Abstract
Purpose
A randomized pilot trial evaluated the hypothesis that early intervention lessens sexual dysfunction in the first year on aromatase inhibitors. A secondary aim was comparing the efficacy of two vaginal moisturizers.
Methods
Fifty-seven postmenopausal women with early stage breast cancer starting aromatase inhibitors were randomized to three treatment groups. All received a handout on managing sexual and other side effects. The Usual Care group received no additional therapy. The Active Treatment groups received a 6-month supply of a vaginal moisturizer (hyaluronic acid-based in Active Group-H and prebiotic in Active Group-P) and a vaginal lubricant and dilator, plus access to an educational website and phone coaching. Questionnaires completed at baseline, 6, and 12 months included the Female Sexual Function Index (FSFI), Menopausal Sexual Interest Questionnaire (MSIQ), Female Sexual Distress Scale-Revised (FSDS-R), and a menopausal symptom scale.
Results
Forty-nine women (86%) provided follow-up data. Mean age was 59 and 77% were non-Hispanic Caucasian. Sexual function was impaired at baseline, but remained stable over 12 months for all groups. The combined active treatment group had less dyspareunia (P = 0.07) and sexual distress (P = 0.02) at 6 months than the Usual Care group. At 6 months, the Active-H group improved significantly more than the Active-P group on FSFI total score (P = 0.04).
Conclusions
Sexual counseling helped women maintain stable sexual function on aromatase inhibitors. Active intervention resulted in better outcomes at 6 months.
Implications for Cancer Survivors
This promising pilot trial suggests a need for more research on preventive counseling to maintain sexual function during aromatase inhibitor treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase inhibitors are the preferred adjuvant endocrine therapy for postmenopausal women with estrogen-sensitive early stage breast cancer [1]. Disease-free survival is superior compared to tamoxifen, and 10 years of therapy may be better than 5 [2]. In premenopausal women with estrogen positive tumors, aromatase inhibitors have a slight advantage over tamoxifen when combined with ovarian suppression [3]. However, a recent American Society of Clinical Oncology Clinical Practice Update suggests that individual factors be used to choose between aromatase inhibitors versus tamoxifen for younger women at high risk of recurrence [4].
With increasing numbers of women taking aromatase inhibitors, sexual dysfunction remains a neglected side effect. Severe vaginal atrophy and dyspareunia are far more likely with aromatase inhibitors than tamoxifen in postmenopausal women [5] or during ovarian suppression [3, 4]. Problems with sexual desire and subjective arousal are also more common, [3–5] either secondary to painful sex or as a direct effect of estrogen deprivation in the brain. Serum androgen levels are unchanged during aromatase inhibitor therapy, [6] but growing evidence suggests that estradiol, rather than testosterone, facilitates sexual desire in the female mammalian brain [7].
Trials of aromatase inhibitor therapy typically include only a few questionnaire items measuring sexual function, resulting in continued underestimates of the prevalence and severity of problems [2, 3, 5, 8]. Differences in sexual dysfunction were not significant during a 5-year extension of aromatase inhibitor therapy between women on letrozole versus placebo, but vaginal atrophy and pain were already established from the previous 5 years of treatment. Investigators in the SOFT and TEXT trials acknowledge inadequate assessment of sexual function [3]. Both reports emphasize that global quality of life scores changed little. However, health-related quality of life in survivors of localized breast cancer is typically similar to norms for healthy peers, despite bothersome symptoms documented by specific scales [9, 10]. Assessments of quality of life are also subject to response shift, [11] with cancer survivors recalibrating their ratings across time as they habituate to long-term treatment effects.
Sexual dysfunction related to aromatase inhibitors may also go unrecognized because half of women over age 50 in the USA are sexually inactive, lacking a functional partner [12]. Unpartnered women on aromatase inhibitors are less distressed about sexual problems [8]. Although women continue taking aromatase inhibitors despite sexual dysfunction, they are the group most likely to seek help in sexuality clinics in cancer centers [13].
