Abstract
Here, we report characterization of an RbcS promoter and its transit peptide for use in chloroplast-targeted expression. RbcS3 that encodes a small subunit of ribulose bisphosphate carboxylase/oxygenase is of great interest due to its green tissue-specific expression such as leaf and leaf sheaths. We isolated the RbcS3 promoter and its transit peptide sequence and linked to GFP for characterization in transgenic plants. Our qRT-PCR analysis on leaves and roots of transgenic plants revealed that GFP transcript levels were higher by fivefold in leaves of RbcS3:TP3:GFP transgenic lines than in those of the RbcS3:GFP transgenic lines, suggesting that the transcript levels were increased by chloroplast-targeted expression, as shown in previously characterized RbcS1:TP1 system. The transit peptide sequence TP3 was sufficient to target GFP to chloroplasts as observed in protoplasts of RbcS3:TP3:GFP transgenic lines. GFP fluorescence in RbcS3:GFP plants was detected only in the cytosol. The GFP protein levels in leaves of RbcS3:TP3:GFP plants were 0.12–0.23 % of the total soluble leaf proteins. These levels were higher or comparable to those of PGD1:GFP plants (GFP driven by a constitutive promoter PGD1). Taken together, these results indicate that the fusion of the RbcS3 promoter to its transit peptide, RbcS3:TP3, provides a chloroplast-targeting expression system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is the most plentiful protein in the leaves of light-grown plants and catalyzes the rate-limiting stage of CO2 fixation in photosynthesis. Rubisco is composed of two subunits, large subunit (RbcL) and small subunit (RbcS). The 55-kDa in size RbcL is encoded in chloroplast genome, whereas the 15-kDa in size RbcS is encoded in the nucleus and targeted to chloroplasts by their transit peptide (TP). As shown in Kyozuka et al. (1993), RbcS1 gene originated from Oryza sativa L. cv IR26 was characterized the properties that are light-regulated and mesophyll-specific expressed. Previous report showed high GFP mRNA and protein levels reaching up to 10 % of the total soluble protein. The correct cleavage site of TP:GFP is between the cysteine (residue 47) and methionine (residue 48) during transport to chloroplast of the reporter gene in transgenic plants (Jang et al. 1999). The high level of GFP protein accumulation, primarily reflected in the increase of the mRNA levels, was due to the presence of the functional RbcS1 transit peptide (Jang et al. 2002). Furthermore, the paralogs of RbcS genes have highly conserved coding regions although the levels and patterns of expression can vary.
The RBCS3C gene expression pattern and promoter sequences have been well studied among the five members of a tomato RbcS gene family (RBCS1, 2, 3A, 3B, 3C) (Sugita and Gruissem 1987; Sugita et al. 1987; Manzara et al. 1991; Wanner and Gruissem 1991; Carrasco et al. 1993; Manzara et al. 1993; Meier et al. 1995). The other individual gene of this family has various expression patterns of during temporal treatment and in specific organs. In common, all the five members are expressed high levels in cotyledons and leaves grown on light conditions, but the expression is not detected in roots. The tomato RBCS gene expression is controlled by light similar to other photosynthetic genes. After moving the plant to immediate dark conditions, the transcript levels of RBCS2 and RBCS3A are decreased, while the levels of RBCS1, RBCS3B and RBCS3C come to be undetectable. Soon after placing the plant under light, levels of mRNA rapidly turn back to those of normal conditions. The soybean SRS1 and SRS4 out of six genes of RbcS were expressed topmost and characterized in detail (Berry-Lowe et al. 1982; Shirley et al. 1990). These two genes are highly expressed in leaves grown on light conditions. It means that expression of the two genes is controlled by light. As same as the tomato RbcS genes, after transferring plants to dark conditions, the mRNA levels of both genes are rapidly decreased and turn back to normal levels by restoring to light conditions.
In this study, we identified four different rice small subunit of Rubisco genes by analyzing protein alignment and phylogenic tree. Among them, the RbcS3 promoter and its transit peptide were chosen to drive transgene expression in rice. Several transgenic lines were produced and GFP expression levels were analyzed at the transcript and protein levels. The RbcS3 promoter and its transit peptide were found to be as efficient as RbcS1 for alternative chloroplast-targeted expression.
