Abstract
Purpose
This study explored whether sociodemographic and health-related characteristics moderated mHealth PA intervention effects on total and moderate-to-vigorous physical activity (MVPA) at 6 months, relative to a self-help condition among young adult cancer survivors (YACS).
Methods
We conducted exploratory secondary analyses of data from a randomized controlled trial among 280 YACS. All participants received digital tools; intervention participants also received lessons, adaptive goals, tailored feedback, text messages, and Facebook prompts. Potential moderators were assessed in baseline questionnaires. PA was measured at baseline and 6 months with accelerometers. Linear model repeated measures analyses examined within- and between-group PA changes stratified by levels of potential moderator variables.
Results
Over 6 months, the intervention produced MVPA increases that were ≥ 30 min/week compared with the self-help among participants who were males (28.1 vs. -7.7, p = .0243), identified with racial/ethnic minority groups (35.2 vs. -8.0, p = .0006), had baseline BMI of 25–30 (25.4 vs. -7.2, p = .0034), or stage III/IV cancer diagnosis (26.0 vs. -6.8, p = .0041). Intervention participants who were ages 26–35, college graduates, married/living with a partner, had a solid tumor, or no baseline comorbidities had modest MVPA increases over 6 months compared to the self-help (ps = .0163-.0492). Baseline characteristics did not moderate intervention effects on total PA.
Conclusions
The mHealth intervention was more effective than a self-help group at improving MVPA among subgroups of YACS defined by characteristics (sex, race, BMI, cancer stage) that may be useful for tailoring PA interventions.
Implications for cancer survivors
These potential moderators can guide future optimization of PA interventions for YACS.
ClinicalTrials.gov identifier
NCT03569605.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly 84,000 young adults, ages 18–39, are diagnosed with cancer in the US annually [1, 2], and there is high demand for physical activity (PA) interventions among young adult cancer survivors (YACS) [3, 4]. Given the benefits of regular PA for cancer survivors, including improved fitness and health-related quality of life (HRQOL) [5], it is critical to address these needs among YACS who may face several decades at risk for long-term and late effects of cancer [6]. Yet few PA interventions have been designed for and evaluated among YACS [4, 7]. Most have been short-term pilot studies among small samples and demonstrated mixed effects [8,9,10,11,12,13], and there is limited evidence to inform the development and optimization of PA interventions that are tailored to YACS’ unique contexts and preferences [7]. There is a need to identify moderators of PA intervention effects, which can guide intervention tailoring for YACS [13] and expand our understanding of subgroups of YACS for whom, and under what conditions, various intervention approaches may be effective.
Previous studies examining moderators of exercise training effects among cancer survivors have focused on HRQOL [14,15,16,17,18,19] and exercise training outcomes (e.g., aerobic fitness, body composition) [16,17,18, 20, 21], older populations [14], and patients in treatment [16, 17, 22]. Most of these studies evaluated supervised exercise interventions and have yielded inconsistent findings. Variables previously identified as moderators of exercise intervention effects on HRQOL have included age [16, 17, 20, 23], ethnicity [19], marital status [14, 16, 17], time since diagnosis [14, 24], and history of chemotherapy [23]. To date, limited research has explored moderators of PA interventions among cancer survivors [24,25,26], and little is known about sociodemographic and health-related characteristics of YACS that may moderate response to PA behavior change interventions [4].
Technology has enabled delivery of theory-based mHealth PA interventions, which are responsive to YACS’ preferences and enhance potential for scalability. Few studies have examined moderators of theory-based PA interventions aimed at promoting behavior change and adherence to PA among cancer survivors [23, 24, 27,28,29,30]. These studies primarily have focused on breast cancer survivors and have reported age [23, 28], marital status [24, 27, 28], time since diagnosis [24, 27], cancer stage [27, 28], cancer treatments [23, 24, 27], and psychosocial factors [28,29,30] as moderators of interventions effects on PA behaviors [24, 28,29,30] and HRQOL outcomes [23, 24, 27]. Interventions have demonstrated greater benefits on moderate-to-vigorous PA (MVPA) among breast cancer survivors who were older [28], married [24, 28], further from diagnosis [24], and had a lower cancer stage [28]. Overall, there is limited evidence regarding moderators of digital PA intervention effects in cancer survivors, and these have yet to be examined among YACS.
We previously reported that both a theory-based mHealth PA intervention and self-help group improved accelerometer-measured MVPA over 6 months in a nationwide sample of YACS [31]. Although findings indicated no between-group differences in total PA changes at 6 months (primary outcome), intervention participants increased MVPA by twice as much as self-help participants (24.7 vs. 11.4 min/week), and baseline MVPA was a moderator of intervention effects, such that intervention effects on MVPA were more pronounced among those starting with low levels of MVPA (i.e., 1–30 min/week) [31]. As we were interested in identifying other potential moderators of intervention effects, the purpose of this study was to explore whether intervention effects on post-intervention outcomes over 6 months (total and MVPA) differed across sociodemographic (e.g., age, sex, race) and health-related variables (e.g., cancer treatment, time since diagnosis). To our knowledge, this is the first study to explore moderators of PA intervention effects among YACS. Given the exploratory aim of the current study, we examined sociodemographic and health-related variables based on previous research indicating that they moderated intervention effects on adherence to PA behaviors [24, 28].
