Abstract
The paper presents a detailed ecological investigation of mangroves (trees and palm) along Carigara Bay in Leyte, Philippines by comparing the diversity, vegetation structure, species composition, and indicator species among forest types (riverine and fringe) and zones (landward, middleward, and seaward/along water) as well as by examining their relationships with environmental variables. A total of 22 mangrove species, belonging to 12 families were documented wherein the most abundant was Sonneratia alba, followed by Nypa fruticans, then by Avicennia rumphiana. It was found that the diversity (Shannon-Wiener) of riverine mangroves (0.94 ± 0.07; 1.20 ± 0.04) was significantly higher than the fringe at the middleward and seaward/along the water (p < 0.001). In the fringe mangrove forests, the mangrove species Aegiceras corniculatum was associated with the middleward zone, and Camptostemon philippinensis, Aegiceras floridum, Rhizophora mucronata, Sonneratia alba, and Lumnitzera littorea were associated with the seaward zone, whereas landward zone of fringe and all the zones in riverine were generally associated by species with low to optimum salt tolerances such as Nypa fruticans, and Avicennia rumphiana as the most abundant. As well, a total of 14 mangroves have been identified as indicator species. Lastly, mangrove species can be generally classified as riverine and fringing based on the environmental factors explaining their distributions, and it has been found that soil porosity, water content, soil salinity, and distance from the sea or river’s edge were the most significant environmental factors that determine diversity patterns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are communities of trees or shrubs thriving along tidal flats and coastlines extending inland along rivers, streams, and their tributaries with brackish waters (Sebidos and Galinato 1996). They are one of the most exceptional flora groups in the world and grow on the coastlines of tropical and sub-tropical countries and are well adapted to extreme conditions such as high salinity and temperature (Goloran et al. 2020). Importantly, mangrove forests are one of the vital ecosystems in tropical countries that provide various natural products and ecological services, including their role in climate change mitigation (Dangan-Galon et al. 2016).
In a mangrove ecosystem, mangroves are considered the primary producers, interacting with the associated aquatic fauna, and physical factors of the coastal environment, providing different ecological services (e.g., soil erosion control and trapping of sediments) (Baleta and Casalamitao 2016). During typhoons, the mangrove ecosystem acts as a natural barrier and reduces the risk of coastal flooding and soil erosion. To coastal communities, mangroves are valuable resources for building materials, fodder for livestock, herbal medicine, and a source of livelihood. It also serves as a nursery for different species of marine life and even terrestrial species by providing them with habitat, food, and protection against predators (Kauffman and Bhomia 2017; Pototan et al. 2021). Mangroves sequester and store large quantities of carbon, which, when disturbed may shift into a carbon source of greenhouse gases. Therefore, they are very important when considering climate change adaptation and mitigation practices (Kauffman and Bhomia 2017).
Mangrove ecosystems today are facing intense pressure due to destruction by humans for various developmental needs. Moreover, the ecological significance of this unique ecosystem is not at all understood (Sreelekshmi et al. 2018). Primavera (2000) stated that overexploitation, conversion to agricultural ponds, and industry and residential areas are attributed to the reduction of mangrove forest cover. The loss of these ecosystems has resulted in a decrease in beneficial services that they provide such as food provision, storm surge protection, climate regulation, as well as cultural and spiritual benefits (Primavera 2000). Ultimately, the degradation and depletion of mangrove forests with the loss of their ecosystem services affect local communities that are dependent on them (Quevedo et al. 2020).
The Philippines is home to at least 39 mangrove species out of the 60 species found in the Indo-Pacific area, making the country one of those with the highest species diversity for mangroves in the region (Primavera et al. 2004; Dangan-Galon et al. 2016). This high diversity of mangroves can be attributed to the country’s geographical location wherein it is located along the tropical bands where the mangroves thrive (Garcia et al. 2014). As well, other environmental factors such as rainfall, freshwater runoff, nutrient inputs, and soil quality can also determine the occurrence and structural diversity of these mangroves (Cintron and Novelli 1984).
Despite the country’s notable high diversity of mangroves, comprehensive ecological studies on the assemblage of mangrove plants, and especially the environmental factors that can influence their occurrence and distribution remain scarcely studied (Dangan-Galon et al. 2016; Raganas and Magcale-Macandog 2020; Pototan et al. 2021). Therefore, the present study was conducted a) to determine any difference in abundance, richness, diversity, and vegetation structures (DBH, height, stem density, canopy cover) of mangroves across mangrove forest types (fringe and riverine) and zones (landward, middleward, and seaward/river); b) to examine how species assemblage differ between mangrove forest types and zones; c) to determine an indicator mangrove species in every zone of fringe and riverine mangrove ecosystems; d) to examine how environmental variables influence the distribution of mangrove plants; and e) to determine which environmental variables influence the abundance, richness, and diversity of mangroves.
Materials and methods
Study area
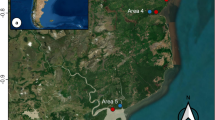
The study was conducted on the mangrove forest areas along the Carigara Bay in Leyte Island (Fig. 1). Mangrove ecosystems of the bay are represented by stands of fringe and riverine mangrove forests distributed among the five surrounding coastal municipalities (Capoocan, Carigara, Barugo, San Miguel, and Babatngon). However, significant areas of mangrove forests along the bay have been lost because of land use conversion mainly due to aquaculture and settlements.
Map of the study area, and study sites (fringe and riverine mangroves) along the Carigara Bay in Leyte, Philippines (adapted from Decena et al. 2023)
The climate of the study area is characterized as equatorial rainforest-fully humid (Kottek et al. 2006). It has no dry season and has more or less evenly distributed rainfall throughout the year. The warmest month is April with a mean annual temperature of 27 ºC and pronounced wetness occurring in the months of November, December, and January with annual total precipitation of 2293 mm (Quiñones and Asio 2015; Marteleira 2019).
