Abstract
Purpose
Integrating mHealth into the cancer care continuum may be an effective strategy to improve cancer survivorship care by supporting self-management. We aim to assess the effectiveness of mHealth applications (apps) for self-management in improving pain, psychological distress, fatigue, or sleep outcomes in adult cancer survivors.
Methods
Experimental quantitative studies evaluating apps aiming to support self-management for adult cancer survivors and reporting pain, psychological distress, fatigue, or sleep outcomes were included. PubMed, Web of Science, Embase, CINAHL, PsycINFO, Scopus, and CENTRAL databases were searched from inception through December 2017. Risk of bias was assessed using the Cochrane risk of bias tool (PROSPERO registration number CRD42017081182).
Results
Seven studies of six mHealth interventions (n = 949 participants) were included. Two randomized controlled trials (RCTs), one quasi-RCT, one non-RCT, and three single-arm studies involved survivors with a mix of cancer types. The most common app features were symptom questionnaires (n = 5) and progress tracking (n = 5). Four studies reported outcomes for pain, with three showing improvements. Two studies reported psychological distress outcomes, showing mixed results. Four studies reported improvements in fatigue post-intervention or in the intervention compared with control group, but the changes were not all statistically significant. Two studies reported improvements in sleep outcomes.
Conclusions
There is emerging evidence that mHealth interventions that support self-management can improve pain and fatigue outcomes in cancer survivors, and some promise for psychological distress and sleep outcomes. Further development and investigation of mHealth is needed, incorporating targeted, evidence-based models of care into app design.
Implications for Cancer Survivors
mHealth interventions can improve outcomes for cancer survivors and have significant potential to benefit this growing population due to their reach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
It has been estimated that there were 2.1 billion smartphone users in the world in 2016 [1] and over 100,000 mobile software applications (apps) for health management currently available on Google Play and iTunes [2]. Healthcare interventions delivered via personal mobile device (mHealth) have been shown to be acceptable to users, who have identified benefits such as convenience, access to personalized information, greater awareness of own health, and ability to interconnect with other apps, other users, and health professionals [3, 4]. Furthermore, the high uptake of mHealth and the projected growth of this industry indicate interest from users and developers alike [4].
Integrating mHealth into the cancer care continuum may be an effective strategy to improve cancer survivorship care by supporting self-management. Prior research focusing on the design and usability of interventions for this population has yielded guidelines for development and implementation [5,6,7]. However, much of this research also indicated the need for further investigation into the ability of mHealth to improve the lives of cancer survivors. Uncertainty remains regarding whether mHealth can measurably improve outcomes relating to health issues that commonly affect this population [8].
Cancer survivors suffer from a variety of symptoms that can impact their quality of life when uncontrolled or poorly managed [9]. Cancer-related fatigue is estimated to affect between 58 and 90% of cancer survivors, making it the most prevalent symptom [10]. An analysis of the 2002 National Health Survey (USA) revealed pain, psychological distress, and insomnia in 34%, 26% and 30% of cancer survivors, respectively, significantly higher than in controls representing the general population [11]. These symptoms are often considered as part of a symptom cluster as they frequently occur together and may be linked by common biological processes [8]. They are often under-diagnosed and difficult to treat, and can persist for years after the completion of treatment, impacting survivors’ engagement in work, personal, and social activities [9]. Evidence for the efficacy of interventions for cancer-related symptoms of pain, fatigue, distress, and sleep is mixed. Brebach and colleagues found high acceptability of telephone-delivered interventions for distress; however, uptake was low and efficacy not clearly evident to date [12]. A recent review of self-management interventions for cancer survivors found it difficult to draw conclusions of their impact due to the heterogeneity of the interventions but highlighted that sustainability of the interventions was poor, suggesting that cancer survivors need a type of intervention that can be readily used in daily life [13]. Pharmacological treatment of cancer-related fatigue and insomnia is commonplace but is only recommended for short periods, while evidence for non-pharmacological approaches is building and includes exercise programs, yoga, cognitive behavioral therapy for insomnia, and sleep hygiene awareness [9].
mHealth apps that incorporate evidence-based interventions into their design may have the potential to deliver high quality, accessible care. However, in order to be cost effective and broadly accessible, interventions need to be evaluated in terms of their effectiveness in supporting self-management [5]. Uptake and usage should also be assessed, in order to identify socio-demographic characteristics associated with improved outcomes and populations most likely to benefit [14].
