Abstract
Phosphorylation of substrate proteins by mitogen-activated protein kinases (MPKs) determines the specific cellular responses elicited by a particular extracellular stimulus. However, downstream targets of plant MPKs remain poorly characterized. In this study, 29 putative substrates of AtMPK3, AtMPK4 and AtMPK6 were identified by solid-phase phosphorylation screening of a λ phage expression library constructed from combined mRNAs from salt-treated, pathogen-treated and mechanically wounded Arabidopsis seedlings. To test the efficiency of this screening, we performed in vitro kinase assay with 10 recombinant fusion proteins. All proteins were phosphorylated by AtMPK3, AtMPK4 and AtMPK6, indicating the efficiency of this screening procedure. To confirm phosphorylation of isolated substrates by plant MPKs, we performed in-gel kinase assays. All test substrates were strongly phosphorylated by wounding or H2O2-activated AtMPK3 and AtMPK6. Three substrates, encoded by genes At2g41430, At2g41900, and At3g16770, were strongly phosphorylated, suggesting a function as AtMPK substrates. The type of screening provides a powerful way for identifying potential substrates of MAP kinases responsive to biotic and abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mitogen-activated protein kinase (MPK) cascade constitutes an essential intracellular signal transduction module that controls a multitude of cellular responses in most eukaryotes (Ahn 1993; Colcombet and Hirt 2008; Pitzschke et al. 2009). The MPK cascade is composed of three classes of molecules: MPKs, MPK kinases (MEK), and MPK kinase kinases (MEKK). MPK cascades are activated in response to a variety of internal and external stimuli. Signaling through an MPK cascade is initiated when the Ser/Thr kinase MEKK phosphorylates and thereby activates the MEK. MEK is a dual specificity Thr/Tyr kinase. Activated MEK in turn phosphorylates and activates the Ser/Thr kinase MPK (Fiil et al. 2009). Phosphorylation by activated MPKs modulates the site of subcellular localization, protein stability, transcriptional activity, and/or interaction with other proteins in their target range of transcription factors and protein kinases. This ability of MPKs thus contributes to the generation of appropriate cellular responses.
The signaling network of MPK cascades in yeast and mammalian cells are better characterized in comparison with those of plant systems. These studies have established that MPK signaling networks are complex. The complexity of MPK signaling networks can be appreciated by considering the extracellular signal-regulated kinases 1 and 2 (ERK1/2). They represent two mammalian MPKs that transmit many extracellular signals to 160 substrates known so far including transcription factors, protein kinases, protein phosphatases, cytoskeletal elements, and a variety of signaling regulators (Yoon and Seger 2006). The signaling networks of plant MPK cascades are expected to be equally complex, considering, for example, possible combinations of 20 MPKs, 10 MEKs and 60 MEKKs in Arabidopsis. The number of MPK networks in plant is suggested to be much higher than the corresponding numbers in yeast and mammalian systems (Ichimura et al. 2002). The large number of MPKs and their upstream kinases enhances complexity, interconnectedness and functional redundancy in plant signaling networks.
MPKs are mainly involved in biotic and abiotic signaling, and in processes such as hormonal and developmental signaling in Arabidopsis (Colcombet and Hirt 2008; Sinha et al. 2011). Especially, MPK3, MPK4 and MPK6 among the 20 MPKs mainly function in a variety of distinct processes ranging from environmental stress responses to developmental processes (Colcombet and Hirt 2008). For example, MPK6 is involved in not only H2O2, O3, PAMPs, osmotic shock, JA, ET and ABA signaling pathways, but also in developmental processes such as stomatal patterning and embryo development (Asai et al. 2002; Droillard et al. 2002; Ahlfors et al. 2004; Teige et al. 2004; Miles et al. 2005; Bush and Krysan 2007; Takahashi et al. 2007; Wang et al. 2007; Yoo et al. 2008). However, it is not possible to conclude that the three well-known MPKs, namely MPK3, MPK4 and MPK6 are specific, or solely responsible, for involvement in the many different signaling pathways because of a lack of exploratory tools, such as specific antibodies, to precisely analyze other MPKs.