Study sites
Fringe mangroves
The fringe mangroves considered in the study were those mangrove forest stands bordering the beach/coastline of the bay. Two stands of this mangrove forest were sampled, one stand is in Barangay Mawodpawod, and the other one is in Barangay Malpag, both in the municipality of San Miguel. The two stands are separated by a small stream and the sampling location from these stands was 500 m away from each other. The stands of fringe mangroves sampled were about 60–200 m wide from the landward to the seaward zone. The nearest community was about 500 m away, though minor disturbances could be observed in the mangrove areas such as the cutting of branches and harvesting leaves of Nypa fruticans (Thunb.) Wurmb. (nipa palm) for making nipa shingles. Also, people from the community collect other resources from the mangrove forests such as mud crabs and varieties of edible mollusks.
Riverine mangroves
Likewise, two riverine mangrove stands located near the estuary from two different rivers draining toward Carigara Bay were sampled. The first mangrove stand was in Bagacay River in Barangay Bagacay of the municipality of San Miguel. The river is approximately 3.2 km in length, originating from its headstream from the western side of the Babatngon Range, which brings freshwater and nutrients to the mangroves and coastal ecosystems. The riverine mangrove stand was located 200 m from the mouth of the river and was adjacent to a highway. In the landward portion of the mangrove stand was a small community comprising 15–20 houses, but no signs of significant clearing or conversion of mangrove areas were observed. The other riverine mangrove stand was located along the Minuhang River in Barangay Minuhang of the municipality of Barugo. The mangrove stand was approximately 800 m from the estuary or sea, with settlements on the opposite side of the river. The river is approximately 4.4 km in length and originates from hilly areas located in the southern direction of the river system. In general, both mangrove stands were pristinely characterized by the abundance of large-sized mangrove trees (> 100 cm DBH), though minor disturbances were observed such as the cutting of small branches or small mangrove trees, as well as harvesting of nipa leaves for making nipa shingles.
Plot establishment and sampling
Reconnaissance surveys were conducted first to identify mangrove stands and sites to be sampled. The geographic location of each sampling site was determined using a handheld GPS. All the field samplings took place between July 2022 to February 2023.
In the fringe mangrove forest stands, a 125 m-long transect line (Kauffman et al. 2011) was established parallel to the coastline in each zone (landward, middleward, and seaward) in every site. The transect line in the landward zone was laid 15 m from the adjacent terrestrial forest, as well as transect line at the seaward zone was laid approximately 15 m from the ecotone. In the riverine mangroves, a transect line of the same length was laid at one side of the bank, parallel to the river. Similarly, the riverine mangrove forest stand was divided into three zones, the landward which is adjacent to the terrestrial forest or ecosystem, middleward or interior, and along the water that is close to the bank. The transect lines were also established at the same distance from the ecotones.
To sample mangrove trees including palm, a 7 m radius circular plot with an area of 154 m2 was demarcated along the transect line at 25 m intervals. There were 36 plots established for each mangrove type (fringe and riverine), bringing the total number of plots to 72. All standing trees with a DBH of ≥ 5 cm inside the plot were identified, counted, and measured for diameter-at-breast height (DBH) and height. The DBH was measured at 1.37 m above the ground, however, in the field, since there are anomalies in terms of stem structure (Kauffman and Donato 2012), adjustments were made accordingly. For trees with tall buttresses exceeding 1.37 m above ground level, stem diameter was measured at the point directly above the buttress. For stilt-rooted species, stem diameter was measured above the highest stilt root (Clough and Scott 1989; Komiyama et al. 2005). For some individuals with prop roots extending well into the canopy, tree diameter was measured above the stilt roots, where a true main stem exists. Additionally, the height of the tree was visually determined using a 2-m long calibrated pole (Madeira et al. 2009; Decena et al. 2022). Other non-tree mangroves or mangrove associates were also noted.
All the samples were identified up to the species level using “The Field Guide to Philippine Mangroves” by Primavera (2009), and “Handbook of Mangroves in the Philippines-Panay” by Primavera et al. (2004). Each of the mangrove species was photographed including the whole tree, leaves, fruits, and flowers, for photo vouchering purposes.
Environmental parameters
To examine the effects of environmental factors on the diversity, assemblage, and distribution of mangroves, edaphic factors were collected or examined. A soil core sample was collected inside and closer to the center of each of the circular plots using a 1 m constructed half-cylindrical steel sampler with an internal diameter of 6 cm. The core sample was divided into depth intervals of 0–15, 15–30, 30–50, 50– 100, and > 100 cm, and a sub-sample of 5 cm thick was extracted from the center of each layer for laboratory analysis. The soil samples were analyzed for some selected physical parameters such as gravimetric water content (GWC), volumetric water content (VWC), dry bulk density (DBD), and porosity. Water content and dry bulk densities were determined through the oven drying method, while porosity was derived from dry bulk density values.
Interstitial soil salinity was measured from each plot with the use of a 5 ml plastic syringe and a hand-held salt meter (ATAGO). The measurements were performed in three random locations inside the plot through the auger boreholes or by digging shallow holes using a machete and allowing the soil water to fill the whole for about 5 min. In some instances, deeper wholes were created in elevated areas and waited for a longer time to extract water samples. Also, care was taken to prevent surface water from flowing to the whole, as surface water can be less saline than soil water.
Soil depth was measured in three random locations inside the plot. The measurements were done by inserting a 2 m long steel or by using a straight wooden pole in deeper areas until reaching the impenetrable layer such as bedrock or coral fragment deposits.
Lastly, the distance (m) of each plot from the sea or river was determined with the use of a distance measuring tool in Google Earth images.
Data analysis
Diversity indices at the plot level for mangrove species were calculated. All the data were tested for normality using the Kolmogorov-Smirnov test. The Generalised Linear Models (GLMs) were performed to determine the effects of mangrove forest types (fringe and riverine) and mangrove zones (landward, middleward, and seaward/along water) on the diversity indices (abundance, richness, and Shannon-Wiener). The GLMs analyses used Poisson or negative binomial distribution with a log link function for count data (abundance and richness) and gamma distribution with a log link function for continuous data (diversity). Post-hoc tests were performed whenever there were significant variations at α = 0.05, using pairwise comparisons. Moreover, to determine the effects of mangrove forest types and mangrove zones on the vegetation structures (DBH, height, stem density, canopy cover), the two-way ANOVA was performed. Then, Tukey’s post-hoc analysis was performed whenever there were significant differences at α = 0.05. Statistical analyses such as normality, GLMs, and two-way ANOVA were performed using the SPSS version 20 for Windows.