Materials and methods
Multiple sequence alignments and phylogenetic analysis
Phylogenetic analysis of amino acid sequences from four rice rubisco small subunit (RbcS) genes was carried out using CLUSTALW program which is a multiple sequence alignment tool (Larkin et al. 2007).
Plant materials
Genomic DNA (gDNA) isolation manner from cv. Nipponbare (Oryza sativa L. var. Japonica) was followed by Park et al. (2013b). Through the rice transformation in cv. Nakdong and propagation, ten independent lines from the T3 generations of transgenic plants transformed-Promoter:GFP constructs were selected for subsequent detailed analysis.
Semi-qRT-PCR analysis of endogenous gene expression
To investigate expression of endogenous gene, plants grown in a greenhouse were sampled on the subsequent time and growth stages that are 30 and 60 days after germination (DAG), before heading stage (BH) and dry seed. Total RNAs from the ground powders were then isolated using the GeneAll RiboEx™ LS Kit (GeneAll Biotech, Korea). First-strand cDNA from the isolated total RNAs was synthesized using cDNA synthesis kits (Fermentas, Burlington, Canada). Using the gene-specific primers as shown in Table S2, the RT-PCR was performed with 50 ng of cDNA template. The cycle carried out RT-PCR is as follows as previously reported (Park et al. 2013b); 98 °C for 10 min, followed by 29 cycles at 98 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, with one cycle at 10 °C for 10 min. To verify RNA loading, the OsCc1 (AF399666) and OsUbi1 (AK121590) genes were used.
Constructs and transgenic rice production
The promoter RbcS3:TP and RbcS3 fragments were isolated using the promoter-isolated primers as shown in Table S2 and PrimeSTAR DNA polymerase (Takara Bio Inc., Japan). The PCR cycle was set as previously described by Park et al. (2010). The primer pairs are listed in supplementary Table S2. The isolated promoter was recombined into the rice transformation vector as previously described by Park et al. (2010). The rice transformation vector consisted of the CaMV 35S promoter link to the bar gene for use with conventional herbicide-based plant selection. Through tri-parental mating assay, the vectors were integrated into Agrobacterium tumefaciens LBA4404 strain. Calli proliferated from the seeds of rice (cv. Nakdong) were co-cultivated with those Agrobacterium (Hiei et al. 1994), grown the regenerating medium containing with 4 mg/L phosphinothricin until producing transgenics as previously described by Park et al. (2013a).
qRT-PCR analysis
Total RNA was purified from leaves and roots of 30 DAG transgenic and non-transgenic (NT) rice plants using the GeneAll RiboEx™ LS Kit (GeneAll Biotech, Korea) and followed by First-strand cDNA synthesis kit (Fermentas, Burlington, Canada). The real-time qRT-PCR was performed with 50 ng of cDNA template, using the GFP-specific primers listed in Table S2 and a 2× Real-time PCR Pre-mixture with Evagreen (SolGent, Seoul, Korea). The thermocycling was as follows: at 95 °C for 10 min followed by 40 cycles of 95 °C for 20 s, 58 °C for 20 s, 72 °C for 20 s in a 20 μl volume. The fluorescence from the amplicons was detected by a Stratagene Mx3000p Real-Time PCR machine and Mx3005P software v2.02 (Stratagene, La Jolla, CA, USA) as previously described (Jang et al. 2014). Gene-specific primer pairs were designed by testing melting curve analysis as reported in Park et al. (2010). All the qRT-PCR results were calculated from triplicate reactions per sample. The comparative experiments were performed and the results were analyzed using Mx3005P software v2.02 (Stratagene, La Jolla, CA, USA). A reference gene, OsUbi1 (AK121590), was used to normalize the individual cDNA template.