In addition, it is difficult to identify the substrates of MPK simply by homology analysis, because their myriads of substrates are not conserved evolutionarily (Sörensson et al. 2012). Thus, in comparison to yeast and mammalian MPKs, relatively few MPK substrates have been identified in plants. In Arabidopsis, substrates that have been identified and characterized to date include ACS6 (1-aminocyclopropane-1-carboxylic acid synthase), MKS1 (MAP Kinase 4 Substrate 1), EIN3 (Ethylene Insensitive 3), ERF104 (Ethylene-response factor 104), SPCH (SPEECHLESS), ZAT10, AS1 (Asymmetric Leaves 1), the MYB44, NIA2, WRKY1, WRKY33 transcription factors, VIP1(VirE2-Interacting Protein 1), PPS3 (Protein Phosphorylated by StMPK1), and PHOS32 (Liu and Zhang 2004; Andreasson et al. 2005; Katou et al. 2005; Menke et al. 2005; Djamei et al. 2007; Lampard et al. 2008; Merkouropoulos et al. 2008; Yoo et al. 2008; Wang et al. 2010; Mao et al. 2011; Nguyen et al. 2012; Park et al. 2013). In addition, a tobacco protein, MAP65-1 (Microtubule Associated Protein 65-1) has also been identified as an MPK substrate (Sasabe et al. 2006). Although several plant MPK substrates have been identified, the steps that connect each of the Arabidopsis MPKs originating from an environmental stimulus to the resultant cellular responses remain unclear.
High-throughput approaches have recently been applied to analyze the complex MPK signaling networks in Arabidopsis. One study identified 48 and 39 putative substrates of AtMPK3 and AtMPK6, respectively, employing a protein microarray strategy (Feilner et al. 2005). A second study by screening a protein microarray reported identification of about 570 putative substrates for 10 different AtMPKs (Popescu et al. 2009). Transcription factors were the largest group of putative MPK substrates uncovered by these protein microarray analyses. However, a majority of the identified proteins have not been confirmed as real MPK substrates.
To identify novel Arabidopsis MPK substrates, we constructed a cDNA expression library in λ phage using RNA purified from biotic and abiotic stress-treated Arabidopsis seedlings. This expression library was screened using rice Blast- and wound-induced MAP kinase 1 (OsBWMK1) as the probe in a solid-phase phosphorylation screen. OsBWMK1 is an MPK that is rapidly induced in response to fungal infection and mechanical wounding (He et al. 1999; Cheong et al. 2003). OsBWMK1 showed higher kinase activity than activated AtMPK3, AtMPK4 and AtMPK6 in our system. All 29 potential MPK substrates identified by this screen were phosphorylated by AtMPK3, AtMPK4 and AtMPK6 in an in vitro kinase assay. These results provide new insights into the integration of MPK signaling pathways with the post-translational regulation of stress responses in Arabidopsis.
Materials and methods
Construction of λGEX5 Arabidopsis cDNA library
The phage expression vector λGEX5, a λgt11-derived vector was modified by insertion of the pGEX-PUC-3T plasmid nucleotide sequence (Fukunaga and Hunter 1997). For construction of the cDNA library, total RNA was extracted from Arabidopsis seedlings that had been subjected to 200 mM NaCl, wounding and pathogen (Pseudomonas syringae) treatments. Poly(A)+ RNA was obtained using a mRNA separator kit (Clontech Laboratories, Palo Alto, CA, USA). Double-stranded cDNA was synthesized with oligo (dT) primer using a cDNA synthesis kit (Stratagene, La Jolla, CA, USA), and ligated to an adaptor which consisted of 5′-phosphorylated oligonucleotides, 5′-pCCAGCACCTGCA-3′ and 5′-pAGGTGCTGG-3′. cDNAs were then size-fractionated by agarose-gel electrophoresis and cDNAs larger than 400 bp were ligated to the SfiI-digested GEX5 arms and packaged into bacteriophage particles. The cDNA library was amplified by passage through E. coli strain BB4.
