Abstract
A protocol of plant regeneration from shoot tips and optimization of Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var. italica) cv. Green Marvel have been developed. Shoot tip response was assessed on Murashige and Skoog (MS) medium supplemented with different concentrations of zeatin. The highest regeneration with a maximum of 13 shoots per explant was obtained on MS medium containing 1.5 mg l−1 zeatin. Primary selection of putative transformed explants was performed on the optimized regeneration medium (MS medium containing 1.5 mg l−1 zeatin and 80 mg l−1 kanamycin) for 60 days. The effects of pre-culture, acetosyringone and growth of bacterial culture were studied. Explants precultured on callus induction medium for 4 days prior to inoculation with A. tumefaciens with 200 μM acetosyringone resulted in improved transformation frequency. The Agrobacterium culture dilution of 1:5 and inoculation time of 30 min increased the efficiency of transformation of shoot tip explants. The results also indicated that 150 mg l−1 ampicillin alone was adequate to eradicate Agrobacterium growth in the SRM incorporated with the respective minimum inhibitory concentration of 80 mg l−1 kanamycin. The polymerase chain reaction (PCR) and Southern blot assays confirmed the transgenic status of the broccoli cv. Green Marvel regenerants. A transformation efficiency of 5 % was achieved based on the positive PCR results using the optimized procedure. The expression of luciferase reporter gene in the transformed cells and the transcription of AtHSP101 using RT-PCR further confirmed the transgenic status of the regenerated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broccoli (Brassica oleracea var. italica) is one of the most nutritious vegetable crops rich in calcium, antioxidants, vitamin A, vitamin K, β-carotene, riboflavin, and iron (Suri et al. 2005). Genetic improvement programs could be a reliable approach to tackle the cultivation problems of the broccoli plants. Genetic transformation is effective and less time-consuming compared to other methods of genetic improvement including conventional breeding (Cardoza and Stewart 2003; Hong et al. 2009). The presence of an efficient transformation protocol is the basic needs in genetic improvement of a crop via Agrobacterium-mediated transformation. The most extensively applied technique for genetic transformation of Brassica spp, including cabbage and broccoli, is the Agrobacterium tumefaciens-mediated method (Cardoza and Stewart 2003, 2004). Agrobacterium rhizogenes has also been applied for transformation of several members of the Brassica family (Henzi et al. 2000). However, in A. rhizogenes-mediated transformation, production of transgenic plants with abnormal phenotypes after regeneration from the hairy-root cultures is usually observed, which is a disadvantage of this method (Puddephat et al. 2001; Young et al. 2003). The pre-requisite to the realization of such gene transfer method is the availability of an efficient plant regeneration system. Several important factors that affect the transformation efficiency need to be optimized for achieving a high frequency of Agrobacterium-mediated transformation (Opabode 2006). The factors include pre-treatment of explants (Hiei et al. 2006), bacterial concentration (Rafat et al. 2010) and immersion time (Xing et al. 2007), co-cultivation condition (Vasudevan et al. 2007) as well as bacterial strain, plant genotype and explant type (Chen et al. 2001).
‘Green Marvel’ is one of the commonly grown broccoli cultivars in the cool climate regions of the world. The cultivar, which could also be grown on the highlands of the tropics, responds adversely to extreme temperatures and high humidity in the lowland; thus gene transformation is essential for improving its tolerance against heat stress. Heat shock proteins (HSPs) are functionally related proteins involved in the folding and unfolding of other proteins. Their expression is increased when cells are exposed to elevated temperatures or other stresses (Narberhaus 2010; Omar et al. 2011). HSP101 appears to play a major and specific role in conferring acquired thermotolerance (Wahid et al. 2007; Su and Li 2008). Transgenic broccoli plants have shown improved tolerance to dehydration, as well as to other types of stresses (salt, heavy metals, and hydrogen peroxide) (Vinocur and Altman 2005). The production of heat-tolerant rice via Agrobacterium-mediated transformation with Arabidopsis thaliana HSP 101 (AtHSP101) cDNA (Katiyar-Agarwal et al. 2003) established the crucial role of AtHSP101 in the heat tolerance characteristic (Hong and Vierling 2000; Queitsch et al. 2000; Rafat et al. 2010).
The main objectives of this study were to establish an efficient regeneration system using shoot tip culture in broccoli cv. Green Marvel followed by the introduction of AtHSP101 cDNA into the cultivar via Agrobacterium tumefaciens-mediated transformation and the optimization of parameters that affect the transformation as the first step to increase the heat tolerance characteristic of the crop.