To gather benchmark data for the current intervention, we surveyed 129 postmenopausal women from UT MD Anderson’s Medical Breast Registry who had been prescribed aromatase inhibitors 18–24 months previously [8]. On the Female Sexual Function Index (FSFI), a widely used multiple-choice questionnaire [14, 15], 93% scored in the dysfunctional range and 75% of this group was distressed about sexual problems. Only 52% of women had been sexually active when endocrine therapy began, but 79% of that group developed new sexual problems. Twenty-four percent discontinued partner sex completely because of dyspareunia.
Aims
We describe a pilot randomized trial in women starting aromatase inhibitors. Women already were postmenopausal with previous breast cancer treatment, so some sexual dysfunction was expected at baseline. Based on our previous work [8, 16, 17], we hypothesized that a combination of sexual counseling, vaginal moisturizers, lubricants, dilation, and pelvic floor muscle exercises would prevent further deterioration of sexual function, compared to usual care. A written handout alone was compared to active treatment. As a secondary aim, we compared the efficacy of two nonhormonal, over-the-counter vaginal moisturizers that had recently become available. Because all women in the pilot trial received written education, data from the benchmark survey provided a historical comparison group more representative of usual care.
Methods
Recruitment
Women were recruited from the breast medical oncology clinics at UT MD Anderson Cancer Center and its satellite clinics between June 2013 and September, 2014. Women were eligible to participate if they had estrogen receptor-positive, localized breast cancer, were postmenopausal, had been prescribed an aromatase inhibitor as their first, adjuvant endocrine therapy, had taken it for less than 4 weeks, were at least age 18, had a sexual partnership of at least 6 months’ duration, and had made at least one attempt at sexual activity in the past 12 months. Women were excluded if unable to speak English or if on estrogen or androgen therapy. Since all were postmenopausal, none were on concurrent medication for ovarian suppression.
The protocol was approved by MD Anderson’s Institutional Review Board. Informed consent was obtained from all individual participants included in the study. Women were randomized, using minimization [18] to three treatment groups based on: age (<65 vs. ≥ 65), ethnicity (non-hispanic white vs. minority), and education (≤ some college vs. ≥ 4-year degree). Minimization was accomplished with a tracking database created for the study.
Intervention procedures
Given results of our benchmark survey [8], we gave all women in the study a booklet, Why It Is Important to Take Your Aromatase Inhibitor (available on request from the corresponding author) encouraging adherence to endocrine therapy. Information was provided on switching between aromatase inhibitors or to tamoxifen, and on self-help strategies and resources for problems with arthralgia, vaginal dryness and pain during sex, hot flashes, and loss of bone density.
Usual Care women had no other intervention. The two active treatment groups differed only in the brand of vaginal moisturizer they received, each getting a 6-month supply. Active Group-H received an over-the-counter product containing a form of hyaluronic acid. Active Group-P received an over-the-counter moisturizer labeled as prebiotic to promote healthy lactobacilli. Both were gels inserted with an applicator at bedtime. Women were instructed to use the moisturizer daily during week 1, and then 2–3 times weekly. Women also received a water-based vaginal lubricant to apply before and during sexual activity, and a silicone vaginal dilator, with size depending on whether the woman was experiencing dyspareunia at baseline (Soulsource® 1 in. × 4 in.) or not (1.15 in. × 4.5 in.). Women were advised to have penetrative sex with a partner and/or to use the dilator at least twice a week.
All women in active treatment received access to a password-protected, website providing detailed help with women’s cancer-related sexual problems [17]. Six phone coaching calls were scheduled during the 12-week treatment period, plus 3 monthly follow-up calls. Calls lasted 15–30 min and included standard questions on frequency of sex, use of the moisturizer, lubricant, and dilator, satisfaction with the vaginal moisturizer, and bother with genital irritation, hot flashes, or joint pain/stiffness.