Screening of the cDNA library by solid-phase phosphorylation
The Arabidopsis cDNA library was plated on E. coli BB4 at a density of 1.5 × 104 plaques per 150 mm agar plate. The screening of the cDNA library was carried out as previously described (Fukunaga and Hunter 1997). OsBWMK1, a rice blast- and wound-induced MAP kinase, was used for cDNA expression library screening (He et al. 1999). Positive clones were purified by secondary screening and the phage DNA was prepared from plate lysates. The phage DNA was digested with NotI and the cDNA-containing plasmid (pGEX-PUC-3T) was recovered by self-ligation followed by transformation of E. coli strain XL-1 Blue. The transformed bacteria were used for plasmid preparation and isolation of GST-fusion protein.
Analysis of phosphorylated proteins
The protein expression procedure was carried out as described previously (Park et al. 2002). An aliquot (10 μl) of the glutathione-sepharose bound GST-fusion protein was incubated for 30 min at 30 °C with 200 ng of BWMK1 in 20 μl of the kinase buffer [20 mM HEPES–NaOH (pH 7.4), 10 mM MgCl2 and 1 mM DTT] in the presence of [γ-32P]ATP (0.5 μCi). After phosphorylation, the reaction mixture was added to 4× SDS sample buffer and then separated on a 12.5% polyacrylamide gel. Phosphorylated proteins were detected by exposure of the gel to X-ray films for 12–48 h at −70 °C with intensifying screens.
Purification of recombinant proteins
Expression and affinity purification of Glutathione S-transferase (GST) fusion proteins (OsBWMK1, AtMPK3, AtMPK4, AtMPK6) were performed as follows. E. coli BL21(pLysS) cells transformed with the GST-fusion constructs were grown for overnight at 37 °C and sub-cultured until the OD600nm reached 0.5. Expression of the GST-fusion proteins was induced with 0.5 mM IPTG. Cells were incubated at 30 °C for 3 h, harvested and then lysed. Beads of Glutathione-Sepharose 4B were added to the supernatant and mixed by inversion for 30 min at 4 °C. The Sepharose beads were washed three times with 1× PBS. The GST-fusion proteins were eluted from the Sepharose beads with 10 mM reduced glutathione in 50 mM Tris–HCl (pH 8.0).
Plant materials and preparation of protein extracts
Arabidopsis thaliana ecotype Columbia was used. The mpk6 knockout mutant line (SALK_062471) was obtained from the Arabidopsis Biological Resource Center (ABRC). Plants were grown at 22 °C in a growth chamber under 120 μEm−2 s−1 light intensity and 16-h-light/8-h-dark photoperiod. Plants were wounded by nipping twice with a forceps. For oxidative stress, plants were sprayed with 1 mM H2O2. Plant samples were frozen in liquid nitrogen at the appropriate times after each treatment for 5, 10 and 15 min. For protein extraction, the frozen plants were ground in liquid nitrogen, and then thawed in extraction buffer (50 mM HEPES–KOH pH 7.5, 5 mM EDTA, 5 mM EGTA, 2 mM DTT, 25 mM NaF, 1 mM Na3VO4, 50 mM β-glycerophosphate, 20% glycerol, 2 μg ml−1 leupeptin, 2 μg ml−1 pepstatin A, and 2 mM PMSF). After centrifugation at 15,000×g for 30 min at 4 °C, the supernatants were transferred into clean tubes, frozen in liquid nitrogen, and stored at −80 °C for later use.