The non-metric multidimensional scaling (NMDS) ordination was used to examine the difference in mangrove species assemblage between mangrove forest types and zones. The NMDS analysis was performed whereby the ordination was constructed from the Bray-Curtis dissimilarity matrix of pairwise dissimilarities between plots based on the abundance data. The NMDS was performed using the function “metaMDS” from the R package vegan (Oksanen 2019). In constructing the ordination diagram, twenty random starting configurations were used, with the final configuration that minimized the stress of the ordination configuration retained for plotting. In addition, to support the results of the NMDS ordination, the Analysis of Similarities (ANOSIM) permutation tests in the vegan package of R (Oksanen 2019), with 5,000 random permutations of the dissimilarity matrix were performed in testing statistically the differences in mangrove species assemblage.
An indicator species analysis (Dufrêne and Legendre 1997) was performed to identify mangrove species that were associated with or indicators of certain zones in fringe and riverine mangrove ecosystems. The analysis was carried out using the “multipatt” function of the R package indicspecies (De Cáceres et al. 2020). The statistical significance of this relationship was tested using a permutation test (De Cáceres et al. 2020).
To examine the distribution of mangrove species with environmental variables, linear vectors were fitted into the NMDS ordination using the function “envfit” in the vegan package of R (Oksanen 2019). The analysis was performed using the same species abundance data, and environmental variables fitted into the ordination included gravimetric water, volumetric water content, dry bulk density, porosity, salinity, soil depth, and distance from the sea/river. Afterward, the significance of each environmental variable was examined using a permutation test with 1000 random permutations.
Lastly, the relationships between the abundance, species richness, and diversity of mangroves with environmental variables were explored using the Generalized Additive Model (GAM) in R package mgcv (Wood 2019). The GAM was performed using Poisson error structure and logarithmic link functions for count data (abundance and species richness) whereas Gaussian error structure and identity link function were used for continuous data (diversity). The environmental variables were tested for multicollinearity, and only those with correlations (r) < 0.65 were retained for the analysis. Firstly, the GAM analysis was performed with a full model fitted with smooth-terms for all the selected environmental variables. In the initial fitting, some of the environmental variables appeared to be best fitted by smooth-terms with effective degrees of freedom (edf) equal to one indicating simple linear relationships. Thus, in the succeeding fitting, these terms were expressed into linear terms. The selection of the final model was performed by dropping the least significant environmental variables one at a time until Akaike’s Information Criterion (AIC) no longer improved. The shape of the response curves associated with each term was illustrated by plotting the partial effects.
The analyses such as NMDS, indicator species, fitting of linear vectors, and GAM were carried out in R 4.1.0 (R Core Team 2021).
Results
Species richness and diversity
In this study, a total of 1651 mangrove individuals (trees and palm) with 22 species, belonging to 12 families were documented (Table 1). The most abundant species was Sonneratia alba J. Smith (mangrove apple) dominating the fringe mangrove forests, followed by Nypa fruticans (Thunb.) Wurmb. (nipa palm), then by Avicennia rumphiana Hall. f. which both dominated the riverine mangrove forests. A single mangrove tree species, Rhizophora stylosa Griff. (spotted mangrove), was documented only outside study plots. In terms of the threats status based on the International Union for Conservation of Nature (IUCN) red list criteria (IUCN 2023), the mangrove species including Camptostemon philippinensis (Vidal) Becc. and Avicennia rumphiana Hall. f. are already classified as endangered (EN), and vulnerable (VU), respectively, two species such as Aegiceras floridum Roem. and Schult. (black mangrove) and Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove) are near threatened (NT), and the rest of the remaining species are classified as least concern (LC). In addition to mangrove trees and palm, other mangroves of different habits (shrubs, vines, and fern) and mangrove associates were encountered though not included in the study. These other mangroves included Acanthus ebracteatus Vahl (holly mangrove), Acanthus ilicifolius Lour. (holly-leaved acanthus), Acanthus volubilis Wall. (sea holly), Acrostichum speciosum Willd. (mangrove fern), Brownlowia tersa (L.) Kosterm. (Dungun Air), and Finlaysonia obovata Wall. (Finlayson’s creep), and the mangrove associates included Glochidion littorale Benth. (monkey apple), Hibiscus tiliaceus L. (sea hibiscus), Lepiniopsis cf. ternatensis Valeton, Nauclea orientalis (L.) L. (yellow cheesewood), Syzygium sp., Terminalia catappa L. (beach almond), and Utania philippinensis (K.M.Wong & Sugau) K.M.Wong, Sugumaran & Sugau.
The results of the GLMs analysis showed that the abundance and species richness of mangroves did not differ significantly between mangrove forest types (p > 0.05) or zones (p > 0.05) (Table 2; Fig. 2a & b). Meanwhile, it is the diversity (Shannon–Wiener) that differed significantly both between forest types (p < 0.001) and zones (p = 0.040), as well as with significant interaction (p < 0.001) (Table 2; Fig. 2c). Specifically, the diversity of riverine mangroves (0.94 ± 0.07; 1.20 ± 0.04) was significantly higher than the fringe at the middleward and seaward/along the water, respectively, however fringe mangrove diversity (1.13 ± 0.07) was significantly higher than the riverine at the landward zone. It is also worth noting that diversity significantly decreased and increased from landward to searward/along water for fringe and riverine mangrove forests, respectively.
Vegetation structures
Both the DBH and stem density did not differ significantly between mangrove forest types or zones (Table 3; Fig. 3a & c). Meanwhile, tree height was observed to be significantly higher in the landward zone (7.00 ± 0.31) compared to seaward/along the water (riverine) (5.72 ± 0.36 m), but not with the middleward (Table 3; Fig. 3b). As well, the canopy cover in the fringe mangroves was significantly higher both in the landward (86.61 ± 1.77) and middleward (84.94 ± 2.25) compared to seaward/along the water (65.27 ± 2.84%). For canopy cover in the riverine mangroves, it was significantly higher in the landward (84.31 ± 1.22) compared with the middleward (74.00 ± 3.24%) but did not differ significantly with seaward/along the water (Table 3; Fig. 3d).