Methods
Study design
We conducted a secondary exploratory analysis using data from the IMproving Physical Activity after Cancer Treatment (IMPACT) trial, a 12-month randomized controlled trial of an mHealth PA intervention among YACS. Details on the study protocol and interventions have been published [31,32,33]. The primary aim of the intervention was to increase accelerometer-measured total PA minutes/week at 6 months, and a secondary aim was to increase MVPA. Comparisons of study groups on total PA and MVPA at 6 and 12 months were previously reported [31, 33]. The current study explored whether baseline characteristics moderated intervention effects on accelerometer-measured total PA and MVPA at 6 months (primary timepoint post-intervention) relative to the self-help condition.
Participants and procedures
A total of 280 YACS were recruited from around the US. Baseline characteristics of the sample and details on recruitment approaches were previously reported [31, 32, 34]. All participants were YACS, ages 18–39, diagnosed with cancer ≤ 10 years, post-treatment, and engaging in less than recommended levels of PA for cancer survivors (i.e., < 150 min/week of MVPA; measured by accelerometer). All participants provided informed consent via an online questionnaire. Following completion of baseline assessments of sociodemographic, health-related and PA measures, participants were randomized to either an intervention or self-help condition. Participants completed additional PA assessments at 6 months. All study procedures were reviewed and approved by the Oncology Protocol Review Committee and Institutional Review Board of the University of North Carolina at Chapel Hill.
Study interventions are detailed elsewhere [31, 32]. Briefly, all participants received digital tools (i.e., activity tracker (Fitbit, San Francisco, CA), smart scale (BodyTrace, New York, NY)), an individual videochat session, and access to arm-specific closed Facebook group. Additionally, over the study period, intervention group participants had access to a mobile website with weekly activity goals that adapted to individuals’ recent PA behaviors, behavioral lessons (weekly in months 1–3, biweekly in months 4–6, bimonthly in months 7–12), tailored feedback (weekly in months 1–6, bimonthly in months 7–12), and publicly available web resources. Intervention participants also received text messages (5 per week in months 1–6, 1 per week in months 7–12) and prompts to engage within the arm-specific Facebook group (≤ 5 per week).
Measures
Minutes/week of total and MVPA were assessed at baseline and 6 months with accelerometers (ActiGraph GT3X+, Pensacola, FL) as described previously [31, 32]. YACS were asked to wear accelerometers continuously for 7 days; wear was valid if the participant had ≥ 4 days with ≥ 10 h of wear and at least one weekend day [35]. Accelerometer-measured minute-level total (light, moderate, or vigorous intensity) and MVPA data were used to derive ≥ 10-minute bouts using a standard algorithm [36, 37], and total and MVPA minutes/week were calculated [(5*weekday average) + (2* weekend day average)]. We used bouts as pre-specified in our study protocol [32], which was established prior to PA guidelines that removed the 10-minute bout requirement [38]. Data on sociodemographic and health-related variables were collected during screening or in baseline questionnaires.
Moderators
Sociodemographic variables collected through online screening questionnaires included date of birth (used to calculate baseline age:18–25, 26–35, 36–<40), sex, race, ethnicity, and residence (rural/urban). Participant zip codes were used to classify urbanization level of residence using 2013 Rural-Urban Continuum Codes (RUCC; 1–3 rural, 4–9 urban). Marital status (married/living with partner vs. other), educational attainment (≤some college vs. ≥college graduate), and number of children living in their home (≥ 1 vs. 0) were collected in online baseline questionnaires.
Health-related variables. Single-item questions during telephone screening asked about time first diagnosed with cancer, cancer type, and cancer stage. Data on cancer treatments and time since end of treatments were collected in the baseline questionnaire (“Have you ever received any of the following treatments for your cancer?” chemotherapy, radiation, surgery, bone marrow transplant/stem cell transplant, other;“When did you finish treatment for your cancer?”). Treatment variables were dichotomized (yes vs. no). Number of comorbid conditions were derived from baseline questions about health conditions (e.g., high blood pressure, diabetes, depression, thyroid disorder) and dichotomized as (0 vs. ≥1). Self-reported height and objective weight at baseline were collected through a single-item question and smart scales (Body Trace, New York, NY; average of 3 weights), respectively, and used to derive body mass index (kg/m2) categories (underweight/normal: <25; overweight: 25-<30; obesity: ≥30). HRQOL was assessed at baseline with the Medical Outcomes Study 36-Item Short Form (SF-36) [39, 40], which includes 36 items with 8 subscales (physical functioning, role limitations-physical, role limitations-emotional, social functioning, pain, mental health, vitality, general health). Subscale components were calculated, transformed to scores from 0 to 100 (higher scores indicate more positive outcomes), and summed to derive Physical Component Summary (PCS) and Mental Component Summary (MCS) scores. Subscale and component summary scores were dichotomized (< 50 vs. ≥50). The SF-36 has been used with YACS [8, 41] and demonstrated reliability and validity among cancer survivors (α > 0.70 for subscales) [40].