Therefore, this systematic review aims to assess the role of mHealth interventions for cancer survivors, specifically considering:
-
1.
Does mHealth used for self-management improve pain, psychological distress, fatigue, or sleep outcomes in cancer survivors?
-
2.
What factors influence uptake and usage of mHealth used for self-management among cancer survivors? What effect do uptake and usage have on pain, psychological distress, fatigue, and sleep outcomes?
Method
The protocol for this systematic review was registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO), registration number CRD42017081182. The review was conducted adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [15].
Selection criteria
Experimental quantitative studies evaluating mHealth apps for self-management for adult cancer survivors, reporting outcomes relating to pain, psychological distress, fatigue, or sleep, were included in the review. The full eligibility criteria are shown in Box 1. Studies were selected for inclusion if the intervention supported self-management, as defined by the Institute of Medicine’s (IOM) 2003 report, as “the systematic provision of education and supportive interventions by health care staff to increase patients’ skills and confidence in managing their health problems, including regular assessment of progress and problems, goal setting and problem-solving support” [16, p. 57]. The criteria for inclusion or exclusion of an article were chosen to identify high quality research articles reporting studies conducted in a population representative of the general population of cancer survivors that investigated mHealth apps designed for self-management for analysis in this review.
Box 1 Eligibility criteria for study inclusion in this systematic review
Inclusion criteria: 1) Experimental quantitative design, including pilot and single-arm studies. 2) Population of cancer survivors aged 18+ years with any cancer type/stage, including those receiving anticancer treatment, those in remission, those considered cured, and those in the terminal phases of the disease. 3) An application downloaded onto a personal mobile device, either smartphone or tablet (mHealth) was a major part of the intervention. 4) The intervention involved some component of self-management, including interventions that involve caregivers or health professionals in a secondary capacity. 5) Study measured and reported at least one outcome in one or more of four domains: pain, psychological distress, fatigue, or sleep. 6) Full-text report of study outcomes published in peer-reviewed journal in English. Exclusion criteria: 1) Studies of health professionals, caregivers, or mixed populations where outcomes for cancer survivors could not be extracted. 2) Participants < 18 years; however, studies involving survivors who were children at the time of diagnosis but aged 18+ years at the time of participation were included. 3) Studies of interventions delivered via website, messaging service, telephone, or videoconference only without a mobile app component (e.g., apps solely focused on delivering videoconferencing or messaging) were excluded. |
Search strategy
Searches of PubMed, Web of Science, Embase, CINAHL (via Web of Science), PsycINFO (via EBSCOhost), Scopus, and CENTRAL databases from inception through 2017 were performed by one author (EHS) to identify relevant studies, using search terms related to (1) cancer survivors (population); (2) mobile health applications (intervention); and (3) pain, psychological distress, fatigue, or sleep (outcomes). Relevant search terms were identified using Medical Subject Headings (MeSH) and database thesauri. Table 1 summarizes the search strategy, and the full search string including dates of coverage for each database can be found in Supplement 1. The date of final searches was 26 December 2017. Search results were captured using citation management software, and duplicates were removed. The reference lists of relevant articles were hand searched, and additional records identified were added to the database search results.
Study selection
Titles and abstracts of articles found via searches were considered against the eligibility criteria by one author (EHS). If an article met the criteria or it was unclear whether an article met the more specific criteria, the full text was reviewed. The full text of ambiguous articles was reviewed and discussed by all authors. A selection of 20 full-text articles (the first 10 articles included, and the first 10 excluded by EHS) were reviewed by a second author (DL or SL). The reasons for inclusion or exclusion were discussed by all three authors for consensus and to ensure the screening process was reliable and consistent. Three articles that had been selected for inclusion were subsequently excluded.
Data collection
The following information was extracted from studies which met the selection criteria for inclusion: publication date, primary authors, country of study, source of funding, conflicts of interest, study design and sample size, characteristics of the population, features of the intervention, results relating to pain, psychological distress, fatigue, or sleep outcomes, and intervention uptake or use. It was intended to extract data relating to associations between uptake or use and other factors (e.g., sociodemographic variables) to identify factors influencing mHealth app uptake or use, but no studies reported such data. Similarly, it was intended to extract data on any associations between uptake or use and review outcomes (i.e., pain, psychological distress, fatigue, or pain), but data were not available.