Species composition
The NMDS ordination analysis exhibited differentiation in mangrove species composition between mangrove forest types and zonation, as indicated by minimal overlapping of at least two polygons (Fig. 4). This difference in species composition was further supported by the highly significant results of the ANOSIM test (ANOSIM R = 0.599, P < 0.001). Based on the NMDS ordination, mangrove species are associated with certain zones or mangrove forest types. For example, Aegiceras corniculatum (L.) Blanco (river mangrove) was associated with the middleward zone of fringe mangrove forests, and the species including Camptostemon philippinensis (Vidal) Becc., Aegiceras floridum Roem. and Schult. (black mangrove), Rhizophora mucronata Lam. (Asiatic mangrove), Sonneratia alba J. Smith (mangrove apple), and Lumnitzera littorea (Jack) Voigt. (black mangrove) are associated with the seaward zone, still of fringe mangrove forests. In addition, all the rest of the species such as Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove), Avicennia officinalis L. (Indian mangrove), Xylocarpus granatum Koen. (cedar mangrove), Rhizophora apiculata Blume (tall-stilt mangrove), Excoecaria agallocha L. (blind-your-eye mangrove), Avicennia alba Blume, Avicennia marina (Forsk.) Vierh. (grey mangrove), Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove), Scyphiphora hydrophyllacea Gaertn. (yamstick mangrove), Avicennia rumphiana Hall. f., Heritiera littoralis, Nypa fruticans (Thunb.) Wurmb. (nipa palm), Ceriops tagal (Perr.) C.B. Rob. (spurred mangrove), and Osbornia octodonta F. Muell. (myrtle mangrove) are associated with all the zones (landward, middleward, along water) of riverine mangrove forests and the landward zone of fringe mangrove forests. It is also important to note that the overlapping of plots or polygons for the landward zone of the fringe mangrove forests to that of the riverine mangrove forests due to the presence of common mangrove species could indicate similarities in environmental conditions between them.
The NMDS of species composition of mangroves (trees and palm) among the different zones for both fringe and riverine mangrove ecosystems along the Carigara Bay in Leyte, Philippines, with habitat polygons and species (two letters symbol) distribution. Fringe (landward) (FLW) – open square, Fringe (middleward) (FMW)-open circle, Fringe (seaward) (FSW)-open triangle, Riverine (landward) (RLW)-solid square, Riverine (middleward) (RMW)-solid circle, Riverine (along water) (RAW)-solid triangle. The species abbreviations are listed in Table 1
Mangrove species indicator
The indicator species analysis revealed a total of 14 mangroves that serve as indicator species, of which six species are indicators for a single zone whereas all others (eight species) are indicators for a combination of two or more zones from either single or both mangrove forest types (Table 4; Fig. 5). In the fringe mangrove forests, Scyphiphora hydrophyllacea Gaertn. (yamstick mangrove) and Osbornia octodonta F. Muell. (myrtle mangrove) are indicators for the landward zone, while the Aegiceras corniculatum (L.) Blanco (river mangrove) is for the middleward zone. The Rhizophora apiculata Blume (tall-stilt mangrove), and Sonneratia alba J. Smith (mangrove apple) are indicators for landward plus middleward, and middleward plus seaward, respectively, of fringe mangrove forest. For riverine mangrove forests, Ceriops tagal (Perr.) C.B. Rob. (spurred mangrove) is an indicator species for the middleward, and Avicennia alba Blume and Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove) are for along the water. Mangrove species that are indicators for multiple zones in fringe mangrove forests include Nypa fruticans (Thunb.) Wurmb. (nipa palm) (landward and along water), Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove) (landward and middleward), and Avicennia officinalis L. (Indian mangrove) (all the zones). Moreover, mangrove species that are indicators of multiple zones from both mangrove forest types are Avicennia marina (Forsk.) Vierh. (grey mangrove) (landward and middleward of fringe, and along the water of riverine mangrove forests), Excoecaria agallocha L. (blind-your-eye mangrove) (landward of fringe, and landward and middleward of riverine mangrove forests), and Avicennia rumphiana Hall. f. (landward of fringe, and all the zones of riverine mangrove forests).
Indicator species identified from the mangrove ecosystems along the Carigara Bay in Leyte, Philippines, (a) Aegiceras corniculatum, (b) Avicennia alba, (c) Avicennia marina, (d) Avicennia officinalis, (e) Avicennia rumphiana, (f) Bruguiera cylindrica, (g) Ceriops decandra, (h) Ceriops tagal, (i) Excoecaria agallocha, (j) Nypa fruticans, (k) Osbornia octodonta, (l) Rhizophora apiculata, (m) Scyphiphora hydrophyllacea, and (n) Sonneratia alba
Species distribution with environmental variables
Fitting the linear vector into the ordination space indicates that all the environmental variables considered in the analysis explained the distribution pattern of most mangrove species (Fig. 6). As shown in Table 5, the permutation test indicates that all the environmental variables significantly contributed to the distribution pattern of mangrove species with P ≤ 0.05. Generally, the explanatory power of the environmental variables is equal except for distance to sea/river. Again, as reflected in Fig. 6, the environmental variables such as soil depth, distance to sea/river, and dry bulk density generally explain the occurrence of mangrove species including Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove), Avicennia officinalis L. (Indian mangrove), Excoecaria agallocha L. (blind-your-eye mangrove), Scyphiphora hydrophyllacea Gaertn. (yamstick mangrove), Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove), Heritiera littoralis Dryand. ex W. Ait. (looking-glass mangrove), Nypa fruticans (Thunb.) Wurmb. (nipa palm), and Avicennia rumphiana Hall. f. On the other side, the environmental variables, namely volumetric water content, gravimetric water content, soil salinity, and soil porosity generally explain the occurrence of Avicennia alba Blume, Xylocarpus granatum Koen. (cedar mangrove), Aegiceras corniculatum (L.) Blanco (river mangrove), Rhizophora apiculata Blume (tall-stilt mangrove), Avicennia marina (Forsk.) Vierh. (grey mangrove), Camptostemon philippinensis (Vidal) Becc., Aegiceras floridum Roem. and Schult. (black mangrove), Sonneratia alba J. Smith (mangrove apple), and Rhizophora mucronata Lam. (Asiatic mangrove).