Statistical analyses
Descriptive statistics were used to summarize baseline participant characteristics. To examine whether any of these characteristics moderated the effects of the intervention relative to the self-help group, we stratified the analyses by levels of the potential moderators. Within each level of the potential moderator, we conducted repeated measures analyses using generalized estimating equation (GEE) analyses to compare the effects of the intervention and self-help groups on total PA and MVPA outcomes. Each model included the time effect, the intervention effect, and the interaction between the time and intervention effect; outcomes were adjusted for accelerometer wear time. For participants within each level of the moderator, we estimated PA changes over time from baseline to 6 months within groups, as well as differences between groups, in changes over time from baseline to 6 months. We chose to stratify the levels of the moderators instead of fitting models with three-way interactions to improve the interpretability of the results. In the results, we focus on reporting the quantity of these estimates and between-group differences in changes over time that were clinically meaningful at ≥ 30 min/week [42, 43], because analyses were exploratory, and this study was not powered a priori to detect group-time interaction effects within subgroups.
Results
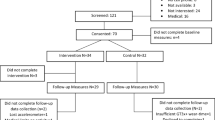
Two-hundred eighty participants were randomized to the intervention (n = 140) or self-help (n = 140) group from August 2018-October 2019 [31, 32, 34]. The CONSORT diagram was presented previously [31]. At baseline, participants were aged 33.4 (SD = 4.8) years and had been diagnosed 3.7 (SD = 2.4) years prior to enrollment. Most participants reported being females (82%), non-Hispanic White (77%), college graduates (71%), and married/living with a partner (62%). Nearly half reported a stage I/II cancer diagnosis (49%) and having ≥ 1 comorbid conditions (39%). Participants reported history of cancer treatments as follows: chemotherapy (61%), radiation therapy (46%), and surgery (86%). Since most participants resided in urban locations (89%), had a history of surgery (86%), and endorsed more favorable health states on most SF-36 subscales (i.e., ≥ 50; 95% physical functioning, 93% social functioning, 85% mental health, 83% pain), we exclude results related to these potential moderator variables due to inadequate sample sizes for each moderator subgroup.
No variables moderated intervention effects on total PA (Supplementary Table). Moderators of intervention effects on MVPA are presented in Table 1. Figure 1 displays estimated change in MVPA minutes/week over 6 months for each group separately by potential moderator variables. Intervention effects on MVPA were more favorable compared with the self-help group, with between-group differences ≥ 30 min/week, among males (intervention: +28.1 vs. self-help: -7.7; mean difference (95% CI): +35.8 (4.7, 67.0); p = .0243), participants who identified as American Indian/Alaska Native, Black, Asian, Hispanic or of mixed race/ethnicity (+35.2 vs. -8.0; mean difference (95% CI): +43.7 (16.5, 71.0); p = .0006), and participants with baseline BMI of 25–<30 (+25.4 vs. -7.2; mean difference (95% CI): +32.7 (10.8, 54.5); p = .0034). Intervention effects on MVPA minutes/week were also more favorable, with smaller between-group differences compared with the self-help, among participants who were ages 26–35 (+26.7 vs. +7.8; mean difference (95% CI): +18.9 (3.1, 34.7); p = .0193), college graduates (+29.3 vs. +12.2; mean difference (95% CI): +17.0 (0.1, 34.0); p = .0492), or married/living with a partner (+30.4 vs. +8.7; mean difference (95% CI): +21.7 (4.0, 39.4); p = .0163).
When considering baseline health-related characteristics, the intervention had more favorable effects on MVPA minutes/week, compared with the self-help group, among participants diagnosed with stage III/IV cancer (+26.0 vs. -6.8; mean difference (95% CI): +32.9 (10.4, 55.3); p = .0041). Additionally, the intervention appeared more effective than the self-help group, though with smaller between-group differences, among participants with a solid tumor type (+21.6 vs. +4.3; mean difference (95% CI): +17.2 (2.1, 32.4); p = .0254) and participants with no baseline comorbid conditions (+33.5 vs. +13.5; mean difference (95% CI): +20.0 (1.0, 39.1); p = .0395). When exploring effects by HRQOL variables, the intervention had greater effects on MVPA, compared with self-help, for participants reporting higher baseline scores on role limitations-physical (+29.6 vs. +11.5; mean difference (95% CI): +18.1 (1.3, 34.9); p = .0346). There were within-group MVPA increases in the intervention group, but not the self-help, in both categories of MCS and PCS (i.e., < 50, ≥50) (between-group ps = .1022–.4433). Having a child at home, years since diagnosis, time since end of treatment, history of chemotherapy, history of radiation, and SF-36 subscales (role limitations-emotional, vitality, general health) were not moderators of intervention effects on MVPA over 6 months.