Protoplast isolation and transient transformation
Leaf sheaths from 10-day-old seedlings were cut into 1-mm disks using a sharp razor blade. The pieces were immediately transferred into a 15-ml digestion solution composed of 0.6 M mannitol, 1.5 % cellulase R-10 (Yakult, Japan), 0.75 % macerozyme R-10 (Yakult, Japan), 1 mM CaC12, 0.1 % bovine serum albumin, 5 mM beta-mercaptoethanol and 0.6 mM 4-morpholineethanesulfonic acid (MES) pH 5.7, and incubated for 4–5 h at 28 °C with gentle shaking in the dark conditions. Digested samples were then filtered twice first using a 70-μm pore sixe nylon mesh (BD Bioscience, USA) and second with 40-μm pore to collect the protoplasts. The collected protoplast were then re-suspended in 20 ml of washing buffer (W5) composed of 154 mM NaCl, 125 mM CaC12, 5 mM KC1, 5 mM d-glucose and 2 mM MES, pH 5.7. The protoplast suspensions were centrifuged at 320×g for 8 min to pellet the protoplasts, which were then re-suspended smoothly in 1 ml MMg solution with consisting of 0.6 M mannitol, 15 mM MgCl2and 4 mM MES, pH 5.7. The protoplasts were counted and quantified using a hemocytometer under a microscope (CX21; Olympus, Tokyo, Japan). The constructs of 35S:TP3:GFP, 35S:TP1:GFP and 35S:GFP were then transfected with the isolated protoplasts using polyethylene glycol-mediated transformation. After 16-h incubation at 28 °C, the transiently transformed protoplasts were gathered by carrying out centrifugation at 300×g for 5 min.
Observation of GFP fluorescence
The GFP fluorescence of rice leaf protoplasts isolated from the transgenic plant or transformed temporally was visualized and taken a picture using a confocal laser scanning microscope (Leica TCS SP8 STED, Wetzlar, Germany). GFP fluorescence of dry seeds was detected under a stereomicroscope (SZX9-3122, Olympus, Tokyo, Japan) with GFP filter set as described by Park et al. (2010) and digital camera (C5060-ZOOM, Olympus).
Immunoblotting analysis
Total soluble proteins of 2-week-old leaves were extracted using the extraction buffer containing 50 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM dithiothreitol and 1 %Triton X-100 and 100 μM phenylmethylsulphonylfluoride (PMSF). After measuring protein concentrations using the Bradford manner (Bio-Rad, Hercules, CA, USA), protein bands were separated on a 12 % SDS–polyacrylamide gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Millipore Co., Billerica, MA, USA) using a wet transfer tool (Hoefer, San Francisco, CA, USA). The immunoreactive proteins were detected using anti-GFP anti-serum from mouse (Santa cruz biotechnology Inc., CA, USA). The chemi-luminescence signals were detected using ECL kit (GE Healthcare, Buckinghamshire, UK) and visualized a FusionSL (Vilber Lourmat, France) imaging system. Recombinant GFP proteins were used as a positive control (Abcam, Cambridge, UK).
Results
To develop a chloroplast expression system in rice, phylogenic analysis of the four small subunit of Rubisco (RbcS) genes was performed as shown in Fig. 1. Comparison of the nucleotide sequences revealed differences between genes (Fig. S1). All four genes encode similar amino acid sequences with some differences found only in the transit peptide region (Fig. 1b). The predominant expressions of the four RbcS genes in green tissues such as leaf blade, leaf sheath and stem were found by the spatio-temporal transcript profiling (Fig. S2). Expression of the RbcS1 and RbcS2 genes was also detected in flower organs and endosperms whereas that of RbcS3 and RbcS4 genes was not. We have therefore chosen RbcS3 for us to develop a green tissue-specific chloroplast-targeting system in transgenic plants. The endogenous expression of RbcS3 gene was confirmed by semi-qRT-PCR in six different tissues of rice. These were leaf and root tissues at 30 and 60 days after germination (DAG), panicles before pollination and dry seeds (Fig. 2a). The RbcS3 gene was highly expressed only in green tissues, leaves and green panicles at the heading stage which was similar to RbcS1 gene expression.