NMDS ordination displaying the magnitude and direction of the fitted vectors (environmental variables) and species distributions among the different zones for both fringe and riverine mangrove ecosystems along the Carigara Bay in Leyte, Philippines. Species abbreviations are listed in Table 1
Relationship between mangrove abundance, species richness, and diversity with environmental variables
To evaluate the relationship between diversity indices and environmental variables, GAM analysis was performed, however environmental variables (gravimetric water content, dry bulk density, and soil depth) with high multicollinearity (r > 0.65) were excluded from the analysis (Table 6). The GAM analysis on mangrove abundance results in a best-supported model consisting of three explanatory variables, soil porosity, volumetric water content, and soil salinity (Table 7). The linear term of the model shows a positive relationship between mangrove abundance and soil porosity, indicating that mangrove abundance increases with increasing soil porosity (Fig. 7a). Meanwhile, the smooth-terms of the model show a non-linear relationship between mangrove abundance with volumetric water content and soil salinity, whereby abundance decreased with intermediate soil water content, and increasing soil salinity (Fig. 7b & c).
For mangrove species richness, the best-supported model consists of two explanatory environmental variables which included volumetric water content, and soil salinity for the linear term and smooth-term, respectively. However, these environmental variables are less important in explaining the variation in species richness as indicated by a non-significant P value (P > 0.05) (Table 7).
Lastly, the best-supported model for mangrove diversity (Shannon–Wiener) includes three environmental variables such as volumetric water content, soil salinity, and distance to sea/river (Table 7). The model has smooth-terms only showing a positive non-linear relationship with at least two environmental variables, where diversity increased with increasing volumetric water content and distance to sea/river (Fig. 8a & c). In contrast, the remaining smooth-term shows a negative non-linear relationship, whereby mangrove diversity decreased with increasing soil salinity (Fig. 8b).
Discussion
Mangrove diversity
In the Indo-Pacific region, the Philippines is regarded with a high species diversity of mangroves of which 39 species can be found in the country (Primavera et al. 2004). The present study documented a total of 22 mangrove tree/palm species (one species documented outside the plot), as well as six non-tree/palm mangroves, and six mangrove associates. The number of mangrove tree/palm species found along Carigara Bay was similar or comparable to the mangrove forests in other areas or islands in the country such as Tacloban, Leyte Island (21 species, Patindol and Casas 2019), Calauit Island (24 species, Malabrigo et al. 2016), and Puerto Princesa Bay, Palawan Island (25 species, Dangan-Galon et al. 2016), but higher than documented in the coastal areas of San Juan, Batangas (11 species, Gevaña et al. 2008), Ajuy and Pedada Bays, Panay Island (13 species, Sinfuego and Buot 2014), and estuarine area of Maligaya, Palanan, Isabela (14 species, Baleta and Casalamitao 2016). Likewise, the number of mangrove species in the study area was higher than in other tropical mangrove ecosystems such as in the tropical lagoon, Setiu Malaysia (17 species, Islam et al. 2022), but comparable to Belitung Island, Indonesia (20 species, Irawan et al. 2021). The mangrove species documented in the study area constitute 56 to 72% (including the non-tree/palm mangroves) of the country’s mangrove species, indicating the need for protection against anthropogenic disturbances (e.g., land use conversion) (Primavera et al. 2004). In addition, the overall dominant species in terms of abundance was Sonneratia alba J. Smith (mangrove apple), which differs from other coastal areas wherein the most dominant are other mangrove species including Rhizophora apiculata Blume (tall-stilt mangrove), and Nypa fruticans (Thunb.) Wurmb. (nipa palm) as reported in the studies of Dangan-Galon et al. (2016) and Baleta and Casalamitao (2016).
The overall average mangrove diversity (Shannon–Wiener) was 0.82 ± 0.05, which was comparable to the findings of Patindol and Casas (2019) for mangrove forests in the coastal areas of Tacloban in Leyte with an average diversity of 0.91, but a little higher than the recorded diversity (0.64) by Dangan-Galon et al. (2016) for mangrove forests along Puerto Princesa Bay, Palawan Island. Generally, diversity in the riverine was higher compared to fringe mangrove forests, particularly in the middleward and the landward zones. A total of 14 species were recorded in the riverine mangrove forests which were mainly dominated by Nypa fruticans (Thunb.) Wurmb. (nipa palm) and Avicennia rumphiana Hall. f. This observed difference was similar to the findings of Singh (2020) who found greater diversity as well as species richness for the mangrove plant community in estuarine or riverine areas. This higher diversity of mangroves in riverine areas can be attributed to the reduced salinity due to freshwater inputs, reduction of exposure to sulfates, and increase in sediments and nutrients (Singh 2020). Moreover, Utawale et al. (1973) reported that the coastal type of mangroves has less diversity, however, the present study showed that fringe mangroves have higher diversity compared to the riverine, though for the landward zone only. This high diversity in the landward zone of fringe mangrove forests could indicate similar conditions in riverine areas with reduced salinity which eventually supports a greater number of mangrove species. Such reduction in salinity in the landwards can be the result of the dilution of groundwater with freshwater from the fluvial origin such as runoff and infiltration (Vilarrúbia 2000). As observed in the study area, there were small intermittent channels of freshwater as well as groundwater from inland that supplies freshwater to the landward zone, where these were evident during rainy periods. This could explain the presence of some individuals of Nypa fruticans (Thunb.) Wurmb. (nipa palm), a mangrove species that thrives where there are freshwater inputs (Islam et al. 2022). On the other hand, reduced diversity in the middleward and seaward in fringe mangrove forests can be explained by the presence of fewer species, these zones were mainly dominated by Sonneratia alba J. Smith (mangrove apple), and in many locations, only the said mangrove species could be observed.