Main outcome Measures
Questionnaires were completed by mail at baseline, 6 and 12 months. Up to 3 reminder calls were made at each point. Questionnaires were not scored if missing items exceeded the range for validity. Demographic and medical factors were assessed. The primary outcome was the total score on the Female Sexual Function Index (FSFI), a well-validated measure of sexual function and satisfaction. A score of 26.55 or below indicates sexual dysfunction [14]. Subscales measure desire, arousal, orgasm, pain, and satisfaction. Women with cancer have often been sexually inactive within the 4-week reporting period, resulting in bias to score as dysfunctional [15]. Therefore, we included the Menopausal Sexual Interest Questionnaire (MSIQ) [19] which was a sensitive measure in a trial of our internet-based intervention [17]. Subscales measure sexual desire, responsiveness, and orgasmic capacity [19]. Since some women are not concerned about sexual function, a measure of distress about sexual problems was included. The 13-item Female Sexual Distress Scale-Revised (FSDS-R) [20] has excellent discriminant validity between normal and sexually dysfunctional women, as well as high validity and test-retest reliability. A score of 11 or more indicates distress about sexual function. We included 2 subscales of the Breast Cancer Prevention Trial Symptom Scale (BESS): dyspareunia and gynecologic symptoms [21]. Items at follow-up assessed coping strategies women used for sexual problems. At follow-up, items assessed whether a woman had discontinued her aromatase inhibitor and her daily adherence in the past 2 weeks. We mailed a pHem-Alert Home Test Kit® for vaginal pH at each assessment [22, 23].
Statistical analysis
Means and/or frequencies were calculated for demographic variables for each group at each time point. To determine if there were differences between the 3 groups at 6 and 12 months, analyses of covariance (ANCOVA) were computed for each independent variable, controlling for baseline scores. The same analyses were computed comparing the combined active treatment women to those in usual care, or combining all treatment groups across time.
Results
Participants
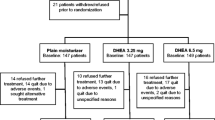
Identification of eligible women was more difficult than anticipated. During the 15-month recruitment period, we estimate about 750 women began adjuvant aromatase inhibitor therapy [8]. About 50% of women invited to participate were not sexually active, similar to rates in national surveys of aging women [12]. Eighty eligible women were interested in participating after review of the informed consent by phone. However, 19 (24%) withdrew after receiving the written consent and baseline questionnaires and 4 (5%) just after that point, on learning their treatment assignment. Of 57 women who completed baseline questionnaires, 8 dropped out before completing follow-up assessments (14%). At 6-month follow-up, N was 49 and at 12 months, 46. The number of women in tables varies slightly because of attrition and/or missing data. All active treatment women completed at least one coaching call. Out of 9 planned calls, the mean number achieved was 5.71 ± 2.76. The number of calls was not significantly different for the Active-P and Active-H groups.
Demographic and medical variables
Treatment groups did not differ significantly on age, ethnicity, type of breast surgery, history of chemotherapy, educational level, or comorbidities. None of the variables predicted dropping out. For 57 women who completed baseline questionnaires, mean age was 59 ± 8 (range 46 to 80). Seventy-seven percent of women were non-Hispanic Caucasian, 11% Hispanic, 5% African-American, 4% Asian-American, and 4% other. Ten percent had a high school degree, 30% some college, 30% a college degree, and 30% a postgraduate degree. Sixty-four percent had breast conservation, 6% unilateral mastectomy without reconstruction, 21% unilateral mastectomy with reconstruction, and 9% bilateral mastectomy with reconstruction. Forty percent had been treated with chemotherapy. Thirty-seven percent were taking prescription medicine for depression, anxiety, pain or hot flashes. On a checklist listing noncancer medical comorbidities, women reported a mean of 0.86 (range 0 to 4).
Factors influencing sexual function at baseline
Table 1 presents mean (SD) scores for questionnaires across the three assessment points for the Usual Care group, Active Group-H, and Active Group-P. The final column presents scores for 109 women adhering to AI therapy for 18–24 months in our benchmark survey [8], a recent historical comparison group not given educational handouts. Higher scores indicate better function on all scores except the FSDS-R and the BESS symptom subscales.