Materials and methods
Plant material and seed germination
Hybrid seeds of broccoli cv. Green Marvel were bought from Kepong, Kuala Lumpur, Selangor, Malaysia. The seeds were placed under running tap water for 15 min followed by immersion and shaking in 70 % ethanol for 1 min. The seeds were rinsed once with sterile distilled water followed by immersion and shaking in NaOCl (1.05 % sodium hypochlorite) with 1–2 drop(s) Tween 20 for 10 min. Finally, the seeds were rinsed with sterile distilled water three times and cultured on a germination medium consisting of half-strength MS (Murashige and Skoog 1962) salts supplemented with 2.8 g l−1 phytagel and 30 g l−1 sucrose. The sterilization of seeds was carried out according to Ravanfar et al. (2014b) with some modifications.
Explant preparation and shoot regeneration
Well-defined shoot tips (8 mm in height) were isolated from 6-day-old seedlings and placed vertically on medium containing different concentrations of zeatin (0, 0.1, 0.5,1, 1.5, 2 and 3 mg l−1) for shoot regeneration. Each treatment consisted of three replications and each replication per treatment contained 10 explants. Parameters recorded were the percentage of explants producing shoots and mean number of shoots produced per explant at week 8 of culture.
Bacterial strains, binary plasmid vectors, and transformation procedure
The A. tumefaciens strain LBA4404 carrying the binary plasmid vector pGreen 0049 was used in the transformation experiments. The pGreen0049 vector contains the antibiotic resistance gene (kanamycin) as the plant selectable marker (Fig. 1). Therefore, the minimum inhibitory concentration (MIC) of kanamycin was assessed by culturing shoot tip explants on a shoot regeneration medium consisting of MS salts supplemented with the best zeatin concentration and 0, 10, 20, 30, 40, 60, 70, 80, 90 and 100 mg l−1 kanamycin. In the following experiment, the effect of acetosyringone (AS) (0 and 200 µM) in combination with pre-culture treatments [without pre-culture, 4 days of pre-culture on callus induction medium (CIM) and 4 days of pre-culture on SRM] on efficiency of transformation was assessed by the application of the bacterial dilution of 1:10 and 5 min inoculation time of Rafat et al. (2010). CIM consisted of MS medium supplemented with 1 mg l−1 6-benzylaminopurine (BAP) and 0.1 mg l−1 naphthalene acetic acid (NAA). The LBA4404 strain harboring the pGreen 0049 vector was grown in Luria–Bertani (LB) broth containing 50 µg l−1 kanamycin and incubated overnight at 28 °C with shaking at 200 rpm until the OD 600 reached 1.6. AS at 0 and 200 µM was added to the Agrobacterium growth medium and the shoot tip explants were given the pre-culture treatments prior to inoculation into the medium. In the next experiment, the effect of Agrobacterium (OD600 = 1.6) dilution of 1:5, 1:10 and 1:15 and inoculation time of 5, 15 and 30 min were assessed on the efficiency of transformation using the best pre-culture combination obtained earlier. After the infection with Agrobacterium, the shoot tip explants were co-cultivated for 4 days in the dark at 25 °C on SRM containing MIC of 80 mg l−1 kanamycin for the selection of putative transformed shoots. The SRM also contained 150 mg l−1 ampicillin to eradicate Agrobacterium growth. Non-inoculated shoot tip explants were regenerated on SRM as a control. The parameters recorded were the percentage of regeneration of survived explants (explants that remained green) and mean number of shoots obtained per survived explant. The experiments were arranged in a Completely Randomized Design. Each treatment was replicated three times and each replication per treatment contained 10 explants. Data were analysed using the analysis of variance (ANOVA). Duncan New Multiple Range Test (DNMRT) at α = 5 % was used for comparison between treatment means. Data on the percentage of putative shoot formation in the experiments combining AS with preculture and bacterial dilution with inoculation time were transformed to log10 (Y + 0.1) due to high coefficient of variation.