Mangrove forest structure
Though no significant variations of DBH were detected for mangrove trees in the study area, the observed average DBH values were 15.50 ± 0.70 and 18.43 ± 1.48 for fringe and riverine mangrove forests, respectively. The DBH values were higher when compared to mangrove forest sites in Tacloban, Leyte (8.95 cm, Patindol and Casas 2019), but lower than from San Juan Batangas, Philippines (28.03 cm, Gevaña et al. 2008). The recorded large DBH values were for Avicennia rumphiana Hall. f., both in fringe and riverine mangrove forests, with the largest value of 152.15 cm in the riverine mangrove forests. However, large-sized, and adult mangrove trees (> 100 cm DBH) were exclusively observed in the riverine mangrove ecosystems and from Avicennia rumphiana Hall. f. only, which can be an indication of a mature forest (Kiruba-Sankar et al. 2017). For tree height, taller mangroves were observed in the landward (7.00 ± 0.31 m), which is comparable to the tree height (6.15 m) of coastal mangroves of Tacloban in Leyte (Patindol and Casas 2019), but lower than in Setiu Lagoon Peninsular, Malaysia (14.65 m, Islam et al. 2022). The overall stem densities of mangroves were 1553.03 ± 106.96 and 1424.97 ± 145.64 stems ha−1, lower compared to mangrove forests of Zambezi River Delta, Mozambique (Trettin et al. 2015), and Pongara National Park, Gabon Estuary (Trettin et al. 2021). The relatively low tree density for mangrove forests particularly in riverine areas indicates good structural development (Cintron and Schaefer-Novelli 1983). Lastly, canopy cover was generally lower in the middleward and seaward/along water. The creation of a canopy gap can be a key driver to the natural regeneration of tropical mangroves (Kathiresan and Bingam 2001). As observed in the riverine mangrove forests, regenerants were common particularly along the water, mainly composed of Avicennia marina (Forsk.) Vierh. (grey mangrove), and Ceriops tagal (Perr.) C.B. Rob. (spurred mangrove). On the other side, the occurrence of a dense canopy does not allow full penetration of sunlight, which is a necessary factor for the growth of plants (Kiruba-Sankar et al. 2017).
Variation in mangrove species composition
The NMDS ordination showed the presence of variation in mangrove species composition between zones and mangrove forest types (Fig. 4). According to Trettin et al. (2015), the variations in site conditions are implied to drive species zonation for mangroves. In the study area, the middleward of fringe mangrove forests was strongly associated with a single species only, specifically Aegiceras corniculatum (L.) Blanco (river mangrove). This shrub to small tree mangrove species was commonly present in the middleward zone in clusters, where it thrives in the muddy substrate, and under the regular influence of tides. However, this species was also observed to occur in tidal creeks and river mouths (Primavera et al. 2004). Meanwhile, the mangrove species such as Camptostemon philippinensis (Vidal) Becc., Aegiceras floridum Roem. and Schult. (black mangrove), Rhizophora mucronata Lam. (Asiatic mangrove), Sonneratia alba J. Smith (mangrove apple), and Lumnitzera littorea (Jack) Voigt. (black mangrove) were strongly associated with the seaward zone of fringe mangrove forests, as also likely observed in previous studies by Yuliana et al. (2019), and Raganas and Magcale-Macandog (2020). The mangrove species are known to thrive closest to the sea with the highly saline conditions and can survive in inundated substrate conditions for a long time (Primavera et al. 2004, Crase et al. 2013, Raganas and Magcale-Macandog 2020). Conspicuously, among the aforementioned species, Sonneratia alba J. Smith (mangrove apple) was the most dominant species forming a monospecific stand, and thrives even in a coralline substrate, with very minimal sediment deposits. Moreover, the mangrove Rhizophora mucronata Lam. (Asiatic mangrove) could be found in other locations besides the seaward, where the species is also strongly associated with the soft muds of estuarine rivers and tidal creeks (Primavera et al. 2004). Alternatively, all the zones in the riverine and landward zone in fringe mangrove forests share most of the mangrove species, with the most abundant species such as Nypa fruticans (Thunb.) Wurmb. (nipa palm), Avicennia rumphiana Hall. f., and Avicennia marina (Forsk.) Vierh. (grey mangrove). Most of the mangrove species found among these habitats or zones are generally considered to have low to optimum salinity tolerances (Raganas and Magcale-Macandog 2020). The similarity in species composition among these zones could be strongly associated with the similarity in environmental conditions. The riverine mangrove forests can receive freshwater inputs from the river flow, likewise, the landward zone in fringe mangrove forests receives its freshwater inputs through small freshwater channels and surface run-off, reducing salinity conditions in these environments (Singh 2020). Furthermore, similarities in substrate compositions were noticeable, characterized by muddy or muddy-sandy substrates. Therefore, the mentioned environmental conditions can strongly explain why riverine areas together with the landward zone in fringe mangroves support similar mangrove species assemblage.
Mangrove species indicators
The species indicator analysis further complements the ordination results on the similarity of mangrove species composition. Accordingly, indicator species are those organisms that might serve as an indicator of environmental or habitat quality and therefore can be helpful for monitoring purposes (Amarasinghe et al. 2021). The present study identified two mangrove species as indicators for the landward zone in fringe mangrove forests, these were Scyphiphora hydrophyllacea Gaertn. (yamstick mangrove) and Osbornia octodonta F. Muell. (myrtle mangrove). The mangrove species are characteristically shrubs to small or medium trees, typically found along high tide lines on exposed rocky and sandy shores, and as well on muddy landwards. Both species tolerate high salinity conditions (Primavera et al. 2004). Consistent with the analysis of the similarity in species composition, Aegiceras corniculatum (L.) Blanco (river mangrove) serves as an indicator species for the middleward zone in fringe mangrove forests. The presence of this species indicates a highly saline environment (Primavera et al. 2004), as well as in muddy substrate under the regular effects of tides and waves. Meanwhile, Rhizophora apiculata Blume (tall-stilt mangrove) and Sonneratia alba J. Smith (mangrove apple) were found to be indicator species for both landward and middleward, and both middleward and seaward zones, respectively, in fringe mangrove forests. As observed, Rhizophora apiculata Blume (tall-stilt mangrove) was abundant both in the landward and middleward which formed a monospecific stand in muddy substrate conditions. As well, Sonneratia alba J. Smith (mangrove apple) also formed a monospecific stand in the meddleward especially closest to the sea, and regularly under the influence of tides and waves. The presence Rhizophora apiculata Blume (tall-stilt mangrove) and Sonneratia alba J. Smith (mangrove apple) indicates environments that are subjected to flooding conditions for longer periods, and high salinity (Sreelekshmi et al. 2018; Irawan et al. 2021).