Risk of bias was assessed using the Cochrane risk of bias tool [17]. One author (EHS) performed the assessment in seven domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias) for each individual study. All authors reviewed and agreed on the results of each assessment.
Data synthesis
Aggregate data was used to perform a narrative, descriptive synthesis with studies grouped according to study characteristics, features of the intervention, and impact of the intervention on outcomes reported. Within each category of outcomes (pain, psychological distress, fatigue, sleep), the results were narratively summarized by the investigators (EHS, DL). Data relating to uptake, use, adherence, and user satisfaction were also narratively summarized.
Results
Search results
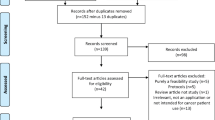
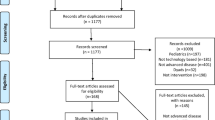
The systematic search revealed 927 original articles. Two hundred eleven articles were assessed at full-text level and seven articles from seven studies were included in the final synthesis. The PRISMA flowchart in Fig. 1 details the selection and screening process.
Characteristics of included studies
Of the seven studies selected for inclusion, two were randomized controlled trials [18, 19], one was quasi-randomized [20], one was non-randomized with a control group [21], and three were single-arm studies [22,23,24]. Sample sizes ranged from 16 [24] to 356 [20], and four of the studies were pilot or feasibility studies. Table 2 details the included studies. Most studies were conducted in the USA [19, 22, 23] or UK [18, 24]. One study was conducted in Korea [20] and one in Sweden [21]. The mean age of participants across all studies was 58.5 years, ranged from 50.3[20] to 69 years [21]. Four studies included participants with a diagnosis of breast cancer [18, 20, 22, 23]; two included participants with a diagnosis of lung cancer [18, 24], colorectal cancer [18, 19], and prostate cancer, [21, 22] respectively. One study also included participants diagnosed with lymphoma [22].
Risk of Bias
The results of the risk of bias assessment performed on each study are demonstrated in Table 3. Five studies [20,21,22,23,24] were found to have a high risk of selection bias due to a non-randomized study design, one study was found to have an unclear risk of selection bias due to inadequate description of the method used to generate a randomized sequence and conceal allocations of participants [19], and only one study was found to have a low risk of selection bias [18]. It is impossible to blind participants and personnel to this type of intervention and so all studies were found to have a high risk of performance bias. No published protocol was found for any of the included studies; thus, the risk of bias due to selective outcome reporting was unclear for all studies. However, as outcome data was self-reported by participants in all studies, the risk of detection bias due to an outcome assessor’s knowledge of which intervention a participant received was low for all studies. One study was found to have a high risk of attrition bias due to high attrition of 48% [18]. Another collected outcome data at three time points (3, 6, and 9 months) but only reported data for one time point (6 months) and so was found to have a high risk of bias due to incomplete outcome data [19]. One study did not report attrition and so the risk of attrition bias was found to be unclear [22].
Characteristics of interventions studied
Six different mHealth apps were used in the seven studies reported in this review. Two studies incorporated the same mHealth app into their design [18, 24], with the app being adapted to suit the population in the second of these studies and the intervention design differing between the two studies. Two interventions aimed to improve the management of patients’ symptoms [18, 21, 24], two interventions aimed to promote health behavior change [19, 20], and two interventions aimed to achieve both of these things [22, 23]. The details of the interventions are summarized in Table 4.
The apps themselves displayed a range of features. The most common of these were self-administered symptom questionnaires [18, 21,22,23,24] and progress tracking over time [19,20,21,22,23], each featured in five interventions. The least common features were survivorship care plans [19] and mind-body exercises provided via app [22]. To engage users, interventions included features such as video demonstrations of prescribed exercises [20, 23], and alerts sent via app [19, 21, 23].
The duration of the recommended period of use ranged from 5 [24] to 24 weeks [19]. All interventions were designed to be used daily. Most of the interventions involved a health care professional. Members of the patients’ treating oncology team were sent patient symptom data for daily monitoring in three of the studies [18, 21, 24]. Exercise programs were provided by a physiotherapist in one intervention [20] and a personal trainer in another [19]. Only one intervention provided an opportunity to network with other users [19]. Three studies tested interventions that commenced while the participant was receiving anticancer treatment [18, 21, 24] while four involved interventions that commenced after the cessation of anticancer treatment [19, 20, 22, 23].