Homologous genes of small subunits of Rubisco (RbcS) in the Oryza sativa L. a The numbers in front and back of black arrow boxes indicate genomic locations of the four different RbcS genes found in rice chromosome 12. The distance is presented by base-pair numbers between two genes. b Protein alignment of the four rice RbcSs. Amino acid alignment was performed with CLUSTALW (Larkin et al. 2007). The N-terminal 47 amino acids filled with green box are transit peptides. c Phylogenic analysis of the four RbcS proteins. Phylogenetic analysis of the amino acid sequences was performed by rooted phylogenetic tree programs
Endogenous expression patterns and structure of rice RbcS3. a Green tissue-specific expression of RbcS3. The endogenous gene expressions were analyzed by semi-qRT-PCR in six different tissues of rice growth. Leaves (L) and roots (R) of 30 and 60 days after germination (DAG) were represented the vegetative stage of growth. Panicles before pollination (less than 10 cm in size) and dry seeds were included. Control genes for equal amount of cDNA loading are OsCc1 (AF399666) and OsUbi1 (AK121590). The gene RbcS1 (Os12g0274700) was used as a positive control for green tissue-specific expression. b Genomic structure of RbcS3 gene. Gray filled boxes represent exons; line between boxes are intron; black bold line is the 5′ upstream region including promoter region; white box is TP3 (transit peptide of RbcS3). The +1 and ATG are referred to as the transcriptional start site and translational start codon, respectively. c Constructs used for rice transformation. RbcS3 promoter (P RbcS3) with and/or without TP3 were linked to the reporter gene green fluorescent protein (GFP) and potato proteinase inhibitor II terminator (T PinII). The phosphinothricin acetyl transferase gene (BAR) as a selection marker gene for rice transformation was connected between the CaMV 35S promoter (P 35S) and the nopaline synthase terminator (T nos)
To investigate RbcS3 promoter and transit peptide (TP3) activities, the promoter region with TP3 (1971 base pairs) and without TP3 (1802 base pairs) was isolated from the genomic DNA of rice (Oryza sativa cv. Nipponbare) by PCR. The isolated PCR fragments were linked to the green fluorescent protein gene GFP and inserted into a rice transformation vector (Fig. 2b,c). Thirty independent transgenic lines were produced through Agrobacterium-mediated rice transformation (Hiei et al. 1994). Copy number analysis of transgenes in transgenic plants was determined through TaqMan qPCR using probes specific for the BAR sequence. Ten T3 homozygous single-copy lines were then selected for further analysis.
To measure promoter activity and tissue specificity, ten independent transgenic lines from RbcS3:TP3:GFP and RbcS3:GFP and three from PGD1:GFP were grown in normal greenhouse conditions for 30 days. Total RNAs were isolated from leaf and root tissues at 30 DAG (Fig. 3a). Levels of GFP mRNAs in leaves RbcS3:TP3:GFP and RbcS3:GFP transgenic lines were determined by qRT-PCR and compared with those of PGD1:GFP transgenic lines. The GFP transcript levels of RbcS3:TP3:GFP transgenic lines were significantly higher than RbcS3:GFP transgenic lines. The TP3 sequence increased the transcript levels more than fivefold (Table S1). The GFP transcripts were not detected in roots of RbcS3:TP3:GFP transgenic lines but the levels in leaves were comparable with those of PGD1:GFP transgenic leaves (Fig. 3). These results suggest that the RbcS3 promoter is highly active in leaf tissues with its own functional transit peptide.
Quantitative comparison of promoter activity and tissue specificity in ten independent lines of RbcS3:TP3:GFP and RbcS3:GFP transgenic plants. Total RNAs were isolated from leaf and root tissues of 30-day-old independent T3 lines of transgenic plants RbcS3:TP3:GFP and RbcS3:GFP. Three lines of PGD1:GFP transgenic plants in the same generation were used as a positive control. a Levels of GFP mRNAs were calculated by analyzing qRT-PCR, normalized with an internal control gene OsUbi1. The fold values were drew a picture relative to the level of GFP mRNAs in the lowest expression transgenic lines (marked with an asterisk). b Range of variation in the promoter activity in different transgenic lines. Red bars were indicated median values of each transgenic construct. Data bars represent the mean ± SD of triplicate measurements
To determine chloroplast localization of transgene product in RbcS3:TP3:GFP transgenic plants, GFP proteins in leaf protoplasts were examined under confocal laser scanning microscope. Leaf protoplasts isolated from transgenic plants were used for subcellular localization (Fig. 4a). Red autofluorescence from chlorophylls was detected in normal leaf chloroplasts. The GFP fluorescence of the RbcS3:TP3:GFP transgenic protoplasts overlapped with the red fluorescence of chloroplasts while the fluorescence of RbcS3:GFP was detected only in the cytosol. PGD1 and maize Ubi1 promoters were used as controls for cytosolic localization (Fig. 4a). To make a comparison of subcellular targeting efficiency between two transit peptides TP3 and TP1, transient expression assays were performed using wild-type protoplasts. Protoplasts were temporally transformed with three different constructs consisting of TP3:GFP, TP1:GFP or simple GFP driven by the CaMV 35S promoter (Fig. 4b). The GFP fluorescence of 35S:GFP-transformed protoplasts is specifically localized in cytosol, whereas the GFP fluorescence of constructs carrying the TP3 and TP1 is specifically localized in chloroplasts. Moreover, the chloroplasts targeting efficiency of TP3 and TP1 appears to be comparable, as judged by the level of GFP fluorescence in 35S:TP3:GFP- and 35S:TP1:GFP-transformed protoplasts (Fig. 4b).