In-gel kinase assay
The in-gel kinase assay was performed as described previously (Zhang et al. 1993; Liu et al. 2010). 10 μg of protein from total protein extracts of wild type and atmpk6 mutant plants treated by wounding or application of 1 mM H2O2, respectively, was separated on 10% SDS–polyacrylamide gels embedded with 0.2 mg/ml myelin basic protein (MBP) as a substrate. After electrophoresis, gels were washed three times with wash buffer (25 mM Tris–HCl pH 7.5, 0.5 mM DTT, 0.1 mM Na3VO4, 5 mM NaF, 0.5 mg/ml bovine serum albumin, 0.1% TritonX-100). The proteins were then renatured in renaturing buffer (25 mM Tris–HCl pH 7.5, 1 mM DTT, 0.1 mM Na3VO4, 5 mM NaF) for overnight at 4 °C. The gels were incubated in reaction buffer (25 mM Tris–HCl pH 7.5, 2 mM EGTA, 12 mM MgCl2, 1 mM DTT, 0.1 mM Na3VO4) at room temperature for 30 min, and then phosphorylated in 20 ml reaction buffer containing 250 nM ATP and 50 μCi [γ-32P]ATP at room temperature for 1.5 h. The reaction was terminated by transferring the gels into stop solution (5% trichloroacetic acid and 1% sodium pyrophosphate). Gels were washed five times with the stop solution for 5 h at room temperature and then dried on Whatman 3 MM paper and exposed to X-ray film (Fuji Photo Film, Tokyo, Japan) or a BAS2500 imaging plate (Fuji Photo Film, Tokyo, Japan).
In vitro kinase assays
Recombinant GST-tagged MPKs were used to phosphorylate recombinant putative substrate proteins. GST-tagged proteins were purified by using Glutathione-Sepharose 4B columns (GE Healthcare, Piscataway, NJ, USA). Purified MPKs (200 ng) and substrates (500 ng) were incubated in 20 μl of kinase reaction buffer (50 mM Tris–HCl, pH 7.5, 10 mM MgCl2 and 10 μCi of γ-32P-ATP) at 30 °C for 30 min. The reactions were stopped by the addition of SDS-loading buffer. Phosphorylated substrates were visualized by autoradiography after electrophoresis on 12.5% polyacrylamide gels.
Results
Isolation of MPK phosphorylation substrates from an Arabidopsis cDNA expression library
To identify new substrates for AtMPK3, AtMPK4 and AtMPK6, we used a solid-phase phosphorylation screening method (Fukunaga and Hunter 1997). First, we established a λ phage cDNA expression library constructed from combined mRNAs from salt-treated, pathogen-treated and mechanically wounded Arabidopsis seedlings. After the induction and the immobilization of fusion proteins from the expression library, we performed in vitro solid-phase phosphorylation assays using MEK-activated recombinant AtMPK3, AtMPK4 and AtMPK6 as kinases. However, we could not isolate positive clones from such assays, although high amounts of MPKs (up to 10 μg ml−1) were used (data not shown). Thereafter, we used a hyperactive kinase, recombinant OsBWMK1, as the probe, owing to its more than 100-fold higher kinase activity compared to AtMPK3, AtMPK4 and AtMPK6 (data not shown). Furthermore, OsBWMK1 has been shown to exhibit strong kinase activity without activation by an upstream kinase (Cheong et al. 2003). By the solid-phase phosphorylation screening with OsBWMK1 as the kinase, 130 positive clones were isolated from 1 × 105 clones (Fig. 1). Among these 130 clones, we rescued 29 clones containing correct open reading frames in expression plasmids by sequencing. The fusion proteins were expressed in E. coli and purified by Glutathione S-transferase affinity chromatography. The phosphorylation of isolated fusion proteins by OsBWMK1 were verified by in vitro kinase assay (Fig. 1). As a result, 29 clones were isolated as new putative substrates of MAP kinase (Table 1). When we compared these isolates with entries in databases, 18 clones had previously identified as putative kinase substrates in the Plant Protein Phosphorylation DataBase (PPPDB; http://www.p3db.org), although information about which kinases phosphorylates them does not exist. The remaining 11 isolates represented novel MPK substrates (Table 1).