AtHSP101 cDNA was cloned into pGreen0049. Expression of AtHSP101 was driven by the CaMV 35S promoter. Source: http://www.pgreen.ac.uk/JIT/pG0049.htm
Molecular analyses of transformants
Genomic DNA was extracted from 100 mg fresh leaves of putative transformants on week 14 after co-cultivation and the control plants (regenerated from non-inoculated explants) using DNeasy Plant Mini Kit QIAGEN. Two pairs of primers were designed and used in the polymerase chain reaction (PCR) to amplify the 500 and 690 bp AtHSP101 cDNA region from the total DNA isolated from the shoot tip explants. Based on the 500-bp AtHSP101 sequence, a forward (5′ GACATCATGGTGTGCGAATCC 3′) and a reverse primer (5′ GGCCCAACGTTTTCTGTGAGC 3′) and for the 690-bp AtHSP101 a forward (5′ GGCGAGGGTAAAGTCTGAGG 3′) and a reverse (5′ GATTCGCACACCATGATGTCC 3′) primer were designed. PCR amplification was carried out using Green Taq (2×) protocol (Green PCR Master Mix) in 50 µl reaction volume containing 25 µl Master Mix PCR buffer, 1 µM forward primer (5 µl), 1 µM reverse primer (5 µl), 1 µg genomic DNA (5 µl), and water (nuclease-free) (10 µl). The PCR conditions were set for an initial denaturation step of 3 min at 95 °C and subsequent 36 cycles at 95 °C for 45 s, 53.6 °C for 30 s (for 500 bp AtHSP101 cDNA) or 56.5 °C for 30 s (for 690 bp AtHSP101 cDNA), 72 °C for 1 min/kb followed by a final extension step at 72 °C for 7 min. PCR amplified products were analyzed by electrophoresis in 1 % (w/v) agarose–ethidium bromide gel.
For Southern blot analysis, genomic DNA from transgenic broccoli leaf tissues was extracted using cetyltrimethylammonium bromide (CTAB) protocol (Doyle and Doyle 1990) with some modifications. Dig High Prime DNA Labeling and Detection Starter Kit II (Roche) was used for the detection of independent transgenic lines of broccoli cv. Green Marvel. The step for Southern blot analysis consisted of DNA labeling, DNA transfer and fixation, hybridization with a probe and immunological detection. For DNA labeling, DIG-labeled DNA probes were regenerated with DIG-High Prime according to the random primed labeling technique. PCR product amplified with forward primer: 5′ GGCGAGGGTAAAGTCTGAGG 3′ and reverse primer: 5′ GATTCGCACACCATGATGTCC 3′ and extracted from 1 % agarose gel was used to prepare the probes for Southern hybridization. The probes contained a common region to HSP gene. For DNA transfer and fixation, 20 µg genomic DNA isolated from fresh leaves of three shoot tip transgenic broccoli lines and the control were digested with EcoR1 enzyme whose restriction site is absent in the T-DNA. The digested genomic DNAs were fractionated in 1 % agarose gel and then blotted on nylon membrane through capillary transfer by placing the nylon membrane on the gel and incubating in 20× SSC for 17 h. For hybridization of membrane with the probe, the optimum hybridization temperature was 42.31 °C calculated according to the manual structure of Dig High Prime DNA Labeling and Detection Starter Kit II (Roche). Finally, for immunological detection, the membrane was incubated in 20 ml antibody solution containing 150 U/ml Anti-digoxigenin-Ap for binding to the DIG-labeled probe. The membrane was covered with 1 ml disodium (CSPD) (substrate for alkaline phosphatase) and incubated for 10 min at 37 °C. After 1–5 min at 25 °C the blue bands (chemiluminescence) were captured using a Canon camera in the dark room.
In the luciferase assay, cell lysates were prepared from the control plants and the eight transformants, one at a time, according to the protocol of Ow et al. (1986) with some modifications. The cell lysate (20 µl) was mixed with 100 µl of luciferase assay reagent and the light produced was measured using a luminometer (Luciferase Assay Kit, Instruction Manual). The results were expressed in terms of relative light units (RLU) per microgram of protein. In the RT-PCR assay, total RNA was extracted from 100 mg of fresh leaves of the transgenic lines and control plants using RNeasy Plant Mini Kit QIAGEN according to the manufacturer’s specifications. The QIAGEN OneStep RT-PCR Kit was used with specifically designed forward (5′ GGCGAGGGTAAAGTCTGAGG 3′) and reverse (5′ GATTCGCACACCATGATGTCC 3′) AtHSP101 primers to screen the integration of AtHSP101 cDNA and for detection of the transcripts. Reaction conditions were set up following the Kit’s protocol. The RT-PCR amplification products were visualized by electrophoresis in 1 % (w/v) agarose–ethidium bromide gel.
