak choi in response to vernalization. PLoS One 10:e0141446
Teutonico RA, Osborn TC (1994) Mapping of RFLP and qualitative trait loci in Brassica rapa and comparison to the linkage maps of B. napus, B. oleracea, and Arabidopsis thaliana. Theoret Appl Genet 89:885–894
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F et al (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Wang F, Li L, Li H, Liu L, Zhang Y, Gao J, Wang X (2012) Transcriptome analysis of rosette and folding leaves in Chinese cabbage using high-throughput RNA sequencing. Genomics 99:299–307
Wang A, Hu J, Huang X, Li X, Zhou G, Yan Z (2016) Comparative transcriptome analysis reveals heat-responsive genes in Chinese cabbage (Brassica rapa ssp. chinensis). Front Plant Sci 7:939
Wang J, Qiu Y, Cheng F, Chen X, Zhang X, Wang H, Song J, Duan M, Yang H, Li X (2017) Genome-wide identification, characterization, and evolutionary analysis of flowering genes in radish (Raphanus sativus L.). BMC Genom 18:981
Weber AP, Weber KL, Carr K, Wilkerson C, Ohlrogge JB (2007) Sampling the Arabidopsis transcriptome with massively parallel pyrosequencing. Plant Physiol 144:32–42
Wullschleger SD, Weston DJ (2012) Modeling the molecular and climatic controls on flowering. New Phytol 194:599–601
Yang KA, Lim CJ, Hong JK, Jin ZL, Hong JC, Yun DJ, Chung WS, Lee SY, Cho MJ, Lim CO (2005) Identification of Chinese cabbage genes up-regulated by prolonged cold by using microarray analysis. Plant Sci 168:959–966
Acknowledgements
This work was supported by Agricultural Biotechnology Developmental Program (nos. 116091-3) grants from the Ministry of Agriculture, Food and Rural Affairs and KRIBB Research Initiative Program to HS Cho and Y-S Kim.
Author information
Authors and Affiliations
Contributions
HSC and Y-SK conceived and designed the study and wrote the manuscript. WYJ performed bioinformatic data analysis and wrote the manuscript. AL conducted bolting phenotyping, gene expression analysis and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, W.Y., Lee, A., Moon, J.S. et al. Genome-wide identification of flowering time genes associated with vernalization and the regulatory flowering networks in Chinese cabbage. Plant Biotechnol Rep 12, 347–363 (2018). https://doi.org/10.1007/s11816-018-0500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0500-0