Scheme for screening of MPK substrates from an Arabidopsis cDNA expression library by solid-phase phosphorylation. A λ phage cDNA expression library was constructed from combined mRNAs from salt-treated, pathogen-treated and mechanically wounded Arabidopsis seedlings. The Arabidopsis cDNA library was plated on E. coli BB4 at a density of 1.5 × 104 plaques per 150 mm agar plate. OsBWMK1, a rice blast- and wound-induced MAP kinase, was used for cDNA expression library screening. Recombinant GST-tagged MPKs were used to confirm the phosphorylation of the recombinant putative substrate proteins
Confirmation of the phosphorylation of putative substrates by AtMPKs
To test the ability of putative substrates of OsBWMK1 identified by library screening to become phosphorylated by Arabidopsis MPKs, in vitro kinase assays with AtMPK3, AtMPK4, and AtMPK6 were carried out (Fig. 2a–c). Ten of 29 identified substrates of OsBWMK1 were selected including an RNA-binding protein (At1g51510), a member of the dormancy/auxin-associated family proteins (At2g33830), the EARLY RESPONSIVE TO DEHYDRATION15 (ERD15; At2g41430), AtERF72 (At3g16770), an unknown protein (At4g15545), MLO2 (At1g11310), the calcium-binding EF hand family protein (At1g21630), SIT4 phosphatase-associated family protein (At1g30470), a zinc finger (CCCH-type) family protein (At2g41900) and RD29A (At5g52310). They were expressed in bacteria and purified as GST-fused full length proteins. GST-fused MAP kinase substrate 1 (MKS1), which acts as a substrate of AtMPK4 (Andreasson et al. 2005), was used as positive control. The in vitro kinase assays showed that all tested proteins were phosphorylated by AtMPK3, AtMPK4 and AtMPK6 (Fig. 2). The phosphorylation signal of seven of ten proteins by three AtMPKs was show higher than that observed with MKS1 (lane 2), strongly suggesting they could be authentic substrates of AtMPKs with high affinity.
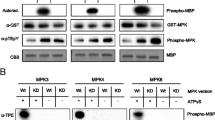
Verification of potential AtMPK substrates. Phosphorylation of potential substrates by purified recombinant MPK3 (a), MPK4 (b) and MPK6 (c). Activated MPK3, MPK4 and MPK6 (500 ng) were used to phosphorylate the substrate proteins (1 µg) in the presence of [γ-32P]ATP. After electrophoresis, proteins were visualized by Coomassie blue (staining), and phosphorylated proteins were visualized by autoradiography (autorad). The positions of putative substrates are indicated by an arrowhead. The positions of the autophosphorylated MPKs are indicated by an asterisk. The purified substrate proteins used in each lane were: GST (lane 1, negative control), MKS1 (lane 2, positive control), At1g11310 (lane 3), At1g21630 (lane 4), At1g30470 (lane 5), At1g51510 (lane 6), At2g33830 (lane 7), At2g41430 (lane 8), At2g41900 (lane 9), At3g16770 (lane 10), At4g15545 (lane 11), and At5g52310 (lane 12). Graphs in the right panel indicate relative intensities of the [32P] phosphoprotein bands in each lane as measured by scanning densitometry of the autoradiogram. Band intensities are expressed relative to that of phosphorylated GST. M, pre-stained protein markers
Phosphorylation of putative substrates by endogenous AtMPKs
Next, we tested whether the putative substrates could be phosphorylated by plant AtMPKs by in-gel kinase assays. AtMPKs in wild type (WT) and MPK6 mutant (mpk6) plants were activated by the treatments of wounding or oxidative stress (H2O2). The activities of AtMPKs were measured by phosphorylation of MBP in a gel, a well-known general MPK substrate (Fig. 3). As a negative control, GST protein was used in assays. As previously reported, the activity of AtMPK6 is rapidly and strongly induced with the treatments of wounding or H2O2 (Ichimura et al. 2000; Kovtun et al. 2000). The activity of AtMPK3 was very weak in WT but significantly increased in mpk6 plants. The reason could be attributed to compensation of AtMPK6 functions in mpk6 plants (Menke et al. 2004). Three of the chosen substrates (At2g41430, At2g41900, and At3g16770) were strongly phosphorylated by AtMPK3 and AtMPK6. However, weak phosphorylation was observed with four substrates (At1g11310, At1g21630, At2g33830, and At4g15545), but this is possibly an effect of incomplete refolding in the reaction solution after denaturation by SDS-PAGE, although repeat experiments were carried out under optimized condition (Fig. 3).