In the riverine mangrove forests, no species is an indicator for the landward zone alone, though Ceriops tagal (Perr.) C.B. Rob. (spurred mangrove) was recognized as an indicator species for middleward, and two species such as Avicennia alba Blume and Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove) as indicators for along the water/river. Meanwhile, mangrove species including Nypa fruticans (Thunb.) Wurmb. (nipa palm), Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove), and Avicennia officinalis L. (Indian mangrove) were identified as indicator species for multiple zones and were widely distributed in riverine mangrove forests. All the mentioned mangrove species are residents of riverine mangrove forests, growing in compact and deep mud or sandy-mud substrates (Primavera et al. 2004). Interestingly, almost all the species including Ceriops tagal (Perr.) C.B. Rob. (spurred mangrove), Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove), Avicennia alba Blume, Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove), and Avicennia officinalis L. (Indian mangrove) were only found in riverine mangrove forests. This may suggest strict environmental requirements for their establishment and growth, particularly, the continuous supply of freshwater that reduces salinity conditions (Raganas and Magcale-Macandog 2020). For example, in the study by Barik et al. (2017), Nypa fruticans (Thunb.) Wurmb. (nipa palm) have been recognized as low-salinity indicator species that primarily inhabit oligohaline to mesohaline zones.
In addition, mangrove species such as Avicennia marina (Forsk.) Vierh. (grey mangrove), Excoecaria agallocha L. (blind-your-eye mangrove), and Avicennia rumphiana Hall. f. were identified as indicator species, generally in the landward zone of fringe and the different zones in riverine mangrove forests. As previously explained, the environmental conditions (e.g., freshwater inputs) from these zones or habitats are likely similar to which the three mangrove species occur.
Species distribution with environmental variables
Environmental factors are known to have a significant influence on the spatial distribution or zonation of mangroves (Trettin et al. 2015, Raganas and Magcale-Macandog 2020, Irawan et al. 2021). The quantitative analysis in the present study revealed that mangrove species can be generally classified as riverine and fringing based on the specific environmental variables that explain their distribution and occurrence. For example, the occurrence of mangrove species including Ceriops decandra (Griff.) Ding Hou (flat-leaved spurred mangrove), Avicennia officinalis L. (Indian mangrove), and Excoecaria agallocha L. (blind-your-eye mangrove) were closely determined by soil depth. These mangroves were commonly found and abundant in riverine mangrove forests, with deep muddy soil substrate often exceeding 2 m, unlike the fringe mangrove forests with thin soil deposits (< 1 m). The distribution of Scyphiphora hydrophyllacea Gaertn. (yamstick mangrove) and Bruguiera cylindrica (L.) Blume (small-leafed orange mangrove) was specifically determined by the distance from the seaward or along the river’s edge. The two species have been primarily observed to occur in the landward most in riverine or fringe mangrove forests. Irawan et al. (2021) also made similar observations and noted that these species grow more tolerant to shorter seawater inundation. Moreover, the increasing soil dry bulk density closely determines the distribution of Heritiera littoralis Dryand. ex W. Ait. (looking-glass mangrove), Nypa fruticans (Thunb.) Wurmb. (nipa palm), and Avicennia rumphiana Hall. f. The mangrove species have been also observed to occur in higher intertidal areas and drylands along forest margins (Primavera et al. 2004). In the study area, the species were growing in the landwards of riverine mangrove forests with denser, and dryer soil, and with mixtures already of mineral materials as indicated by brown or light brown soil color.
Meanwhile, the distribution of fringe mangrove species specifically Aegiceras corniculatum (L.) Blanco (river mangrove), Rhizophora apiculata Blume (tall-stilt mangrove), and Xylocarpus granatum Koen. (cedar mangrove), was closely influenced by increasing water content. The mangroves Aegiceras corniculatum (L.) Blanco (river mangrove), and Rhizophora apiculata Blume (tall-stilt mangrove) were found to be abundant in the middleward zones where the muddy soils were often saturated with seawater due to regular flooding by tides. The occurrence of mangroves particularly Rhizophora apiculata Blume (tall-stilt mangrove) at this zone can be likely explained by the location of the establishment of its larger propagules, which are more likely to be stranded and develop in lower and frequently flooded areas (Sreelekshmi et al. 2018). For Xylocarpus granatum Koen. (cedar mangrove), it was observed in the landward zone of fringe mangrove forests where tides can also reach. The tree species has been commonly described as occurring in the upper intertidal zone of mangrove forests, but mature trees are occasionally found at lower elevations (Allen et al. 2003). Furthermore, the distributions of the group of mangroves consisting of Avicennia marina (Forsk.) Vierh. (grey mangrove), Camptostemon philippinensis (Vidal) Becc., Aegiceras floridum Roem. and Schult. (black mangrove), Sonneratia alba J. Smith (mangrove apple), and Rhizophora mucronata Lam. (Asiatic mangrove) were closely linked to higher soil salinity and soil porosity. These mangrove tree species were observed closest to the sea where they are under the influence of tide for most of the time, and are first to receive the force of incoming waves. The mangrove species are known to be tolerant to high salinity (Sreelekshmi et al. 2018), and their adaption to salt tolerances can be classified as salt accumulators like the Sonneratia alba J. Smith (mangrove apple), the presence of salt-secreting glands as exhibited by Avicennia marina (Forsk.) Vierh. (grey mangrove), and Aegiceras floridum Roem. and Schult. (black mangrove), and salt excluders like Rhizophora mucronata Lam. (Asiatic mangrove) (Md Isa and Suratman 2021). For example, Sonneratia alba J. Smith (mangrove apple) grows in waters between 5 and 50% seawater, according to Ball and Pidsley (1995).