Results and discussion
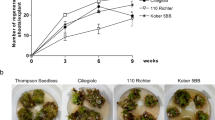
Shoot tip explants placed on MS medium containing different concentrations of zeatin began to elongate and produced shoots after 4 weeks of culture. At week 8 of culture, significant differences were observed between the treatments on percentage of explant forming shoots (Fig. 2a) and mean number of shoots produced per explant (Fig. 2b). Percentage shoot production at 1.5 mg l−1 zeatin was among the highest (96.67 %), while the lowest percentage (70 %) was with 3 mg l−1 zeatin treatment at week 8 of culture. The number of shoots formed from the shoot tip explants was the highest (4.27) at 1.5 mg l−1 zeatin but declined significantly at 2 and 3 mg l−1 zeatin. Therefore, zeatin at 1.5 mg l−1 (Fig. 3a, b) was selected for genetic transformation of broccoli cv. Green Marvel using shoot tip explants in subsequent experiments. The study showed that the different concentrations of zeatin affected shoot development and multiplication from shoot tip explants of broccoli cv. Green Marvel; however, zeatin at 1.5 mg l−1 had effectively broken the apical dominance and caused multiple shoot proliferation. It was also observed that callus was not produced even at high concentrations of zeatin while the shoots produced were normal. The superior effect of zeatin on shoot proliferation from shoot tip explants was earlier reported in Brassica campestris (Lim et al. 1997). In addition, Munir et al. (2008) reported obtaining the best regeneration when B. napus explants were cultured on medium containing 1 mg l−1 zeatin. Other species of Brassica also responded well on medium with 1.5 mg l−1 zeatin (Cardoza and Stewart 2006), which indicates that zeatin is generally good for the Brassica spp. However, Metz et al. (1995) and Ravanfar et al. (2014a) reported high regeneration of peduncle, hypocotyl and petiole explants on medium containing either BAP or thidiazuron (TDZ).
The MIC determination of kanamycin was carried out for the shoot tip explants of broccoli based on the presence of the plant selectable marker gene in the T-DNA region of pGreen vector. Among the highest percentage of regeneration from the survived shoot tip explants (96.67 %) was in the control which differed significantly compared to other treatments except with 10 and 20 mg l−1 kanamycin (Table 1). However, maximum death (100 %) occurred on 90 and 100 mg l−1 kanamycin (Fig. 3c). Treatments without and with low concentrations of kanamycin (0, 10 and 20 mg l−1) produced many shoots from the surviving shoot tip explants at week 8 of culture (Table 1). According to Rafat et al. (2010) if any antibiotic resistance gene as a plant selectable marker is available inside the vector construct, the MIC of the antibiotic has to be determined before transformation to allow the selection of putative transformants. Kanamycin has been used as a selectable marker in genetic transformation of several Brassica species. Metz et al. (1995) investigated the selection of putative transgenics on medium with 25 mg l−1 kanamycin in broccoli peduncle explants. Li et al. (1999) used 15 mg l−1 kanamycin to select transformed shoots from cotyledon explants of B. napus, while Block et al. (1989) and Cao and Earle (2003) applied 50 mg l−1 kanamycin for the selection of putative transformants from B. oleracea hypocotyl explants. Meanwhile Zhao et al. (2008) selected putatively transformed shoots of mustard (B. juncea Coss.) using 30 mg l−1 kanamycin. In this study, the ability of shoot tip explants of B. oleracea cv. Green Marvel to withstand high concentration of kanamycin (80 mg l−1) could be due to the fact that the cells in the shoot tip are more tolerant to kanamycin because of the continuous cell division process that occurs in that region. Results showed that the highest mortality rate occurred on medium containing 90 mg l−1 kanamycin. Therefore, 80 mg l−1 kanamycin was selected as the MIC for effective screening of putative transformants from shoot tip explants of broccoli cv. Green Marvel following Agrobacterium-mediated transformation.
Optimization of plant transformation conditions
Shoot tips were placed on different combinations of preculture and AS treatments for optimizing the Agrobacterium tumefaciens-mediated transformation protocol. Selection of putative transformants from the non-putatives was done by culturing on SRM supplemented with 150 mg l−1 ampicillin and 80 mg l−1 kanamycin for 8 weeks. Shoot tip explants precultured on CIM and exposed to 200 μM AS produced among the highest percentage of putative transformants (23.33 %) (Fig. 4a) as well as among the highest mean number of putative transformants (0.27) at week 8 of culture (Fig. 4b). According to Khan et al. (2009), preculturing, AS treatment and explant source affected transformation frequency. In this study, the highest putative transformant production occurred with preculture and with AS application for shoot tips of broccoli cv. Green Marvel. Preculture and AS application were also required for efficient transformation in other Brassica spp. (Chakrabarty et al. 2002; Rafat et al. 2010; Kuta and Tripathi 2005). In broccoli cv. Green Marvel, preculture of shoot tip explants on CIM as determined in this study was necessary to overcome necrosis and increased the efficiency of transformation.