As illustrated in Table 1, at baseline, Active Group-P reported significantly more pain during sex (FSFI pain subscale) than Active Group-H (P = 0.03). A similar trend was seen on the BESS dyspareunia scale. Differences at baseline in total FSFI scores between the three treatment groups were not significant. General linear modeling explored factors that might affect baseline sexual function. Total FSFI scores were not significantly associated with prior chemotherapy, breast reconstruction, health comorbidities, or educational levels. A significant interaction effect was observed with age (P = 0.03). Within the Active-P group, younger women reported better sexual function. In the other two groups, older women had slightly better FSFI scores, suggesting probable random effects in this small sample, despite the use of minimization.
Outcomes across time
ANCOVAs controlling for baseline scores were calculated to assess changes in scores on each questionnaire at 6 and 12 months. As shown in Table 1, Active Group-H improved significantly more on sexual function/satisfaction (FSFI total score) than Active Group-P at 6 months (P = 0.04). Neither active group (nor the combined active groups) differed significantly in changes across time from the usual care group. Table 2 compares the percentage of women who had sexually dysfunctional FSFI scores (below 26.55) by group and across time. Logistic regression analyses did not find significant differences between treatment groups at any of the three assessments.
MSIQ scores did not change significantly across time between the treatment groups, or in comparing the usual care and active treatment group. However, at 6-month follow-up, an ANCOVA controlling for baseline scores found a trend to more improvement in total MSIQ score for the Active-H group than for the Active-P group (P = 0.16), similar to the analysis of FSFI scores.
At 6-month follow-up, the combined active treatment groups reported significantly less distress on the FSDS-R (mean ± SD 12.92 ± 11.78) than the usual care group (mean ± SD 23.18 ± 16.50), t (42) = 2.41, P = 0.02. On the BESS symptom subscales, at 6-month follow-up the usual care group had more dyspareunia (mean ± SD 3.82 ± 2.88) than the combined active treatment groups (2.96 ± 2.30), P = 0.07. Gynecological symptom scores were not significantly different across time or between groups.
Comparison to women in the benchmark survey
Since women in Usual Care received the study handout, the benchmark sample provides a recent historical comparison with women who had no systematic sexual education. The benchmark sample was significantly older (64 ± 9 years vs. 59 ± 8, t (194) = 3.8284, P = 0.0002), and only 73% were married or in a current relationship. However, the percentage of ethnic minorities and levels of education were similar. An ANCOVA compared the FSFI total score at 12 months for the combined Active Treatment groups (19.95 ± 9.10), Usual Care group (20.15 ± 8.25), and the sexually active women in the benchmark survey (13.05 ± 8.00). Controlling for age and education, the benchmark group had significantly worse sexual function (P = 0.002). A comparison with Table 2 is also relevant, since 93% of the benchmark survey group had total FSFI scores in the abnormal range.
Coping techniques for vaginal dryness and pain
Table 3 presents the results of coping strategies women used in the past 6 months, as well as reports from women in our benchmark survey who had been sexually active when starting aromatase inhibitors [8]. Women in the active treatment groups were notably less likely to stop all partner sex or to ask a doctor’s advice on vaginal dryness or loss of sexual desire, and more likely to use a vaginal moisturizer than women in usual care group or the benchmark survey.
Home vaginal pH assessment
Table 4 presents vaginal pH across time as assessed with the pHem-Alert Home Test Kit®. Scores are only included for assessments when a woman reported taking an aromatase inhibitor, verified by review of medications in medical charts. Scores above 4.5 represent postmenopausal pH levels. The moisturizer used by the Active-H group has a pH of 5.5–6.5, consistent with the reported data. The moisturizer used by the Active-P group claimed to restore a premenopausal pH level. No significant differences were observed in vaginal pH across time or according to treatment group. The mean pH was 6.28, 6.45, and 6.31 at the three assessment points. Normal pH before menopause is ≤4.5. Although the pH was slightly less abnormal in the Active-H group, the difference was present at baseline, making it difficult to interpret.