Outcome data related to pain, psychological distress, fatigue, and sleep
Table 5 presents a summary of the outcomes relating to pain, psychological distress, fatigue, and sleep reported in the selected studies. Outcomes were assessed using validated tools in six of the seven studies, most commonly the European Organization for Research and Treatment (EORTC) quality of life tool (QLQ-C30) [20, 21] or distress thermometer [19, 22]. One study used a symptom questionnaire that was developed for the study [18] Table 6.
Four studies (one quasi-randomized trial, one non-randomized trial, and two single-arm studies) reported data relating to pain outcomes using validated instruments such as the Edmonton Symptom Assessment Scale (ESAS) [24], the Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) [23], and the EORTC QLQ-C30 [20, 21]. Both studies that compared intervention group to control group participants reported lower mean pain scores in the intervention group at follow-up, but in neither case did this reach statistical significance [20, 21]. In contrast, both single-arm studies reported statistically significant differences, but in different directions. Fu et al. [23] reported a decrease in the prevalence of pain at 12 weeks (p = 0.031), while Maguire et al. [24] reported an increase in median pain scores over 5 weeks (p = 0.04).
Two studies (one randomized controlled trial and one single-arm study) examined psychological distress both using the distress thermometer. In a single-arm study, Smith et al. [22] reported a mean reduction in psychological distress of 1.6 (standard deviation, SD 2.8) over the first 4-week period of their study and 1.3 (SD 3.1) over the total 8-week study period. In a randomized controlled trial, Mayer et al. [19] found no difference in the reported psychological distress when comparing an intervention group with a control group (p > 0.17).
Fatigue outcomes improved in four studies (one randomized controlled trial, one quasi-randomized trial, one non-randomized trial, and one single-arm study) that reported data relating to this symptom [18, 20, 21, 24]. The improvement reached statistical significance in two studies. In a randomized trial, Kearney et al. [18] reported a lower average prevalence of fatigue over a 2-week period (maximum four blocks of 2 weeks per participant) of 67% in the intervention group compared with 81% in the group receiving usual care (p = 0.04), using a symptom questionnaire developed for the study. In a non-randomized trial, Sundberg et al. [21] used the EORTC QLQ-C30 and reported lower mean fatigue of 25.7 (SD 21.5) versus 34.3 (SD 22.9) when comparing groups at first follow-up (5 or 8 weeks) (p = 0.047). At second follow-up (17 or 20 weeks), mean fatigue decreased further to 22.8 (SD 19.5) in the intervention group and 29.4 (SD 19.9) in the control group (p = 0.073). Non-statistically significant improvements were shown in two further studies. Maguire et al. [24] reported a decrease in median fatigue scores over 5 weeks using the ESAS (p > 0.05) in a single-arm study, and Uhm et al. [20] reported a lower mean fatigue in intervention group participants compared with control group participants at 12 weeks using the BCLE-SEI (p = 0.517) in a quasi-randomized trial.
Sleep outcomes also improved in two studies (one quasi-randomized trial and one non-randomized trial) that reported data relating to insomnia or sleep disturbance. Both studies used the EORTC QLQ-C30 to measure reports of insomnia. In a non-randomized trial, Sundberg et al. [21] reported lower mean insomnia of 18.6 (SD 25.7) in the intervention group versus 33.9 (SD 32.2) in the control group at first follow-up (5 or 8 weeks) (p = 0.005), and 18.6 (SD 24.7) in the intervention group versus 29.6 (SD 30.8) in the control group at second follow-up (17 or 20 weeks) (p = 0.035). In a quasi-randomized trial, Uhm et al. [20] also reported lower mean insomnia in intervention group participants compared with control group participants at 12 weeks but this did not reach the statistical significance (p = 0.224).
Intervention use
Three studies provided data on the uptake of the apps, reporting that 100% (n = 56) [18], 94% (n = 135) [19], and 100% (n = 66) [21] of participants commenced using the apps provided. Four studies reported that over 90% of participants continued the use of the intervention over the study period [20,21,22, 24]. Participants in the study conducted by Mayer et al. [19] accessed the progress tracking and networking features of the app most frequently. Kearney et al. [18] reported that 54% of the participants in their study continued the use over the study period, and two participants stated that they discontinued the use of the intervention because they did not like the mobile device. Mayer et al. [19] reported a change in app usage over time, showing greatest use in the first week and declining the use over the 6-month period. User satisfaction with the apps (reported by three studies) was high [20, 22, 24].