Effect of preculture and acetosyringone on a percentage of shoot formation and b mean number of putative transformants produced per shoot tip explant of broccoli cv. Green Marvel at week 8 of culture on SRM with 150 mg l−1 ampicillin and 80 mg l−1 kanamycin. Bars followed by the same letter are not significantly different from each other based on DMNRT (p = 0.05). P1W1 without preculture without acetosyringone, P1W2 without preculture with 200 μM acetosyringone, P2W1 precultured on SRM without acetosyringone, P2W2 precultured on SRM with 200 μM acetosyringone, P3W1 precultured on CIM without acetosyringone, P3W2 precultured on CIM with 200 μM acetosyringone
Figure 5a, b, shows that shoot tip explants exposed to a high concentration of Agrobacterium (1:5 dilution) and relatively long period of inoculation (30 min) (Treatment 3) exhibited the highest transformation efficiency. However, Chakrabarty et al. (2002) reported that using 1:10 dilution of A. tumefaciens and 10 min inoculation time resulted in a relatively high percentage (7.6 %) of recovery of transformants. In general, the results in this study confirmed earlier observations that the appropriate bacterial density and inoculation time help to improve transformation frequency of Brassica (Yi et al. 2011). After selecting the putative broccoli transformants, hormone-free MS medium (Ravanfar et al. 2009) without kanamycin was used for elongation and rooting of the transformed shoots for further molecular analysis (Fig. 6).
Effect of bacterial dilution and inoculation time on a percentage of shoot formation and b mean number of putative transformants produced per shoot tip explant of broccoli cv. Green Marvel at week 8 of culture on SRM with 150 mg l−1 ampicillin and 80 mg l−1 kanamycin. Bars followed by the same letter are not significantly different based on DMNRT (p = 0.05). Treatments: 1:1:5 bacterial dilution, 5 min inoculation, 2:1:5 bacterial dilution, 15 min inoculation, 3:1:5 bacterial dilution, 30 min inoculation, 4:1:10 bacterial dilution, 5 min inoculation, 5:1:10 bacterial dilution, 15 min inoculation, 6:1:10 bacterial dilution, 30 min inoculation, treatment 7:1:15 bacterial dilution, 5 min inoculation, 8:1:15 bacterial dilution, 15 min inoculation, 9:1:15 bacterial dilution, 30 min inoculation
Acclimatization of broccoli transformants. a Rooted transgenic broccoli cv. Green Marvel in a culture flask, b after 1 month in plastic pots, c after 3 months and d after 4 months acclimatization at 34 °C in the transgenic greenhouse (Ravanfar et al. 2013) Acclimatization of broccoli transformants. a, b, d Bar 30 mm and c Bar 50 mm
Molecular analyses of AtHSP101 transgenics
The expected amplified DNA fragments from the PCR were 500 and 690 bp for AtHSP101. The resulting samples were subjected to electrophoresis using a 1.0 % agarose gel. Electrophoresis showed that the product sizes were that of the expected amplification sizes. The PCR results indicated stable transformation of the shoot tip explants. Three independent lines showed the expected fragments of 500 and 690 bp for AtHSP101 in the PCR products (Fig. 7a, b). The PCR results also showed a transformation efficiency of 8.33 %. Kong et al. (2009) reported reaching a maximum transformation efficiency of 18.93 % (positive PCR) when cotyledonary nodes of Brassica napus were infected for 10 min with an Agrobacterium suspension of 0.8 OD and containing 200 µM AS. According to Metz et al. (1995), the overall transformation efficiency for the flowering stalk of broccoli was 6.4 %, while for seedling explants it was 6.6, 10 % for hypocotyl explants and only 1.8 % for petiolar explants. For Southern blot analysis, genomic DNA prepared from the transgenic plants and the control was digested with EcoR1, as there is a unique site for the restriction enzyme in the T-DNA region of the binary vectors used. The size of the expected hybridization band was slightly more than 3 kb, due to the size of AtHSP101. Figure 8a shows two different hybridization bands of the AtHSP101 in the shoot tip derived transformants. Meanwhile, from three independent transgenic lines assessed only two lines showed positive