Subcellular targeting patterns in rice leaf protoplasts. a Transgenic plants harboring RbcS3:TP3:GFP and RbcS3:GFP were analyzed in comparison with the control transgenic plants PGD1:GFP and ZmUbi1:GFP (Park et al. 2010). b Chloroplast localization of GFP protein linked to transit peptide of RbcS3 and RbcS1 were conducted by expressing transiently in wild-type protoplast isolated from 10-day-old rice green seedlings. 35S:GFP was used as control of cytosolic localization. Protoplasts were visualized under confocal laser microscope (Leica). GFP gfp channel, Red red channel, Merged merged channel of Red and GFP image. Scale bar 10 µm. c Constructs for the transient expression assays. CaMV 35S promoter (P 35S) with transit peptide of RbcS3 (TP3) or RbcS1 (TP1) and without transit peptide linked to the GFP reporter gene and the CaMV 35S terminator (T 35S). The three constructs were kindly provided by Professor Sun Hwa Ha of Kyung Hee University
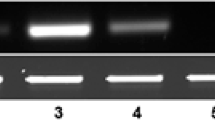
To determine the GFP protein levels in the transgenic plants, western blot analysis was performed as shown in Fig. 5. The GFP protein levels in leaves of RbcS3:TP3:GFP plants were 0.12–0.23 % of the total soluble leaf proteins. These levels were higher or comparable to those of PGD1:GFP plants (GFP driven by the constitutive promoter PGD1). Taken together, these results indicate that the fusion of the RbcS3 promoter to its transit peptide, RbcS3:TP3, provides a chloroplast-targeting expression system.
Levels of GFP protein detected through western blot. Total soluble proteins were extracted in the 2-week-old leaf tissues of RbcS3:TP3:GFP transgenic rice plants. Non-transgenic (NT) and PGD1:GFP (GFP driven by the constitutive promoter PGD1) transgenic plants were used as a negative and a positive control, respectively. Numbers with sharp (hash) indicate transgenic lines. Ten microgram of total soluble proteins was loaded on a 12 % SDS–polyacrylamide gel and immunoblotted with anti-GFP antibodies. Levels of GFP proteins were compared with those of the recombinant GFP (rGFP)
Discussion
In current study, we analyzed four different rice RbcS genes that encode a small subunit of ribulose bisphosphate carboxylase/oxygenase. They share the same levels of similarity in amino acid sequence with diverged transit peptides. We selected the RbcS3 promoter and its transit peptide for characterization to establish a chloroplast-targeted expression system. The RbcS3 gene is expressed only green tissues such as leaves and leaf sheaths. By promoter analysis and chloroplast localization of GFP proteins in protoplasts of RbcS3:TP3 transgenic plants, RbcS3 promoter and its transit peptide (TP3) sequences were found to direct chloroplast-targeted expression. The RbcS3 promoter was highly active in leaf tissues especially in the presence of its functional transit peptide sequence TP3. These results are consistent with results of RbcS1 and TP1 where TP1 enhanced the levels of transcripts significantly (Jang et al. 2002). Furthermore, transient expressions of constructs, 35S:TP3:GFP and 35S:TP1:GFP in rice leaf protoplasts showed that enhanced expression levels are similar between TP3 and TP1.