Discussion
This systematic review examined seven studies of six mHealth interventions to assess whether mHealth used for self-management improves pain, psychological distress, fatigue, or sleep outcomes for cancer survivors. Overall, the findings are promising, demonstrating the emergence of potential benefits of mHealth for this group. The analysis-indicated evidence was strongest for improving fatigue outcomes, mixed for pain and still developing for psychological distress and sleep, as few studies examined these outcomes.
The most promising findings of this review are the improvements in fatigue outcomes. Two of the apps shown to improve fatigue across three studies (one randomized controlled trial, one non-randomized controlled trial, and one single-arm study) [18, 21, 24] were similar, involving tailored self-management advice received via the app after submitting a symptom questionnaire/check-in. This could indicate that apps that gather patient-reported outcomes and provide supported self-management have a role in the management of cancer-related fatigue. A third app, which delivered an exercise program, was also shown to improve fatigue in a quasi-randomized controlled trial [20]. It is known that exercise is effective in combatting fatigue in cancer survivors [25]. In this case, it is possible that the improvement is attributable to the app supporting adherence to the prescribed exercise program, as 93% of users were reported to have continued to follow their programs for the duration of the 12-week study period. Positive findings were also reported for pain outcomes in three studies of varying designs that evaluated three significantly different interventions [20, 21, 23].
Despite these differences, in each instance where improvements in pain or fatigue were seen, the app was designed specifically for the target population, delivered an evidence-based intervention that was customizable, and could be accessed at any time to deliver real-time benefits to the user. These findings highlight a potential mechanism by which mHealth can be used to improve outcomes for cancer survivors, regardless of specific app design features.
In two instances, the use of mHealth interventions did not lead to an improvement in outcomes. Maguire et al. [24] reported an increase in median pain score over time in study participants provided with the app. Notably, this was the only group studied in this review for which outcomes worsened. The design of this study did not include a control group for comparison, and the sample size (16 participants diagnosed with lung cancer receiving radiation treatment) was small. Five participants died during the 5-week study period, which could suggest that the increase in pain scores may be attributable to disease stage or acute side effects of the treatment regimen, which have been shown to include increasing pain during and for 2 weeks after radiation treatment [26].
The second study to show no improvement in outcomes was a randomized controlled trial by Mayer et al. [19], who reported no difference in psychological distress between groups of colon cancer survivors in a randomized controlled trial. The intervention group was provided with an exercise program via mobile app, and the control group was provided with a paper-based version of the same exercise program. Both groups showed an equal reduction in distress, suggesting that the program was equally beneficial for participants regardless of mode of delivery.
Of note, Smith et al. [22] reported a statistically significant reduction in psychological distress in a single-arm study of a group of cancer survivors who had been diagnosed with post-traumatic stress disorder (PTSD) with the trauma being their diagnosis and/or treatment. Participants were provided with an app designed specifically to help self-manage PTSD. The improvements may be partly attributable to the fact that the study participants had higher levels of distress at baseline than the general population of cancer survivors and therefore had a greater capacity to benefit. This study further highlights the potential for apps to produce improvements when designed specifically for users and deliver evidence-based interventions to support self-management in real time. In contrast, only two of the studies analyzed in this review reported sleep outcomes [20, 21], which were reported due to being included as part of the EORTC QLQ-C30 questionnaire aimed to assess quality of life in general. The results of the systematic search did not include any studies that aimed to improve sleep outcomes specifically or any interventions designed to track, monitor, or improve sleep, which could indicating a gap in the current research.
Associations between specific characteristics of the populations studied and the outcomes in terms of symptoms or uptake/usage were not investigated by the studies analyzed. Interestingly, participant age was relatively high across studies, with the mean varying from 50 [20] to 69 [21] years, challenging perceptions that mHealth is less likely to be used by older populations [14]. This is in line with previous research showing that older patients can benefit from mHealth interventions which have been designed with the population in mind [27], and that while smartphone ownership rates may be lower in older populations, their willingness to use and engage with mHealth is equal to that of younger populations [28]. Furthermore, study participants represented populations with a range of common cancer diagnoses (such as breast, lung, bowel, lymphoma, and prostate), both males and females, and populations from a mix of countries, although the USA and UK were represented most frequently. This supports the position that mHealth has a large potential reach that can transcend diverse population characteristics.