hybridization bands carrying two and three copies of the gene. No positive hybridization bands were found in the untransformed plants (control) and line 3. Thus, the Southern blot analysis confirmed that the AtHSP101 gene had been stably integrated into the genome of two transgenic lines. Stable integration of transgene into the genome of other Brassica species has been demonstrated by Southern blot analysis such as in B. oleracea var. botrytis (cauliflower) (Nugent et al. 2006), B. oleracea (Chinese cabbage) (Min et al. 2007) and B. napus (rapeseed) (Cheng et al. 2010). Transgenic plants confirmed by PCR and Southern blot analysis were further analyzed using luciferase assay and RT-PCR for the presence of AtHSP101 transcripts. According to Chen et al. (2001) RT-PCR revealed that ipt gene expression occurred early on the day of detachment of broccoli and Southern blot showed the hybridization analysis of ipt transformants. The luciferase gene has been used extensively as a reporter gene in the verification of transgenic tobacco (Ow et al. 1986) and rice (Sadasivam and Gallie 1994, Cornejo et al. 1993). The results from the luciferase assays indicated that the luciferase gene could be suitably used as a reporter gene for the confirmation of transgenic plants. The activity of the luciferase gene was visualized in the shoot tip-derived broccoli transformants (Fig. 8b). The highest RLU per microgram protein was in shoot tip transformants 3 (1200 RLU) followed by transformant 4 (800) and 2 (500). Non-transformed shoot tip-derived regenerants as controls did not show any RLU value. The transgenic status of the three independent transgenic lines that exhibited luciferase activity was further assessed by RT-PCR. All three transgenic lines showed the expected 690 bp amplified fragment in RT-PCR (Fig. 8c) demonstrating transcriptionally active AtHSP101 gene in the plant genome. The introduction of the AtHSP101 gene into broccoli cv. Green Marvel is the first step towards the production of broccoli plants tolerant to high temperature via genetic transformation.
PCR analysis of DNA from putatively transformed shoot tip-derived regenerants of broccoli cv. Green Marvel using primers of AtHSP101 cDNA. a 500 bp fragments were amplified by the first set of primers and b 690 bp fragments were amplified by the second set of primers. M marker (0333), Co non-transformed plant (control), lanes 1–3 transformed regenerants
Molecular characterization of independent PCR-positive lines of broccoli cv. Green Marvel. a Southern blot analysis, arrow indicates probe of binary vector pGreen 0049 (positive control), Co—non-transformed plant (negative control), lanes 1, 2 and 3 shoot tip-derived transformants. b Luciferase activity in RLU per microgram protein of shoot tip-derived transformants. Co—control, 1–3 shoot tip derived transformants. c RT-PCR analysis of transgenic lines derived from shoot tip explant. M marker, lanes 1–3 transformed lines from shoot tips, Co control
References
Block MD, Brouwer DD, Tenning P (1989) Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol 91:694–701. doi:10.1104/pp.91.2.694
Cao J, Earle ED (2003) Transgene expression in broccoli (Brassica oleracea var. italica) clones propagated in vitro via leaf explants. Plant Cell Rep 21:789–796. doi:10.1007/s00299-003-0589-6
Cardoza V, Stewart CN Jr (2003) Increased Agrobacterium mediated transformation and rooting efficiencies in canola (Brassica napus L.) from hypocotyl explants. Plant Cell Rep 21:599–604. doi:10.1007/s00299-002-0560-y
Cardoza V, Stewart CN Jr (2004) Brassica biotechnology: progress in cellular and molecular biology. In Vitro Cell Dev Biol Plant 40:542–551. doi:10.1079/IVP2004568
Cardoza V, Stewart CN Jr (2006) Agrobacterium protocols, Canola (Brassica napus L.). Meth Mol Biol 343(IV):257–266. doi:10.1385/1-59745-130-4:257
Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL (2002) Agrobacterium-mediated transformation of cauliflower: optimization of protocol and development of Bt-transgenic cauliflower. J Biosci 27:495–502. doi:10.1007/BF02705046