Chloroplast-targeted expression system is useful in the field of plant biotechnology, for example, insect-resistant crops. Insecticidal protein originated from Bacillus thuringiensis (Bt) is introduced into various crops for pest management (Barton et al. 1987; Vaeck et al. 1987; Fischhoff et al. 1987). Although constitutive promoters were often used to drive Bt gene expression in insect-resistant transgenic rice (Wu et al. 1997; Cheng et al. 1998; Alam et al. 1999; Tu et al. 2000; Chen et al. 2005; Tang et al. 2006), chloroplast-targeted expression of the gene seems more attractive for two reasons. Obviously, enhanced levels of the expression are the one that plant biotechnologists are seeking for. In fact, chloroplast-targeted expression of synthetic cry1Ac in transgenic rice increased the levels of plant resistance to rice pests tremendously. This was accomplished using RbcS1 and TP1 (Kim et al. 2009). Secondly, green tissue-specific expression system is desired not only because of the potential side effects derived from constant expression of the endotoxin (Kuiper et al. 2001; Shelton et al. 2002; Conner et al. 2003), but also because this approach helps solve the concerns about food safety of GM crops where there should be no or rare detection of the insecticidal protein at the edible parts of crops such as embryo and endosperm of grains (Kuiper et al. 2001; Shelton et al. 2002; Conner et al. 2003). In our study, GFP fluorescence was not detected in the seeds of RbcS3:TP3:GFP and RbcS1:TP1:GFP transgenic plants (Fig. 6). Thus, the RbcS3:TP3 provides an effective alternative for chloroplast-targeted expression system in crop biotechnology.
Observation of GFP fluorescence in transgenic grains. Two transgenic lines harboring RbcS3:TP3:GFP and RbcS3:GFP were analyzed in comparison with the control transgenic lines. The RbcS1:TP1:GFP and RbcS1:GFP (Jang et al. 2002) are positive controls for RbcS3:TP3:GFP and RbcS3:GFP, respectively. PGD1 (Park et al. 2010) and ZmUbi1 (Cornejo et al. 1993) are known constitutive gene promoters. Light and GFP images were captured using stereomicroscope (Oylmpus). Scale bar 2 mm
References
Alam MF, Datta K, Abrigo E, Oliva N, Tu J, Virmani SS, Datta SK (1999) Transgenic insect resistant maintainer line (IR68899B) for improvement of hybrid rice. Plant Cell Rep 18:572–575
Barton KA, Whiteley HR, Yang NS (1987) Bacillusthuringiensis δ-endotoxin expressed in transgenic Nicotiana tabacum provides resistance to lepidopteran insects. Plant Physiol 85:1103–1109
Berry-Lowe SL, McKnight TD, Shah DM, Meagher RB (1982) The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet 1:483–498
Carrasco P, Manzara T, Gruissem W (1993) Developmental and organ-specific changes in DNA–protein interactions in the tomato rbcS3B and rbcS3C promoter regions. Plant Mol Biol 21:1–15
Chen H, TangW XuCG, Li XH, Lin YJ, Zhang QF (2005) Transgenic indica rice plants harboring a synthetic cry2A∗ gene of Bacillus thuringiensis exhibit enhanced resistance against rice lepidopteran pests. Theor Appl Genet 111:1330–1337
Cheng X, Sardana R, Kaplan H, Altosaar I (1998) Agrobacterium tumefaciens-transformed rice plants expressing synthetic cryIAb and cryIAc genes are highly toxic to striped stem borer and yellow stemborer. Proc Natl Acad Sci USA 95:2767–2772
Conner AJ, Glare TR, Nap JP (2003) The release of genetically modified crops into the environment. Part II. Overview of ecological risk assessment. Plant J 33:19–46
Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581
Fischhoff DA, Bowdish KS, Perlak FJ, Marrone PG, McCoormick SM, Niedermeyer JG, Dean DA, Kusano-Kretzmer K, Mayer EJ, Rochester DE, Rogers SG, Fraley RT (1987) Insect tolerant transgenic tomato plants. Biotechnology 5:807–813
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Jang IC, Nahm BH, Kim JK (1999) Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed 5:453–461
Jang IC, Lee KH, Nahm BH, Kim JK (2002) Chloroplast targeting signal of a rice rbcS gene enhances transgene expression. Mol Breeding 9:81–91
Jang G, Shim JS, Jung C, Song JT, Lee HY, Chung PJ, Kim J-K, Choi YD (2014) Volatile methyl jasmonate is a transmissible form of jasmonate and its biosynthesis is involved in systemic jasmonate response in wounding. Plant Biotechnol Rep 8:409–419