Phosphorylation of putative substrates by endogenous AtMPKs. Proteins were extracted from WT and mpk6 seedlings that had received wounding or H2O2 treatments (1 mM) for the indicated time periods. MPK activities were monitored with an in-gel kinase assay using substrates that were embedded at 1 mg/ml in the running gel. MBP and GST are shown as positive and negative control substrates, respectively. The positions of MPK3, MPK4, and MPK6 on the gel are indicated
In another study, AtMPK3 and AtMPK6 substrates show similarity in their substrates, by phosphorylating the conserved protein motif (L/P-P/X-S-P-R/K) (Sörensson et al. 2012). In addition, we compared the similarity of substrates used to substrates reported from references (Fig. 4). The results indicated that 22 of 29 substrates represented novel substrates suggesting that the method could provide a useful tool for isolating novel substrates of MPKs in plants.
Discussion
The existence of a large family of MPKs with overlapping as well as unique substrate specificities has complicated delineation of MPK signaling pathways in plants. Although a large number of putative Arabidopsis MPK substrates have been identified using high-throughput protein microarray methods, the majority of them have neither been confirmed as AtMPK substrates in planta nor characterized further (Feilner et al. 2005; Popescu et al. 2009). In addition, the MPK3 and MPK6 specifically phosphorylate their substrates on specific serine residues. However, mutations in these serine residues abolish the activities of MPK, which renders them unable to phosphorylate their target substrate (Park et al. 2011).
Our screening method differed from other approaches because it facilitated the necessary tests providing detailed characterization. We used a phage Arabidopsis cDNA expression library constructed in λGEX5 vector to screen for MPK substrates. The advantages offered by the λGEX5 vector for solid-phase phosphorylation screening by the method of Fukunaga and Hunter (1997) are: (1) isolated cDNA clones can be used directly for expression, purification and characterization of the putative substrates identified in the screen, and (2) the GST expression vector can reduce the noise of background phosphorylation. Therefore, the method facilitates identification of physiological substrates for a variety of MPK kinases in plants.
Significantly also, several studies show agreement with our findings (Feilner et al. 2005; Sörensson et al. 2012; Hoehenwarter et al. 2013). By comparing the substrates it was found that three substrates were identical to those found by Hoehenwarter et al. (2013) and Sörensson et al. (2012) and one substrate was similar to one identified by Feilner et al. (2005) (Fig. 4). In the current study, 22 of 29 substrates were identified as novel putative substrates of rice BWMK1 (Table 1).
Among the putative substrates identified in the first round of screening, 10 putative substrates were selected to study phosphorylation activities using AtMPK3, AtMPK4, and AtMPK6 as probes. Seven identified substrates were more strongly phosphorylated than MKS1 by AtMPK3, AtMPK4 and AtMPK6, indicating that they could be highly suitable substrates of AtMPKs (Fig. 2). In addition, an excellent example for the suitability of our screening method is the identification of the microtubule-associated protein 65-7 (MAP65-7) as a substrate in Arabidopsis (Table 1, number 3). Sasabe et al. (2006) reported that phosphorylation of NtMAP65-1, a homolog of AtMAP65-7 in tobacco plants, by an MAP kinase down-regulates its activity of microtubule bundling and stimulates the progression of cytokinesis. The results illustrate the value of the cDNA expression library for identifying putative MPK substrates.
Interestingly, nucleotide diphosphate (NDP) kinase 1 (NDPK1) and NDPK2 were obtained by the solid-phase phosphorylation screening method (Table 1, number 21 and 29). Arabidopsis NDPK2 was expressed by H2O2 treatment and overexpression of AtNDPK2 showed tolerance to multiple stresses such as cold, salt, and oxidative stress (Moon et al. 2003). In addition, AtNDPK2 interacted and activated AtMPK3 and AtMPK6 by phosphorylation (Moon et al. 2003). These data imply that NDPKs and MPKs might function equally or jointly in various plant signaling pathways.