Assessments from phone coaching calls in active treatment groups
Regression models were calculated with all active treatment participants included, for the number of counseling calls regressed with outcome changes from baseline to 12 months (total FSFI, total MSIQ, and FSDS-R). No association reached significance. At each phone call, women were asked if they had used the web intervention [17]. Nineteen (54%) never used it, 10 (29%) used it once, and 6 (17%) more than once. Extent of usage was not significantly associated with outcomes.
Table 5 compares the Active-P and Active-H groups on variables assessed in the coaching calls, including usage of vaginal moisturizer and lubricant, satisfaction with the moisturizer, menopausal symptoms, and weekly exercise. Summed ratings were divided by the number of phone calls to create a mean rating. No significant differences between the two active treatment groups were identified. Women in both groups were somewhat to very satisfied with the vaginal moisturizer and used it about twice a week.
Adherence to endocrine therapy
According to women’s self-reports and a medical chart review, 1 woman in usual care, 1 in Active-P, and 2 in Active-H stopped all endocrine therapy (total 7%). Another 8 switched to tamoxifen (14%). In the benchmark survey, 20 of 139 women (14%) were stopped their aromatase inhibitor (12% off endocrine therapy and 3% on tamoxifen) [8].
Conclusions
Results from this pilot trial support our hypothesis that early intervention can decrease the decline in sexual function seen historically in women taking aromatase inhibitors. Participants in this pilot trial had stable, or mildly improved sexual function across their first year on adjuvant aromatase inhibitors. Reported sexual function and activity with a partner were clearly superior to data from our previous, benchmark survey. On questionnaires measuring sexual function/satisfaction, women in Usual Care, who only received a detailed handout, had similar outcomes to Active Treatment women additionally provided with vaginal moisturizers, lubricants, and dilators along with a website and coaching calls. However, at 6-month follow-up, Active Treatment women were significantly less distressed about sexual function than women in Usual Care and reported less dyspareunia.
For the secondary aim of comparing the efficacy of the two nonhormonal vaginal moisturizers, the 6-month follow-up may be most relevant, since women in active treatment were still using the supplied vaginal moisturizer and lubricant. Women using the moisturizer containing hyaluronic acid had greater improvement on the FSFI and MSIQ at 6 months, as well as less sexual inactivity throughout the year. In several trials, hyaluronic acid-based vaginal moisturizers have been as effective as vaginal estrogen cream or tablets in relieving postmenopausal vaginal dryness and pain [24–26]. However, recent experience at our center and at Memorial Sloan Kettering [13] suggests that women with severe atrophy should use the moisturizer 3–7 times weekly. We recommended 2–3 times a week and actual usage was even more suboptimal, 1–2 times weekly. Despite high satisfaction ratings for both moisturizers, by 12 months, a decline in moisturizer use in the Active-H group (see Table 3) and increase in lubricant use was observed, along with a corresponding mild deterioration in sexual function scores in group Active-H (see Tables 1 and 2). This change may reflect the fact that moisturizers were no longer being supplied by the study and are more expensive than purchasing vaginal lubricants. The Active-H moisturizer appeared particularly effective at 6 months. Neither moisturizer corrected vaginal pH according to home testing.
Episodes of physically stretching the vagina were also suboptimal. Women had sexual activity with a partner an average of once a week. Most did not add a session with the dilator, despite encouragement during phone coaching. Even two episodes of stretching per week may be inadequate to reduce vaginal atrophy. Dilator usage in our cohort was similar to adherence in women treated with pelvic radiotherapy for gastric or gynecologic cancers [27]. Although dilator usage appeared to preserve vaginal size in that trial, the frequency decreased during the year after radiation therapy. Few studies have adequately tested the type and frequency of dilation required to maintain vaginal size in women post-radiation therapy, [28] or even just post-menopause.
Further research is needed to identify and refine the effective elements of this multi-component intervention. No relationship was observed between the number of coaching calls or extent of website usage and sexual outcomes. Only a minority of women in Active Treatment utilized the website, in contrast to frequent use in our previous randomized trial [17]. Perhaps the handout and coaching calls provided adequate care without the website, since women in Active Treatment were less likely than usual care or benchmark women to seek professional help for sexual problems. Reports of coping mechanisms suggest that the handout influenced behavior. Vaginal moisturizer use by Usual Care women was less than in the Active Treatment groups, but higher than use in the benchmark survey sample. Lubricant use was very high at 6 months among usual care women.