Our analysis did not reveal sufficient data to draw conclusions about factors influencing the use of mHealth interventions or factors influencing their effect on pain, psychological distress, fatigue, and sleep outcomes. Although the majority of studies collected some data related to uptake, usage, adherence, or user satisfaction, it was not consistently evaluated or reported. The small number of articles included in the review limited the ability to find common themes across studies; however, it should be noted that user satisfaction and perceptions were consistently positive in the three studies that gathered this data [20, 22, 24].
Evidence for the use of mHealth to support self-management of a range of health-related outcomes among cancer survivors is building. The challenge to provide care to this growing population, many of whom have complex symptoms and co-morbidities, has been identified [29] and calls for novel and innovative interventions and models of care. With 500 million people using mobile apps for health in 2014 [30], there is significant interest in utilizing mHealth as part of these care models. This review has demonstrated promising findings for a positive association between mHealth interventions and improved outcomes in cancer survivors. However, further research is needed in this area to identify clinically significant benefits, potential limitations, factors influencing outcomes and develop guidelines for best practice.
Recommendations
Continued investigation of the effect of mHealth interventions on outcomes in cancer survivors should be undertaken, using tools which are validated and consistent across studies to collect outcome data so that studies can be compared. Results reported in single-arm studies should be confirmed in trials including a control or comparison group. It should be noted that small improvements discovered in clinical trials could translate into large benefits among the general population due to the reach of mHealth technology. Further development of novel mHealth interventions to support self-management should be encouraged, incorporating evidence-based models of care into app design and focusing on addressing the gaps in the needs of the cancer-survivor population which are not currently being met. Uptake and usage should be reported in future studies of outcomes to allow the relationships between these factors and any improvements to be identified, paving the way for the most effective translation of this research into practice.
Strengths and limitations
This review extends the knowledge base for mHealth interventions to support self-management of common symptoms experienced by cancer survivors in terms of their efficacy and use. The review used a rigorous approach to quality assessment, providing a greater understanding of the limitations of research to date. The main limitation of this study was the screening of articles at the title/abstract level being conducted by one author only. However, two other authors were consulted for discussion of any queries relating to the screening process, and the full text of any ambiguous articles was accessed and reviewed by the second author. While every attempt was made to capture all applicable results for inclusion in this systematic review, the possibility remains that a relevant study has not been included for analysis. Authors employed a broad search strategy designed to capture differences in terminology relating to mHealth and to include populations that represented the general population of cancer survivors in order to prevent this eventuality.
Conclusions
There is emerging evidence that mHealth interventions that support self-management can improve pain and fatigue outcomes in cancer survivors, and some promise that psychological distress and sleep outcomes can also be improved. The benefits of the broad reach of mHealth were demonstrated by the improvements realized across cancer types and population groups. Further investigation is required to determine the applications and limitations of these findings and to evaluate the factors such as app design features or population characteristics that contribute to improvements in outcomes.
References
Statista. Number of smartphone users worldwide from 2014 to 2020. 2018. https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/.
Mendiola M, Kalnicki M, Lindenauer S. Valuable features in mobile health apps for patients and consumers: content analysis of apps and user ratings. JMIR mHealth uHealth. 2015;3(2):e40. https://doi.org/10.2196/mhealth.4283.
Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self-care: a qualitative study of user experiences. Report. 2016;11(5):e0156164. https://doi.org/10.1371/journal.pone.0156164.
Birkhoff SD, Smeltzer SC. Perceptions of smartphone user-centered mobile health tracking apps across various chronic illness populations: an integrative review. J Nurs Scholarsh. 2017;49(4):371–8. https://doi.org/10.1111/jnu.12298.
Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498–504. https://doi.org/10.1007/s11764-017-0608-1.
Darlow S, Wen KY. Development testing of mobile health interventions for cancer patient self-management: a review. Health Informatics Journal. 2016;22(3):633–50. https://doi.org/10.1177/1460458215577994.
Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013;15(12). https://doi.org/10.2196/jmir.2661.
Aaronson NK, Mattioli V, Minton O, Weis J, Johansen C, Dalton SO, et al. Beyond treatment - psychosocial and behavioural issues in cancer survivorship research and practice. EJC Suppl. 2014;12(1):54–64. https://doi.org/10.1016/j.ejcsup.2014.03.005.
Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(30):3687–96. https://doi.org/10.1200/JCO.2012.41.7238.
Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006;42(7):846–63. https://doi.org/10.1016/j.ejca.2005.11.026.
Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. Journal of the American Board of Family Medicine : JABFM. 2007;20(5):434–43. https://doi.org/10.3122/jabfm.2007.05.060225.
Brebach R, Sharpe L, Costa DSJ, Rhodes P, Butow P. Psychological intervention targeting distress for cancer patients: a meta-analytic study investigating uptake and adherence. Psychooncology. 2016;25:882–90. https://doi.org/10.1002/pon.4099.
Boland L, Bennett K, Connolly D. Self-management interventions for cancer survivors: a systematic review. Support Care Cancer. 2018;26(5):1585–95. https://doi.org/10.1007/s00520-017-3999-7.
Carroll J, Moorhead A, Bond R, Leblanc W, Petrella R, Fiscella K. Who uses mobile phone health apps and does use matter? A secondary data analytics approach. J Med Internet Res. 2017;19(4):E125. https://doi.org/10.2196/jmir.5604.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Adams KM, Corrigan J. Priority areas for national action : transforming health care quality. Washington, D.C.: National Academies Press; 2003.
The Cochrane Collaboration. Assessing risk of bias in included studies. In: Higgins JPT AD, Sterne JAC, editor. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Online: The Cochrane Collaboration; 2011.
Kearney N, McCann L, Norrie J, Taylor L, Gray P, McGee-Lennon M, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009;17(4):437–44. https://doi.org/10.1007/s00520-008-0515-0.
Mayer DK, Landucci G, Awoyinka L, Atwood AK, Carmack CL, Demark-Wahnefried W, et al. SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? J Cancer Surviv. 2017;12:1–13. https://doi.org/10.1007/s11764-017-0647-7.
Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017;161(3):443–52. https://doi.org/10.1007/s10549-016-4065-8.
Sundberg K, Wengstrom Y, Blomberg K, Halleberg-Nyman M, Frank C, Langius-Eklof A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer. 2017;25:2195–204. https://doi.org/10.1007/s00520-017-3625-8.
Smith SK, Kuhn E, O'Donnell J, Koontz BF, Nelson N, Molloy K, et al. Cancer distress coach: pilot study of a mobile app for managing posttraumatic stress. Psychooncology. 2016. https://doi.org/10.1002/pon.4363.
Fu MR, Axelrod D, Guth AA, Rampertaap K, El-Shammaa N, Hiotis K, et al. mHealth self-care interventions: managing symptoms following breast cancer treatment. Mhealth. 2016;2:28. https://doi.org/10.21037/mhealth.2016.07.03.
Maguire R, Ream E, Richardson A, Connaghan J, Johnston B, Kotronoulas G, et al. Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs. 2015;38(2):E37–47. https://doi.org/10.1097/NCC.0000000000000150.
Kessels E, Husson O, Van Der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;Volume 14:479–94.
Langendijk JA, Aaronson NK, de Jong JM, ten Velde GP, Muller MJ, Lamers RJ, et al. Prospective study on quality of life before and after radical radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2001;19(8):2123–33. https://doi.org/10.1200/JCO.2001.19.8.2123.
Gilbert B, Goodman E, Chadda A, Hatfield D, Forman D, Panch T. The role of mobile health in elderly populations. Current Geriatrics Reports. 2015;4(4):347–52. https://doi.org/10.1007/s13670-015-0145-6.
Abelson JS, Symer M, Peters A, Charlson M, Yeo H. Mobile health apps and recovery after surgery: what are patients willing to do? Am J Surg. 2017;214(4):616–22. https://doi.org/10.1016/j.amjsurg.2017.06.009.
Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomark Prev. 2011;20(10):1996. https://doi.org/10.1158/1055-9965.EPI-11-0729.
Rho M, Kim H, Chung K, Choi I. Factors influencing the acceptance of telemedicine for diabetes management. The Journal of Networks, Software Tools and Applications. 2015;18(1):321–31. https://doi.org/10.1007/s10586-014-0356-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Hernandez Silva, E., Lawler, S. & Langbecker, D. The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: a systematic review. J Cancer Surviv 13, 97–107 (2019). https://doi.org/10.1007/s11764-018-0730-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-018-0730-8