Changes in accelerometer-measured MVPA over 6 months by group stratified by moderators at baseline. Abbreviation: MVPA, moderate-to-vigorous physical activity. A Stratified by sex. B Stratified by race and ethnicity (White (non-Hispanic) vs. American Indian or Alaska Native, Asian, Black or African American, White (Hispanic), Multiple races, Other. C Stratified by body mass index (< 25 vs. 25-<30vs. ≥ 30). D Stratified by cancer stage (I/II vs. III/IV vs. other/no stage). E Stratified by baseline age groups (18–25 vs. 26–35 vs. 36–<40). F Stratified by education (≤ some college vs. ≥ college degree). G Stratified by marital/partner status (not partnered vs. married or living with partner). H Stratified by cancer type (not solid vs. solid). I Stratified by comorbid conditions (0 vs. ≥1 or more)
Discussion
This study is one of the first to explore baseline sociodemographic and health-related characteristics as moderators of mHealth intervention effects on PA outcomes in YACS, providing evidence to enhance tailoring and optimization of future PA interventions for YACS. While baseline characteristics did not moderate intervention effects on total PA, the intervention produced differential effects on MVPA over 6 months relative to the self-help condition among specific subgroups. Among YACS who were males, identified as having an underrepresented racial/ethnic background, had a BMI of 25–30, or stage III/IV cancer at baseline, the intervention was more favorable for promoting clinically meaningful MVPA increases relative to the self-help condition. Additionally, those who were ages 26–35, college graduates, married/partnered, reported having a solid tumor, or no comorbid conditions at baseline achieved more MVPA when receiving the intervention compared with self-help. Overall, these findings indicate potential subgroups of YACS who may benefit from receiving digital tools alone, and others that may require more support from a comprehensive theory-based mHealth PA intervention integrating digital tools to achieve meaningful health benefits.
Few studies have reported on the effects of mHealth PA interventions among YACS. To our knowledge, one study has explored heterogeneity in PA intervention effects on outcomes by baseline PA levels among YACS. Belanger and colleagues [8] reported differential effects of targeted print materials among YACS self-reporting ≤ 300 PA minutes/week at baseline; YACS who received print materials reported significantly more PA minutes/week at 3 months (+ 90) compared with a control group that only received national PA guidelines. There are no studies of mHealth PA interventions in YACS, nor studies with accelerometer-measured PA outcomes in YACS, to directly compare our results.
Our findings provide evidence that sex and racial/ethnic background may be moderators of MVPA outcomes in response to an mHealth behavior change intervention designed for YACS. In the current study, YACS who identified as female or non-Hispanic White increased MVPA in both conditions, while males and those identifying with underrepresented racial/ethnic groups increased MVPA if they received the intervention. Most studies of PA among adolescents and young adults (AYAs) with cancer have included samples of predominantly women, with limited power to detect intervention effects by subgroups [7]. Consistent with a large cross-sectional survey of a diverse sample of AYAs which showed that several PA preferences (e.g., sports participation, program delivery, exercise modalities) differed by sex [4], our results suggest that tailoring future PA interventions for YACS by sex could be beneficial. Dieli-Conwright and colleagues have shown Hispanic ethnicity to be a moderator of the effects of an aerobic and resistance training exercise intervention, such that Hispanic breast cancer survivors experienced greater benefits on several measures of metabolic syndrome, physical fitness, and HRQOL, compared with non-Hispanic breast cancer survivors [19, 21]. Given documented disparities in reported MVPA levels among AYA survivors by racial and ethnic groups [44], our findings show the potential for mHealth intervention strategies to promote MVPA among YACS identifying with racial or ethnic minority backgrounds.
Notably, the intervention increased MVPA relative to the self-help group among participants in the overweight range (i.e., BMI 25–<30), suggesting that the strategies used may have successfully addressed some PA barriers in this subgroup, but not among those with higher BMI. Participants with lower BMI demonstrated MVPA improvements in both conditions, while those with higher BMI did not increase MVPA regardless of condition and may have needed additional or alternate intervention enhancements and support. Baseline BMI has been shown to moderate adherence to prescribed exercise sessions among breast cancer survivors randomized to various supervised exercise regimens during chemotherapy [45], and to moderate the effects of exercise training on HRQOL among lymphoma patients [17]. Additionally, higher BMI has been associated with lower reported MVPA among AYAs [44], and both lower educational level and higher BMI have been identified as barriers to PA among adults with cancer [46, 47]. To our knowledge, other PA behavior change interventions among cancer survivors have not demonstrated moderation of MVPA outcomes by baseline BMI or educational attainment. Education level was not a moderator of accelerometer-measured MVPA in a previous trial of a PA behavior change intervention among breast cancer survivors [24], whereas we found that YACS with a college degree or higher achieved more MVPA in response to the intervention compared with self-help participants. It has been established that lower education levels are associated with lower PA among cancer survivors [48,49,50], with limited evidence corroborating this relationship among AYAs [44, 51]. Taken together, as individuals with lower education levels, males, and individuals from racial/ethnic minority groups have been underrepresented in studies of PA behavior among YACS [7, 52] and in behavior change intervention trials in cancer survivors in general [53], mHealth strategies show potential to improve PA among these subgroups and future interventions should work to address the unique needs and preferences of these subgroups. Disparities in MVPA attainment among AYAs by educational attainment, race, and BMI point to the need for PA interventions focused on subgroups potentially at risk for lower MVPA (e.g., those with lower education levels, identify as Black, increased BMI) [44]. Future PA interventions should consider mHealth strategies that are specifically tailored to address disparities among these subgroups of YACS who could potentially derive greater benefits from MVPA and mHealth strategies. Strategies such as embedding theory-based components that leverage existing digital tools and connected health data to provide adaptive PA goals, personalized feedback, or text messages that are tailored to individuals’ characteristics and behavioral contexts may be warranted.