Chen FO, Hwang JY, Charng YY, Sun CW, Yang SF (2001) Transformation of broccoli (Brassica oleracea var. italica) with isopentenyltransferase gene via Agrobacterium tumefaciens for post-harvest yellowing retardation. Mol Breed 7(3):243–257. doi:10.1023/A:1011357320259
Cheng L, Li HP, Qu B, Huang T, Tu JX, Fu TD, Liao YC (2010) Chloroplast transformation of rapeseed (Brassica napus) by particle bombardment of cotyledons. Plant Cell Rep 29(4):371–381. doi:10.1007/s00299-010-0828-6
Cornejo M, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581. doi:10.1007/BF00019304
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Henzi MX, Christey MC, McNeil DL (2000) Factors that influence Agrobacterium rhizogenes-mediated transformation of broccoli (Brassica oleracea L. var. italica). Plant Cell Rep 19:994–999. doi:10.1007/s002990000221
Hiei Y, Ishida Y, Kasaoka K, Komari T (2006) Improved frequency of transformation in rice and maize by treatment of immature embryos with centrifugation and heat prior to infection with Agrobacterium tumefaciens. Plant Cell Tissue Org Cult 87:233–243. doi:10.1007/s11240-006-9157-4
Hong S, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci 97:4392–4397. doi:10.1073/pnas.97.8.4392
Hong ZJ, Yan ZH, Guo TN, Fang TY, Yun ZX, Xiu LY (2009) Several methods to detect the inheritance and resistance to the Diamondback moth in transgenic Chinese cabbage. Afr J Biotech 8(12):2887–2892. doi:10.5897/AJB08.870
Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat-tolerant basmati rice engineered by over-expression of HSP101. Plant Mol Biol 51:677–686. doi:10.1023/A:1022561926676
Khan MMA, Robin ABMAHK, Nazim-ud-dowla MAN, Talukderand SK, Hassan L (2009) Agrobacterium-mediated genetic transformation of two varieties of brassica: optimization of protocol. Bangladesh J Agric Res 34(2):287–301. doi:10.3329/bjar.v34i2.5802
Kong F, Li J, Tan X, Zhang L, Zhang Z, Ma CQX (2009) A new time-saving transformation system for Brassica napus. Afr J Biotech 8(11):2497–2502. doi:10.5897/AJB09.240
Kuta DD, Tripathi L (2005) Agrobacterium-induced hypersensitive necrotic reaction in plant cells: a resistance response against Agrobacterium-mediated DNA transfer. Afr J Biotech 4(8):752–757
Li XB, Zheng SX, Dong WB, Chen GR, Mao HZ, Bai YY (1999) Insect-resistant transgenic plants of Brassica napus and analysis of resistance in the plants. Acta Genet Sin 26(3):262–268
Lim HT, You YS, Park EJ (1997) Plant regeneration and genetic transformation of Brassica campestris ssp. pekinensis via organogenesis. HortScience 32(3):512–513
Metz RD, Dixit R, Earle ED (1995) Agrobacterium tumefaciens mediated transformation of broccoli (Brassica oleracea var. italica) and cabbage (B. oleracea var. capitata). Plant Cell Rep 15:287–292. doi:10.1007/BF00193738
Min BW, Cho YN, Song MJ, Noh TK, Kim BK, Chae WK, Park YS, Choi YD, Harn CH (2007) Successful genetic transformation of Chinese cabbage using phosphomannose isomerase as a selection marker. Plant Cell Rep 26(3):337–344. doi:10.1007/s00299-006-0247-x
Munir M, Rashid H, Rauf M, Chaudhry Z, Bukhari MS (2008) Callus formation and plantlets regeneration from hypocotyl of Brassica napus by using different media combinations. Pak J Bot 40(1):309–315
Murashige T, Skoog FA (1962) revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Narberhaus F (2010) Translational control of bacterial heat shock and virulence genes by temperature-sensing mRNAs. RNA Biol 7(1):84–89
Nugent GD, Coyneb S, Nguyena TT, Kavanaghb TA, Dix PJ (2006) Nuclear and plastid transformation of Brassica oleracea var. botrytis (cauliflower) using PEG-mediated uptake of DNA into protoplasts. Plant Sci 170(1):135–142. doi:10.1016/j.plantsci.2005.08.020
Omar SA, Fu QT, Chen MS, Wang GJ, Song SQ, Elsheery NI, Xu ZF (2011) Identification and expression analysis of two small heat shock protein cDNAs from developing seeds of biodiesel feedstock plant Jatropha curcas. Plant Sci 181(6):632–637. doi:10.1016/j.plantsci.2011.03.004
Opabode JT (2006) Agrobacterium-mediated transformation of plants: emerging factors that influence efficiency. Biotechnol Mol Biol Rev 1:12–20
Ow DW, Wood KV, DeLuk M, Wet JR, Helinski DR, Howell SH (1986) Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234:856–859. doi:10.1126/science.234.4778.856