To prevent sexual dysfunction related to aromatase inhibitors, we need to understand their physiological effects. Aromatase inhibitors decrease proliferation of cells in the vaginal mucosa, decrease staining for progesterone receptors, increase staining for androgen receptors [29], and decrease expression of genes that modulate cell differentiation, proliferation, and adhesion [30]. Replacing estrogen may not be the only solution. Vaginal moisturizers with hyaluronic acid can improve the vaginal maturation index as well as alleviating pain with sex [26]. Promoting genital blood flow may also reduce vaginal atrophy in women, similar to post-prostatectomy penile rehabilitation in men [31–33]. Both increased genital blood flow and tissue expansion with sexual arousal, and neurotransmitters mediating genital changes are similar between genders [34]. Potential modalities to reduce genitourinary atrophy in women include a daily dose of phosphodiesterase-5-inhibitor, vibrator or self-stimulation of the clitoris and vulva several times a week, regular vaginal dilation, or exercising the pelvic floor muscles.
Are there better options than nonhormonal moisturizers and lubricants? Most interventions continue to focus on the safety of low-dose vaginal estrogen, despite its limited efficacy [35–37]. In one pilot, postmenopausal breast cancer survivors who applied liquid lidocaine to the vulva before penetration had less dyspareunia [38]. Although partners did not report penile numbing, lidocaine obviously could reduce pleasurable sensations for both partners. Another feasibility study combined a polycarbophil-based vaginal moisturizer, olive oil as a lubricant during sex, and pelvic floor muscle training [39]. Although pain improved, women using oil- or petroleum-jelly-based vaginal lubricants have far higher rates of colonization with Candida and bacterial vaginosis than those using water- or silicone-based lubricants [40].
An ideal solution would be a selective-estrogen receptor modulator equal or better than tamoxifen or aromatase inhibitors at preventing breast cancer, with ability to improve genitourinary atrophy without elevating risk of uterine cancer. Lasofoxifene is an excellent candidate [41]. Clinical trials are ongoing.
Adherence to aromatase inhibitors was much higher in this trial than is typical, even in a comprehensive cancer center. Encouragement in the handout and coaching calls to manage symptoms by switching to a different aromatase inhibitor or to tamoxifen may have played a role. Aromatase inhibitors provide superior disease-free survival, but not overall survival thus far [1–3]. Many women may better tolerate tamoxifen’s side effect profile, especially as the duration of adjuvant endocrine therapy is extended or is combined with ovarian suppression in premenopausal women.
Limitations of this trial include the small, self-selected sample, particularly given the far greater number of women eligible for the trial who did not chose to participate. Some women who had a phone review of the informed consent decided not to sign the written consent after seeing the baseline questionnaires. The sexually explicit nature of some items may have raised concerns about privacy. Participants in the trial were somewhat younger than those in our benchmark survey, and more likely to be in a sexual relationship [8], suggesting higher distress about sexuality. The 24% rate of dropping out before baseline is typical of our previous studies [17] and those of our colleagues [42]. The company that manufactured the vaginal moisturizer used in the Active-P group was prohibited by the US Food and Drug Administration (FDA) from distributing it in early January 2015 because of unproven claims for its prebiotic properties. By the time we became aware of the legal action, all women in the Active-P group would have completed their 6-month-period supply. One woman stopped using the moisturizer after the first administration because of burning, an eventuality anticipated in the informed consent. The product is currently widely marketed with reformulated ingredients and revised labeling.
Although this is a promising pilot trial, it lacked power to provide definitive answers because of the small N and unanticipated baseline difference between groups in dyspareunia. Nevertheless, it suggests that a preventive approach can mitigate the sexual problems caused by aromatase inhibitor therapy. More research is needed to identify the optimal components of a cost-effective intervention.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thürlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R. Aromatase inhibitors versus tamoxifen in early breast cancer; patient-level meta-analysis of the randomised trials. Lancet. 2015; 386:1341–1352.