Our study identified age and marital status as potential moderators. Although a greater intervention effect on MVPA among participants ages 26–35 was observed, most participants were in this age subgroup, thereby increasing statistical power to detect an effect. The sample included fewer emerging adults, ages 18–25; more research is needed to identify effective intervention strategies for YACS in this age range, as they are less commonly represented in behavioral intervention trials [54]. Previous studies have shown that age may moderate the effects of PA interventions among breast cancer survivors, with some interventions indicating more benefits for older participants in improving physical functioning (> 57 years) [23] and strength and health scores (> 50 years) [20], while another showed stronger effects on aerobic fitness among younger participants (< 50 years) [16]. MVPA outcomes were more favorable among YACS who were married/partnered, which is consistent with previous studies of PA behavior change interventions among breast cancer survivors [24, 28]. This is not unexpected given that social relationships and social support have been identified as facilitators of PA in YACS [55, 56], and research has shown a positive association between social support and PA among AYAs [57].
The intervention had a stronger effect on MVPA than the self-help condition among YACS who reported having stage III/IV cancer. This is contrary to an evaluation of a PA behavior change intervention among breast cancer survivors, which reported no moderation of accelerometer-measured MVPA by cancer stage [24]. We are aware of one previous trial that reported cancer stage as a moderator of PA behavior change intervention effects, with better outcomes on lower extremity pain and physical dysfunction among breast cancer survivors with lower- versus higher-stage disease [27]. However, previous studies in breast cancer and lymphoma patients found that supervised exercise training was more favorable than usual care for improving lean body mass among those with higher-stage, but not lower-stage disease [16, 17]. It is possible the intervention effects among YACS with stage III/IV cancer relate to better response to mHealth intervention strategies, which were designed to address PA barriers and support PA adaptations, over and above the digital tools available to the self-help group. This is notable since a systematic review demonstrated the health benefits of PA for advanced-stage cancer patients and the need for more randomized trials focused on exercise in this population [58]. Given our exploratory analyses and the smaller subgroup of participants with stage III/IV cancer, future replication of these findings and consideration of cancer stage in optimizing PA interventions may be warranted.
Greater MVPA increases, though of smaller magnitudes compared with self-help participants, were observed among intervention participants with no baseline comorbidities or a solid tumor type. These findings differ from a PA behavior change intervention in breast cancer survivors in which comorbidities did not moderate effects on MVPA [24]. In a study of PA determinants among YACS, those reporting no comorbidities were more likely to report meeting PA guidelines [51], so it is plausible that intervention effects were stronger among this subgroup in the current study. Other studies of moderators of PA intervention effects have focused on survivors of individual cancer types, which limits evaluation by subcategories of solid or non-solid tumors. A recent systematic review of 118 studies including 15 cancer types found that reported PA barriers, such as comorbidities, vary across cancer types [59]. Overall, more research is needed to understand how PA intervention effects may vary by cancer type, treatment history, and comorbidities. These may be important variables to consider in future PA interventions for YACS as side effects, comorbidities, and PA barriers related to cancer type and treatments may vary more frequently over time. More precisely tailored and highly adaptive intervention strategies, such as just-in-time adaptive interventions that offer support only during times of need, could potentially address this heterogeneity.
This study contributes to the emerging literature identifying moderators of response to PA interventions among cancer survivors. Study strengths include the randomized controlled design, large sample size and longer duration relative to previous studies in YACS, and accelerometer-measured outcomes. These findings demonstrate the potential for mHealth strategies among subgroups of YACS underrepresented in research (i.e., males, racial/ethnic minority groups) and identified baseline characteristics for which further tailoring and adaptations in future PA interventions may be warranted. Given this was an exploratory study, there are several limitations. Like most previous work, our analyses did not account for combinations of moderators that may identify subgroups who responded favorably to the intervention. The study had limited power to detect group-time interaction effects within subgroups due to large sample size imbalances for some moderator variables (e.g., baseline HRQOL scores). Our measures of cancer stage and comorbidities were self-reported. Finally, findings may not be generalizable to subgroups of YACS not represented in the sample, including non-English speakers or those without internet access.