Based on our screening protocol, we have identified several transcription factors as well as transcriptional regulators as potential AtMPKs substrates. Studies in mammalian systems have shown that phosphorylation of transcription factors by MPKs can alter their activities, localizations, and stabilities (Raman et al. 2007; Turjanski et al. 2007). Indeed, several lines of evidence suggest that transcription factors are also major targets of MPKs in Arabidopsis. Firstly, it has been reported that AtMPKs may phosphorylate transcription factors or transcription-related proteins (Asai et al. 2002; Ichimura et al. 2002; Hoang et al. 2012). Secondly, transcription factors involved in development and stress responses were represented as substrates of AtMPK identified by high-throughput protein microarray approaches (Feilner et al. 2005; Popescu et al. 2009). In agreement with these findings, >30% of the putative MPK substrates identified in our screen represented transcription-related proteins, implying MPK-based phosphorylation of many Arabidopsis transcription factors. Finally, further biological and genetic analysis of the phosphorylation of the novel substrates identified in our study (Table 1) will be necessary to extend our understanding of the role of MPK signaling pathways on the integration of plant responses to various external and internal cues in plants.
Abbreviations
- MPK:
-
Mitogen-activated protein kinase
- BWMK:
-
Blast- and wound-induced MAP kinase
- GST:
-
Glutathione S-transferase
- PAMP:
-
Pathogen-associated molecular pattern
- PPPDB:
-
Plant protein phosphorylation database
- MBP:
-
Myelin basic protein
References
Ahlfors R, Macioszek V, Rudd J, Brosche M, Schlichting R, Scheel D, Kangasjarvi J (2004) Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J 40:512–522
Ahn NG (1993) The MAP kinase cascade. Discovery of a new signal transduction pathway. Mol Cell Biochem 127(128):201–209
Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, Qiu JL, Micheelsen P, Rocher A, Petersen M, Newman MA, Bjørn Nielsen H, Hirt H, Somssich I, Mattsson O, Mundy J (2005) The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24:2579–2589
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415:977–983
Bush SM, Krysan PJ (2007) Mutational evidence that the Arabidopsis MAP kinase MPK6 is involved in anther, inflorescence, and embryo development. J Exp Bot 58:2181–2191
Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, Yoon HW, Chung WS, Lim CO, Lee SY, Cho MJ (2003) BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 132:1961–1972
Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signaling network involved in multiple biological processes. Biochem J 413:217–226
Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H (2007) Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science 318:453–456
Droillard M, Boudsocq M, Barbier-Brygoo H, Lauriere C (2002) Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett 527:43–50
Feilner T, Hultschig C, Lee J, Meyer S, Immink RG, Koenig A, Possling A, Seitz H, Beveridge A, Scheel D, Cahill DJ, Lehrach H, Kreutzberger J, Kersten B (2005) High throughput identification of potential Arabidopsis mitogen-activated protein kinases substrates. Mol Cell Proteom 4:1558–1568
Fiil BK, Petersen K, Petersen M, Mundy J (2009) Gene regulation by MAP kinase cascades. Curr Opin Plant Biol 12:615–621
Fukunaga R, Hunter T (1997) MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J 16:1921–1933
He C, Fong SH, Yang D, Wang GL (1999) BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Mol Plant Microbe Interact 12:1064–1073
Hoang MH, Nguyen XC, Lee K, Kwon YS, Pham HT, Park HC, Yun DJ, Lim CO, Chung WS (2012) Phosphorylation by AtMPK6 is required for the biological function of AtMYB41 in Arabidopsis. Biochem Biophys Res Commun 422:181–186
Hoehenwarter W, Thomas M, Nukarinen E, Egelhofer HR, Weckweth W, Conrath U, Beckers MJG (2013) Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol Cell Proteom 12:369–380
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24:655–665
Ichimura K et al (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Katou S, Yoshioka H, Kawakita K, Rowland O, Jones JD, Mori H, Doke N (2005) Involvement of PPS3 phosphorylated by elicitor-responsive mitogen-activated protein kinases in the regulation of plant cell death. Plant Physiol 139:1914–1926
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97:2940–2945
Lampard GR, Macalister CA, Bergmann DC (2008) Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322:1113–1116