Kim EH, Suh SC, Park BS, Shin KS, Kweon SJ, Han EJ, Park S-H, Kim YS, Kim J-K (2009) Chloroplast-targeted expression of synthetic cry1Ac in transgenic rice as an alternative strategy for increased pest protection. Planta 230:397–405
Kuiper HA, Kleter GA, Noteborn HP, Kok EJ (2001) Assessment of the food safety issues related to genetically modified foods. Plant J 27:503–528
Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K (1993) Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion gene in transgenic rice. Plant Physiol 102:991–1000
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Manzara T, Carrasco P, Gruissem W (1991) Developmental and organ-specific changes in promoter DNA–protein interactions in the tomato rbcS gene family. Plant Cell 3:1305–1316
Manzara T, Carrasco P, Gruissem W (1993) Developmental and organ-specific changes in DNA–protein interactions in the tomato rbcS1, rbcS2 and rbcS3A promoter regions. Plant Mol Biol 21:69–88
Meier I, Callan KL, Fleming AJ, Gruissem W (1995) Organ-specific differential regulation of a promoter subfamily for the ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit genes in tomato. Plant Physiol 107:1105–1118
Park SH, Yi N, Kim YS, Jeong MH, Bang SW, Choi YD, Kim JK (2010) Analysis of five novel putative constitutive gene promoters in transgenic rice plants. J Exp Bot 61:2459–2467
Park SH, Jeong JS, Redilas MCF, Jung H, Bang SW, Kim YS, Kim JK (2013a) Transgenic overexpression of UIP1, an interacter of the 3′ untranslated region of the Rubisco small subunit mRNA, increases rice tolerance to drought. Plant Biotechnol Rep 7:83–90
Park SH, Jeong JS, Redilas MCF, Jung H, Bang SW, Kim YS, Kim JK (2013b) Characterization of the root-predominant gene promoter HPX1 in transgenic rice plants. Plant Biotechnol Rep 7:339–344
Shelton AM, Zhao JZ, Zhao RT (2002) Economic, ecological, food safety and social consequences of the development of Bt transgenic plants. Annu Rev Entomol 47:845–881
Shirley BW, Ham DP, Senecoff JF, Berry-Lowe SL, Zuruh LL, Shah DM, Meagher RB (1990) Comparison of the expression of two highly homologous members of the soybean ribulose-1,5-bisphosphate carboxylase small subunit gene family. Plant Mol Biol 14:909–925
Sugita M, Gruissem W (1987) Developmental, organ-specific, and light-dependent expression of the tomato ribulose-1,5-bisphosphate carboxylase small subunit gene family. Proc Natl Acad Sci USA 84:7104–7108
Sugita M, Manzara T, Pichersky E, Cashmore A, Gruissem W (1987) Genomic organization, sequence analysis and expression of all five genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from tomato. Mol GenGenet 209:247–256
Tang W, Chen H, Xu CG, Li XH, Lin YJ, Zhang QF (2006) Development of insect-resistant transgenic indica rice with a synthetic Cry1C∗ gene. Mol Breed 18:1–10
Tu J, Zhang G, Datta K, Xu C, He Y, Zhang Q, Khush GS, Datta SK (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nat Biotechnol 18:1101–1104
Vaeck M, Reynaerts A, Hofte H, Jansens S, Beukeleer MD, Dean C, Zabeau M, Montagu MV, Leemans J (1987) Transgenic plants protected from insect attack. Nature 328:33–37
Wanner LA, Gruissem W (1991) Expression dynamics of the tomato rbcS gene family during development. Plant Cell 3:1289–1303
Wu C, Fan Y, Zhang C, Oliva N, Datta SK (1997) Transgenic fertile japonica rice plants expressing a modified cry1A(b) gene resistant to yellow stemborer. Plant Cell Rep 17:129–132
Acknowledgments
This research was supported by the Rural Development Administration under the Next-Generation BioGreen 21 Program (Project No. PJ011135 to J.-K.K.). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (Project No. R1A2A1A11051690 to J.-K.K., Project No. R1A1A3012706 to S.-H.P. and Project No. R1A1A1012877 to J.S.J).
Author information
Authors and Affiliations
Corresponding author
Additional information
S.-H. Park and J. S. Jeong contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, SH., Jeong, J.S., Do Choi, Y. et al. Characterization of the rice RbcS3 promoter and its transit peptide for use in chloroplast-targeted expression. Plant Biotechnol Rep 9, 395–403 (2015). https://doi.org/10.1007/s11816-015-0376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-015-0376-1