In conclusion, our findings on potential moderating variables indicate that considering baseline sex, race and ethnicity, BMI, and cancer stage when tailoring future PA interventions for YACS may be warranted. Additionally, further intervention enhancements or alternate strategies may be needed to facilitate improved PA behaviors among subgroups where none were observed (e.g., lower education) as a result of the current intervention. Overall, these results demonstrate the potential of mHealth interventions to promote increased MVPA among subgroups of YACS and underscore the need for more highly tailored and adaptive interventions that are matched to participant characteristics.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–59. https://doi.org/10.3322/caac.21637.
National Institutes of Health. Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Accessed March 25. 2023. https://seer.cancer.gov/statfacts/html/aya.html.
Pugh G, Hough RE, Gravestock HL, Jackson SE, Fisher A. The health behavior information needs and preferences of teenage and young adult cancer survivors. J Adolesc Young Adult Oncol. 2017;6(2):318–26. https://doi.org/10.1089/jayao.2016.0089.
Adams SC, Petrella A, Sabiston CM, et al. Preferences for exercise and physical activity support in adolescent and young adult cancer survivors: a cross-sectional survey. Support Care Cancer. 2021;29(7):4113–27. https://doi.org/10.1007/s00520-020-05897-w.
Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for Cancer survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. https://doi.org/10.1249/MSS.0000000000002116.
Adams SC, Herman J, Lega IC, et al. Young Adult Cancer Survivorship: recommendations for patient Follow-up, Exercise Therapy, and Research. JNCI Cancer Spectr. 2021;5(1). https://doi.org/10.1093/jncics/pkaa099.
Brunet J, Wurz A, Shallwani SM. A scoping review of studies exploring physical activity among adolescents and young adults diagnosed with cancer. Psychooncology. 2018;27(8):1875–88. https://doi.org/10.1002/pon.4743.
Bélanger LJ, Mummery WK, Clark AM, Courneya KS. Effects of targeted print materials on physical activity and quality of life in young adult cancer survivors during and after treatment: an exploratory randomized controlled trial. J Adolesc Young Adult Oncol. 2014;3(2):83–91. https://doi.org/10.1089/jayao.2013.0021.
Rabin C, Dunsiger S, Ness KK, Marcus BH. Internet-based physical activity intervention targeting young adult cancer survivors. J Adolesc Young Adult Oncol. 2011;1(4):188–94.
Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):41–7. https://doi.org/10.1089/jayao.2015.0033.
Valle CG, Tate DF, Mayer DK, Allicock M, Cai J. A randomized trial of a Facebook-based physical activity intervention for young adult cancer survivors. J Cancer Surviv. 2013;7(3):355–68. https://doi.org/10.1007/s11764-013-0279-5.
Johnson AM, Baker KS, Haviland MJ, et al. A pilot randomized controlled trial of a fitbit- and Facebook-based physical activity intervention for Young Adult Cancer survivors. J Adolesc Young Adult Oncol. October 2021;22. https://doi.org/10.1089/jayao.2021.0056.
Wurz A, Brunet J. Exploring the feasibility and acceptability of a mixed-methods pilot randomized controlled trial testing a 12-week physical activity intervention with adolescent and young adult cancer survivors. Pilot Feasibility Stud. 2019;5:154. https://doi.org/10.1186/s40814-019-0530-6.
Buffart LM, Newton RU, Chinapaw MJ, et al. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer. 2015;121(16):2821–30. https://doi.org/10.1002/cncr.29406.
Kalter J, Buffart LM, Korstjens I, et al. Moderators of the effects of group-based physical exercise on cancer survivors’ quality of life. Support Care Cancer. 2015;23(9):2623–31. https://doi.org/10.1007/s00520-015-2622-z.
Courneya KS, McKenzie DC, Mackey JR, et al. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer. 2008;112(8):1845–53. https://doi.org/10.1002/cncr.23379.
Courneya KS, Sellar CM, Stevinson C, et al. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2600–7. https://doi.org/10.1158/1055-9965.EPI-09-0504.
Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–43. https://doi.org/10.1093/annonc/mdu374.
Dieli-Conwright CM, Fox FS, Tripathy D, et al. Hispanic ethnicity as a moderator of the effects of aerobic and resistance exercise on physical fitness and quality-of-life in breast cancer survivors. J Cancer Surviv. 2021;15(1):127–39. https://doi.org/10.1007/s11764-020-00918-3.
Speck RM, Gross CR, Hormes JM, et al. Changes in the body image and relationship scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–30. https://doi.org/10.1007/s10549-009-0550-7.
Dieli-Conwright CM, Sweeney FC, Courneya KS, et al. Hispanic ethnicity as a moderator of the effects of aerobic and resistance exercise in survivors of breast cancer. Cancer. 2019;125(6):910–20. https://doi.org/10.1002/cncr.31879.
Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115(20):4874–84. https://doi.org/10.1002/cncr.24551.
Pinto B, Stein K, Dunsiger S. Peer mentorship to promote physical activity among cancer survivors: effects on quality of life. Psychooncology June. 2015;25. https://doi.org/10.1002/pon.3884.