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Eng J Med. 2016.
Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, Pinotti G, Spazzapan S, Ruhstaller T, Puglisi F, Pavesi L, Parmar V, Regan MM, Pagani O, Fleming GF, Francis PA, Price KN, Coates AS, Gelber RD, Goldhirsch A, Walley BA. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16:848–58.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on ovarian suppression. J Clin Oncol. 2016;34:1689–701.
Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162–8.
Baumgart J, Nilsson K, Stavreus Evers A, Kunovac Kallak T, Kushnir MM, Bergquist J, Sundström Poromaa I. Androgen levels during adjuvant endocrine therapy in postmenopausal breast cancer patients. Climacteric. 2014;17:48–54.
Cappelletti M, Wallen K. Increasing women’s sexual desire: the comparative effectiveness of estrogens and androgens. Horm Behav. 2016;78:178–93.
Schover LR, Baum GP, Fuson LA, Brewster A, Melhem-Bertrandt A. Sexual problems during the first 2 years of adjuvant treatment with aromatase inhibitors. J Sex Med. 2014;11:3102–11.
Groenvold M. Health-related quality of life in early breast cancer. Dan Med Bull. 2010;57:B4814.
Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol. 2016;34:816–24.
Hamidou Z, Dabakuyo TS, Bonnetain F. Impact of response shift on longitudinal quality-of-life assessment in cancer clinical trials. Expert Rev Pharmacoecon Outcomes Res. 2011;11:549–59.
Lutfey KE, Link CL, Rosen RC, Wiegel M, McKinlay JB. Prevalence and correlates of sexual activity and function in women: results from the Boston Area Community Health (BACH) survey. Arch Sex Behav. 2009;38:514–27.
Carter J, Stabile C, Seidel B, Baser RE, Gunn AR, Chi S, Steed RF, Goldfarb S, Goldfrank DJ. Baseline characteristics and concerns of female cancer patients/survivors seeking treatment at a female sexual medicine program. Support Care Cancer. 2015;23:2255–65.
Wiegel M, Meston C, Rosen R. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20.
Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606–18.
Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8:549–59.
Schover LR, Yuan Y, Fellman BM, Odensky E, Lewis PE, Martinetti P. Efficacy trial of an internet-based intervention for cancer-related female sexual dysfunction. J Natl Compr Cancer Netw. 2013;11:1389–97.
Taves DR. The use of minimization in clinical trials. Contemp Clin Trials. 2010;31:180–4.
Rosen RC, Lobo RA, Block BA, Yang HM, Zipfel LM. Menopausal Sexual Interest Questionnaire (MSIQ): a unidimensional scale for the assessment of sexual interest in postmenopausal women. J Sex Marital Ther. 2004;30:235–50.
Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5:357–64.
Cella D, Land SR, Chang C-H, Day R, Costantino JP, Wolmark N, Ganz PA. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–26.
Lindau ST, Hoffmann JN, Lundeen K, Jaszczak A, McClintock MK, Jordan JA. Vaginal self-swab specimen collection in a home-based survey of older women: methods and applications. J Gerontol Social Sciences. 2009;64B(S1):106–18.
Roy S, Caillouette JC, Faden JS, Roy T, Ramos DE. Improving appropriate use of antifungal medications: the role of an over-the-counter vaginal pH self-test device. Infect Dis Obstet Gynecol. 2003;11:209–16.
Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575–84.
Stute P. Is vaginal hyaluronic acid as effective as vaginal estriol for vaginal dryness relief? Arch Gynecol Obstet. 2013;288:1199–201.
Ekin M, Yasar L, Savan K, Temur M, Uhri M, Gencer I, Kıvanç E. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283:539–43.
Law E, Kelvin JF, Thom B, Riedel E, Tom A, Carter J, Alektiar KM, Goodman KA. Prospective study of vaginal dilator use adherence and efficacy following radiotherapy. Radiother Oncol. 2015;116:149–55.
Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;8:CD007291.
Kallak TK, Baumgart J, Göransson E, Nilsson K, Poromaa IS, Stavreus-Evers A. Aromatase inhibitors affect vaginal proliferation and steroid hormone receptors. Menopause. 2014;21:383–90.
Kallak TK, Baumgart J, Nilsson K, Åkerud H, Poromaa IS, Stavreus-Evers A. Vaginal gene expression during treatment with aromatase inhibitors. Clin Breast Cancer. 2015;15:527–35.
Gandaglia G, Suardi N, Cucchiara V, Bianchi M, Shariat SF, Roupret M, Salonia A, Montorsi F, Briganti A. Penile rehabilitation after radical prostatectomy: does it work? Transl Androl Urol. 2015;4:110–23.
Meldrum DR, Burnett AL, Dorey G, Esposito K, Ignarro LJ. Erectile hydraulics: maximizing inflow while minimizing outflow. J Sex Med. 2014;11:1208–20.
Lin HC, Yang WL, Zhang JL, Dai YT, Wang R. Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl. 2013;15:387–90.
Traish AM, Botchevar E, Kim NN. Biochemical factors modulating female genital sexual arousal physiology. J Sex Med. 2010;7:2925–46.
Le Ray I, Dell’Aniello S, Bonnetain F, Azoulay L, Suissa S. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat. 2012;135:603–9.
Committee Opinion No. 659. The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:393–396.
Donders G, Neven P, Moegele M, Lintermans A, Bellen G, Prasauskas V, Grob P, Ortmann O, Buchholz S. Ultra-low-dose estriol and lactobacillus acidophilus vaginal tablets (Gynoflor®) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat. 2014;145:371–9.
Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394–400.
Juraskova I, Jarvis S, Mok K, Peate M, Meiser B, Cheah BC, Mireskandari S, Friedlander M. The acceptability, feasibility, and efficacy (phase l/II study) of the OVERcome (olive oil, vaginal exercise, and moisturizer) intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J Sex Med. 2013;20:2549–58.
Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol. 2013;121:773–80.
Mocellin S, Pilati P, Briarava M, Nitti D. Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials. J Natl Cancer Inst. 2016;108.
Jennings S, Philip EJ, Nelson C, Schuler T, Starr T, Jandorf L, Temple L, Garcia E, Carter J, DuHamel K. Barriers to recruitment in psycho-oncology: unique challenges in conducting research focusing on sexual health in female survivorship. Psychooncology. 2014;23:1192–5.
Acknowledgments
This work was supported by a grant from the University of Texas MD Anderson Cancer Center, Duncan Family Institute for Cancer Prevention and Risk Assessment. Support was provided, in part, by the Patient-Reported Outcomes, Survey, and Population Research (PROSPR) Shared Resource through a Cancer Center Support Grant (CA16672, PI: R. DePinho, MD Anderson Cancer Center), from the National Cancer Institute, National Institutes of Health. Vaginal moisturizer for the study was donated by Fidia Pharma, USA, Inc., and by Laclede, Inc. The authors would like to thank Lisa Fuson, APN, for assistance in recruiting participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pragati Advani, Abenaa Brewster, and George P. Baum declare that they have no conflict of interest. Leslie Schover acknowledges that Fidia Pharma, USA, Inc., and Laclede, Inc., donated vaginal moisturizer for the study. She also holds a financial interest in Will2Love, LLC, a digital health company based in part on the intervention in reference 17.
Ethical approval
All procedures performed in studies involving human participants were performed under an approved protocol and in accordance with the ethical standards of the institutional research committee of the University of Texas MD Anderson Cancer Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Leslie R. Schover retired from University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Rights and permissions
About this article
Cite this article
Advani, P., Brewster, A.M., Baum, G.P. et al. A pilot randomized trial to prevent sexual dysfunction in postmenopausal breast cancer survivors starting adjuvant aromatase inhibitor therapy. J Cancer Surviv 11, 477–485 (2017). https://doi.org/10.1007/s11764-017-0606-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0606-3