Puddephat IJ, Robinson HT, Fenning TM, Barbara DJ, Morton A, Pink DAC (2001) Recovery of phenotypically normal transgenic plants of Brassica oleracea upon Agrobacterium rhizogenes mediated co-transformation and selection of transformed hairy roots by GUS assay. Mol Breed 7:229–242. doi:10.1023/A:1011338322000
Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12:479–492. doi:10.1105/tpc.12.4.479
Rafat A, Aziz MA, Rashid AA, Abdullah SNA, Kamaladini H, Sirchi MHT, Javadi MB (2010) Optimization of Agrobacterium tumefaciens-mediated transformation and shoot regeneration after co-cultivation of cabbage (Brassica oleracea subsp. capitata) cv. KY Cross with AtHSP101 gene. Sci Hortic 124:1–8. doi:10.1016/j.scienta.2009.11.015
Ravanfar SA, Aziz MA, Kadir MA, Rashid AA, Sirchi MHT (2009) Plant regeneration of Brassica oleracea subsp. italica (Broccoli) cv Green Marvel as affected by plant growth regulators. Afr J Biotech 10(29):5614–5619. doi:10.5897/AJB2009.000-9304
Ravanfar SA, Aziz MA, Shabanimofrad M, Samarfard S (2013) Greenhouse evaluation on the performance of heat tolerant transgenic broccoli and genetic diversity analysis using inter simple sequence repeat (ISSR) markers. Electron J Biotech 16(5):1–10. doi:10.2225/vol16-issue5-fulltext-10
Ravanfar SA, Shahida S, Aziz MA, Abdullah SNA, Rashid AA (2014a) Influence of phenyl-urea and adenine-type cytokinins on direct adventitious shoot regeneration of cabbage (Brassica oleracea subsp. capitata) “KY Cross”. Plant Biotech 31(3):275–280. doi:10.5511/plantbiotechnology.14.0514a
Ravanfar SA, Aziz MA, Rashid AA, Shahida S (2014b) In vitro adventitious shoot regeneration from cotyledon explant of Brassica oleracea subsp. italica and Brassica oleracea subsp. capitata using TDZ and NAA. Pak J Bot 46(1):329–335
Sadasivam S, Gallie DR (1994) Isolation and transformation of rice aleurone protoplasts. Plant Cell Rep 13:394–396. doi:10.1007/BF00234145
Su PH, Li HM (2008) Arabidopsis stromal 70-kd heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol 146(3):1231–1241. doi:10.1104/pp.107.114496
Suri SS, Saini ARK, Ramawat KG (2005) High frequency regeneration and Agrobacterium tumefaciens -mediated transformation of broccoli (Brassica oleracea var. italica). Eur J Hortic Sci 70(2):71–78
Vasudevan A, Selvaraj N, Ganapathi A, Choi CW (2007) Agrobacterium-mediated genetic transformation in cucumber (Cucumis sativus L.). Am J Biotechnol Biochem 3:24–32. doi:10.3844/ajbbsp.2007.24.32
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opinion Biotech 16(2):123–132. doi:10.1016/j.copbio.2005.02.001
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Env Exp Bot 61:199–223. doi:10.1016/j.envexpbot.2007.05.011
Xing Y, Yang Q, Ji Q, Luo Y, Zhang Y, Gu K, Wang D (2007) Optimization of Agrobacterium-mediated transformation parameters for sweet potato embryogenic callus using b-glucuronidase (GUS) as a reporter. Afr J Biotech 6:2578–2584. doi:10.5897/AJB2007.000-2411
Yi D, Lei C, Yu-mei L, Mu Z, Yang-yong Z, Zhi-yuan F, Li-mei Y (2011) Transformation of cabbage (Brassica oleracea L. var. capitata) with Bt cry1Ba3 gene for control of diamondback Moth. Agric Sci China 10(11):1693–1700. doi:10.1016/S1671-2927(11)60167-3
Young JM, Kerr A, Sawada H (2003) Genus Agrobacterium. In Bergey’s manual of systematic bacteriology, 2nd edn, vol 2 (in press). Springer, New York
Zhao S, Lei JJ, Chen GJ, Cao BH (2008) Application of kanamycin in transgenic mustard (Brassica juncea Coss.). Hereditas 30(4):501–507. doi:10.3724/SP.J.1005.2008.00501
Acknowledgments
The authors are thankful to the Department of Agriculture Technology, Faculty of Agriculture, Universiti Putra Malaysia, for the laboratory facilities provided and the financial support in the form of a research grant no. 01/01/07/0300RU.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ravanfar, S.A., Aziz, M.A. Shoot tip regeneration and optimization of Agrobacterium tumefaciens-mediated transformation of Broccoli (Brassica oleracea var. italica) cv. Green Marvel. Plant Biotechnol Rep 9, 27–36 (2015). https://doi.org/10.1007/s11816-014-0340-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-014-0340-5