Schleicher E, McAuley E, Courneya KS, et al. Moderators of physical activity and quality of life response to a physical activity intervention for breast cancer survivors. Support Care Cancer. 2022;31(1):53. https://doi.org/10.1007/s00520-022-07477-6.
Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100.
Turner RR, Steed L, Quirk H, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9(9):CD010192. https://doi.org/10.1002/14651858.CD010192.pub3.
Rogers LQ, Courneya KS, Carter SJ, et al. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat. 2016;159(2):283–91. https://doi.org/10.1007/s10549-016-3945-2.
Pinto BM, Dunsiger SI, Kindred MM, Mitchell S. Peer mentoring for physical activity adoption and maintenance among breast cancer survivors: moderators of physical activity outcomes. J Cancer Surviv January. 2022;7. https://doi.org/10.1007/s11764-021-01162-z.
Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. https://doi.org/10.1097/00006199-200701000-00003.
Mama SK, Song J, Ortiz A, et al. Longitudinal social cognitive influences on physical activity and sedentary time in hispanic breast cancer survivors. Psychooncology. 2017;26(2):214–21. https://doi.org/10.1002/pon.4026.
Valle CG, Diamond MA, Heiling HM, et al. Effect of an mHealth intervention on physical activity outcomes among young adult cancer survivors: the IMPACT randomized controlled trial. Cancer. 2023;129(3):461–72. https://doi.org/10.1002/cncr.34556.
Valle CG, Pinto BM, LaRose JG, et al. Promoting physical activity in young adult cancer survivors using mHealth and adaptive tailored feedback strategies: design of the improving physical activity after Cancer Treatment (IMPACT) randomized controlled trial. Contemp Clin Trials. 2021;103:106293. https://doi.org/10.1016/j.cct.2021.106293.
Valle CG, Diamond MA, Heiling HM, et al. Physical activity maintenance among young adult cancer survivors in an mHealth intervention: twelve-month outcomes from the IMPACT randomized controlled trial. Cancer Med. June 2023;14. https://doi.org/10.1002/cam4.6238.
Valle CG, Camp LN, Diamond M, et al. Recruitment of young adult cancer survivors into a randomized controlled trial of an mHealth physical activity intervention. Trials. 2022;23(1):254. https://doi.org/10.1186/s13063-022-06148-5.
Evenson KR, Terry JW. Assessment of differing definitions of accelerometer nonwear time. Res Q Exerc Sport. 2009;80(2):355–62. https://doi.org/10.1080/02701367.2009.10599570.
Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44(10):2009–16. https://doi.org/10.1249/MSS.0b013e318258cb36.
Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. https://doi.org/10.1249/mss.0b013e31815a51b3.
Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for americans. JAMA. 2018;320:2020–8. https://doi.org/10.1001/jama.2018.14854.
Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. User’s manual for the SF-36v2 Health Survey: second edition. Linc RI: QualityMetric Incorporated. 2007.
Reulen RC, Zeegers MP, Jenkinson C, et al. The use of the SF-36 questionnaire in adult survivors of childhood cancer: evaluation of data quality, score reliability, and scaling assumptions. Health Qual Life Outcomes. 2006;4:77. https://doi.org/10.1186/1477-7525-4-77.
Pranikoff S, Ayer Miller VL, Heiling H, et al. Frail young adult cancer survivors experience poor health-related quality of life. Cancer. 2022;128(12):2375–83. https://doi.org/10.1002/cncr.34196.
Singh B, Spence RR, Sandler CX, Tanner J, Hayes SC. Feasibility and effect of a physical activity counselling session with or without provision of an activity tracker on maintenance of physical activity in women with breast cancer - A randomised controlled trial. J Sci Med Sport. 2020;23(3):283–90. https://doi.org/10.1016/j.jsams.2019.09.019.
Hair BY, Hayes S, Tse C-K, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–82. https://doi.org/10.1002/cncr.28630.
Berkman AM, Andersen CR, Tang K, Gilchrist SC, Roth ME. Disparities in physical activity in adolescent and young adult cancer survivors. J Cancer Surviv. 2023;17(3):848–58. https://doi.org/10.1007/s11764-022-01264-2.
Courneya KS, Segal RJ, Gelmon K, et al. Predictors of adherence to different types and doses of supervised exercise during breast cancer chemotherapy. Int J Behav Nutr Phys Act. 2014;11:85. https://doi.org/10.1186/s12966-014-0085-0.
Depenbusch J, Wiskemann J, Haussmann A, et al. Impact and determinants of structural barriers on physical activity in people with Cancer. Int J Behav Med. 2022;29(3):308–20. https://doi.org/10.1007/s12529-021-10014-0.
Romero SAD, Brown JC, Bauml JM, et al. Barriers to physical activity: a study of academic and community cancer survivors with pain. J Cancer Surviv. 2018;12(6):744–52. https://doi.org/10.1007/s11764-018-0711-y.
Naik H, Qiu X, Brown MC, et al. Socioeconomic status and lifestyle behaviours in cancer survivors: smoking and physical activity. Curr Oncol. 2016;23(6):e546–55. https://doi.org/10.3747/co.23.3166.