Liu Y, Zhang S (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16:3386–3399
Liu XM, Kim KE, Kim KC, Nguyen XC, Han HJ, Jung MS, Kim HS, Kim SH, Park HC, Yun DJ, Chung WS (2010) Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry 71:614–618
Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23:1639–1653
Menke FL, van Pelt JA, Pieterse CM, Klessig DF (2004) Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16:897–907
Menke FL, Kang HG, Chen Z, Park JM, Kumar D, Klessig DF (2005) Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol Plant Microbe Interact 18:1027–1034
Merkouropoulos G, Andreasson E, Hess D, Boller T, Peck SC (2008) An Arabidopsis protein phosphorylated in response to microbial elicitation, AtPHOS32, is a substrate of MAP kinases 3 and 6. J Biol Chem 283:10493–10499
Miles GP, Samuel MA, ZhangY Ellis BE (2005) RNA interference-based (RNAi) suppression of AtMPK6, an Arabidopsis mitogen-activated protein kinase, results in hypersensitivity to ozone and misregulation of AtMPK3. Environ Pollut 138:230–237
Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak SS, Kim DH, Nam J, Bahk J, Hong JC, Lee SY, Cho MJ, Lim CO, Yun DJ (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 100:358–363
Nguyen XC, Hoang MH, Kim HS, Lee K, Liu XM, Kim SH, Bahk S, Park HC, Chung WS (2012) Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germination. Biochem Biophys Res Commun 423:703–708
Park HC, Kang YH, Chun HJ, Koo JC, Cheong YH, Kim CY, Kim MC, Chung WS, Kim JC, Yoo JH, Koo YD, Koo SC, Lim CO, Lee SY, Cho MJ (2002) Characterization of a stamen-specific cDNA encoding a novel plant defensin in Chinese cabbage. Plant Mol Biol 50:59–69
Park HC, Song EH, Nguyen XC, Lee K, Kim KE, Kim HS, Lee SM, Kim SH, Bae DW, Yun DJ, Chung WS (2011) Arabidopsis MAP kinase phosphatase-1 is phosphorylated and activated by its substrate AtMPK6. Plant Cell Rep 30:1523–1531
Park HC, Han HJ, Lee SM, Yun DJ, Chung WS (2013) ASYMMETRIC LEAVES1 is phosphorylated by MPK3/6 in Arabidopsis thaliana. J Plant Biol 56:208–215
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12:421–426
Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, Dinesh-Kumar SP (2009) MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev 23:80–92
Raman M, Chen W, Cobb MH (2007) Differential regulation and properties of MAPKs. Oncogene 26:3100–3112
Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y (2006) Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev 20:1004–1014
Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated rotein kinase signaling in plants under abiotic stress. Plant Signal Behav 6:196–203
Sörensson C, Lenman M, Veide-vilg J, Schopper S, Ljungdahl T, Grøtli M, Tamás MJ, Peck SC, Andreasson E (2012) Determination of primary sequence specificity of Arabidopsis MAPKs MPK3 and MPK6 leads to identification of new substrates. Biochem J 446:271–278
Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19:805–818
Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15:141–152
Turjanski AG, Vaqué JP, Gutkind JS (2007) MAP kinases and the control of nuclear events. Oncogene 26:3240–3253
Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19:63–73
Wang P, Du Y, Li Y, Ren D, Song CP (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J (2008) Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signaling. Nature 451:789–795
Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44
Zhang S, Jin CD, Roux SJ (1993) Casein kinase II-type protein kinase from pea cytoplasm and its inactivation by alkaline phosphatase in vitro. Plant Physiol 103:955–962
Acknowledgements
We thank Dr. Tony Hunter, Salk Institute, for the λGEX5 vector and the Arabidopsis Biological Resource Center (ABRC, Ohio State University) for providing atmpk6 mutant plants. This work was supported by grants from the Next-Generation BioGreen 21 Program (#PJ011091) funded by the Rural Development Administration, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2016R1A2B4015859), and partly by Vietnam National Foundation (Grant No. 106-NN.02-2013.30) for Science and Technology Development (NAFOSTED).
Author information
Authors and Affiliations
Corresponding authors
Additional information
H. C. Park and X. C. Nguyen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, H.C., Nguyen, X.C., Bahk, S. et al. Novel MAP kinase substrates identified by solid-phase phosphorylation screening in Arabidopsis thaliana . Plant Biotechnol Rep 10, 415–423 (2016). https://doi.org/10.1007/s11816-016-0412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-016-0412-9