Schmidt ME, Wiskemann J, Ulrich CM, Schneeweiss A, Steindorf K. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol. 2017;56(4):618–27. https://doi.org/10.1080/0284186X.2016.1275776.
Steindorf K, Depenbusch J, Haussmann A, et al. Change patterns and determinants of physical activity differ between breast, prostate, and colorectal cancer patients. Support Care Cancer. 2020;28(7):3207–18. https://doi.org/10.1007/s00520-019-05097-1.
Bélanger LJ, Plotnikoff RC, Clark AM, Courneya KS. Determinants of physical activity in young adult cancer survivors. Am J Health Behav. 2012;36(4):483–94. https://doi.org/10.5993/AJHB.36.4.5.
Munoz AR, Kaiser K, Yanez B, et al. Cancer experiences and health-related quality of life among racial and ethnic minority survivors of young adult cancer: a mixed methods study. Support Care Cancer. 2016;24(12):4861–70. https://doi.org/10.1007/s00520-016-3340-x.
Grimmett C, Corbett T, Brunet J, et al. Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int J Behav Nutr Phys Act. 2019;16(1):37. https://doi.org/10.1186/s12966-019-0787-4.
Gooding HC, Gidding SS, Moran AE, et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a national heart, lung, and blood institute working group. J Am Heart Assoc. 2020;9(19):e016115. https://doi.org/10.1161/JAHA.120.016115.
Wu YP, Yi J, McClellan J, et al. Barriers and facilitators of healthy diet and exercise among adolescent and young adult cancer survivors: implications for behavioral interventions. J Adolesc Young Adult Oncol. 2015;4(4):184–91. https://doi.org/10.1089/jayao.2015.0028.
Adamovich T, Watson R, Murdoch S, et al. Barriers and facilitators to physical activity participation for child, adolescent, and young adult cancer survivors: a systematic review. J Cancer Surviv June. 2022;4. https://doi.org/10.1007/s11764-022-01217-9.
Love C, Sabiston CM. Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psycho-oncology. 2011;20(3):278–86.
Rodríguez-Cañamero S, Cobo-Cuenca AI, Carmona-Torres JM, et al. Impact of physical exercise in advanced-stage cancer patients: systematic review and meta-analysis. Cancer Med. 2022;11(19):3714–27. https://doi.org/10.1002/cam4.4746.
Gildea GC, Spence RR, Jones TL, et al. Barriers, facilitators, perceptions and preferences influencing physical activity participation, and the similarities and differences between cancer types and treatment stages - a systematic rapid review. Prev Med Rep. 2023;34:102255. https://doi.org/10.1016/j.pmedr.2023.102255.
Acknowledgements
We are grateful for the valuable contributions of study team members, including Dr. Donald Rosenstein, Karen Hatley, Dr. Kristen Polzien, Dr. Lindsey Camp, Erin Coffman, Susanna Choi, Kayla Warechowski, and Miriam Chisholm. We thank Lauren Lux, Dr. Andrew Smitherman, Dr. Eliza Park, Dr. Allison Lazard, and community-based organizations that helped with study recruitment. We gratefully acknowledge the young adult cancer survivors who participated in the study.
Funding
This work was supported by the National Cancer Institute (R01CA204965 to CGV); the UNC Connected Health Applications & Interventions Core [funded through the UNC Nutrition Obesity Research Center (National Institute of Diabetes and Digestive and Kidney Diseases-funded; P30DK056350) and the Lineberger Comprehensive Cancer Center (National Cancer Institute-funded; P30 CA016086)]; the UNC Health Registry (funded in part by the UNC Lineberger Comprehensive Cancer Center’s University Cancer Research Fund; and the National Center for Advancing Translational Sciences (UL1TR002489, supporting REDCap and UNC Carolina Data Warehouse for Health).
Author information
Authors and Affiliations
Contributions
Conceptualization: CGV, CMR, DFT; Data curation: DPH, BTN; Formal analysis: HHH, AMD, DPH, BTN; Funding acquisition: CGV, CMR, BMP, JGL, DFT; Investigation: CGV, MAD, BTN; Methodology: CGV, HHH, AMD, DPH, BMP, JGL, DFT; Project administration: CGV, MAD, BTN; Supervision: CGV; Validation: MAD, DPH, BTN; Visualization: CGV, HHH, AMD; Writing – original draft: CGV, HHH, AMD; Writing – review & editing: CGV, HHH, AMD, MAD, DPH, BTN, CMR, BMP, JGL, DFT.
Corresponding author
Ethics declarations
Ethics approval
All procedures were approved by the University of North Carolina at Chapel Hill Oncology Protocol Review Committee (LCCC1707) and Institutional Review Board (#16-3409) and performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valle, C.G., Heiling, H.M., Deal, A.M. et al. Examining sociodemographic and health-related characteristics as moderators of an mHealth intervention on physical activity outcomes in young adult cancer survivors. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-024-01577-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-024-01577-4