Relationship between mangrove diversity with environmental variables
The edaphic factors have been recognized to have a major influence on the mangrove community (Perera et al. 2013; Dangan-Galon et al. 2016; Barik et al. 2017). The present study showed that the abundance of mangroves had a direct positive association with soil porosity, where specifically abundance increases with increasing porosity. The existing relationship could be strongly explained by the higher number of mangrove individuals occurring in the seaward zone of fringe and the landward zone of riverine mangrove forests. The mangrove species such as Sonneratia alba J. Smith (mangrove apple) and Nypa fruticans (Thunb.) Wurmb. (nipa palm) occur abundantly in the previously mentioned zones, respectively, where the substrate has primarily been observed to be very sandy, a substrate type that is associated with higher porosity. Previous studies have indicated that both species preferred sandy substrates (Baleta and Casalamitao 2016, Dharmawan and Pramudji 2020).
It has been noted also that hydrological processes may have a strong influence on mangroves (Cunha-Lignon et al. 2011). With this study, water content significantly influences abundance, where mangroves tend to be least abundant with moderate water content, however, most importantly, mangrove diversity increases with increasing soil water. In the riverine mangrove forests, diversity linearly increased from the landward towards the edge of the river, where the soil becomes more saturated. In this case, the increase in soil water content can be attributed to the freshwater inputs from the river, which lowers the soil salinity, and consequently promotes a higher diversity of mangroves (Singh 2020).
Among the different edaphic factors, it is soil salinity has been identified to be one with the most significant influence in shaping the mangrove ecosystems (Perera et al. 2013, Sreelekshmi et al. 2018, Prasanna et al. 2019, Raganas and Magcale-Macandog 2020). The current study has demonstrated that soil salinity negatively affects both the abundance and diversity of mangroves where both decrease with increasing salinity conditions. This observed pattern corroborated with the result of the study by Perera et al. (2013) for tropical mangrove communities on the northwestern coast of Sri Lanka. In the study area, fringe mangrove forests particularly the seaward are characterized by a reduced number of species, where in many locations, only monospecific stands of salt-tolerant Sonneratia alba J. Smith (mangrove apple) (Md Isa and Suratman 2021) together with other few species were present. Concerning high soil salinity, the establishment of other mangrove species is possibly being prevented as this environmental condition limits water uptake, decreases photosynthesis, and is coupled with other negative impacts such as on reduction in tree density and height (Singh 2020).
Finally, distance to sea or river was another important determinant of mangrove diversity, wherein diversity was found to increase with increasing distance from the sea or river. Though the pattern is not reflected in the case of riverine, the effect of seaward distance on diversity is much more evident in the fringe mangrove forests. Again, the higher diversity in the landward zone of fringe and riverine mangrove forests, in general, can be attributed to lower salinity, and might as well nutrient supply.
Limitations and directions for future research
The current study has some limitations and thus can be a basis for future research. Firstly, the study explored the response of mangrove diversity with limited environmental factors, therefore additional studies are needed to be conducted considering other edaphic factors especially nutrients and other physicochemical properties. Secondly, the study has been conducted in the coastal areas that are typically with fringe and riverine mangrove forests, and possibly including other mangrove forests (e.g., basin and dwarf mangrove forests) in future investigations can further enrich the current understanding of mangrove diversity and assemblage in the region. Lastly, the study was limited only to a single bay or coastal area, similar studies in other parts of the country or neighboring countries will help determine the generality of the current results.
Conclusions
Mangrove forests are among the most productive, and complex ecosystems which are comprised of salt-tolerant plants. The present study has shown that the riverine mangrove forests were the most diverse, and structurally complex, indicating their protection and conservation values. The mangrove species composition varied where the riverine and the landward most fringe mangrove forests were generally associated with species with low to optimum salt-tolerances, while mangrove stands closer to the sea were associated with highly salt-tolerant species. In addition, specific mangrove species were associated with certain or multiple zones and mangrove forest types, which may suggest specific environmental or habitat requirements. Lastly, mangrove species can be generally classified as riverine and fringing based on the environmental variables explaining their distributions, and it has been found that soil porosity, water content, soil salinity, and distance from the sea or river’s edge were the most important environmental factors that determine diversity patterns of mangroves.
Availability of data and material
Interested party may request data and materials from the corresponding author.
References
Allen JA, Krauss KW, Hauff RD (2003) Factors limiting the intertidal distribution of the mangrove species Xylocarpus granatum. Oecologia 135:110–121. https://doi.org/10.1007/s00442-002-1167-2
Amarasinghe AAT, Putra CA, Henkanaththegedara SM, Dwiyahreni AA, Winarni NL, Sunaryo MC, Supriatna J (2021) Herpetofaunal diversity of West Bali National Park, Indonesia with identification of indicator species for long-term monitoring. Global Ecol Conserv 28:e01638. https://doi.org/10.1016/j.gecco.2021.e01638
Baleta FN, Casalamitao RS Jr (2016) Species composition, diversity and abundance of mangroves along the estuarine area of Maligaya, Palanan, Isabela, Philippines. Int J Fish Aquat Stud 4(2):303–307
Ball MC, Pidsley SM (1995) Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata Northern Australia. Funct Ecol 9(1):77–85. https://doi.org/10.2307/2390093
Barik J, Mukhopadhyay A, Ghosh T, Mukhopadhyay SK, Chowdhury SM, Hazra S (2017) Mangrove species distribution and water salinity: an indicator species approach to Sundarban. J Coast Conserv 22:361–368. https://doi.org/10.1007/s11852-017-0584-7
Cintron G, Novelli YS (1984) Methods for studying mangrove structure. In: Snedaker SC, Snedaker JG (eds) The mangrove ecosystem: research methods. United Nations Educational, Scientific and Cultural Organization, Paris, pp 91–113
Cintron G, Schaefer-Novelli Y (1983) Introduction to mangrove ecology. Regional Office of Science and Technology, Latin America and UNESCO, Montevideo
Clough BF, Scott K (1989) Allometric relationships for estimating aboveground biomass in six mangrove species. For Ecol Manage 27:117–127. https://doi.org/10.1016/0378-1127(89)90034-0
Crase B, Liedloff A, Vesk PA, Burgman MA, Wintle BA (2013) Hydroperiod is the main driver of the spatial pattern of dominance in mangrove communities. Glob Ecol Biogeogr 22:806–817. https://doi.org/10.1111/geb.12063
Cunha-Lignon M, Kampel M, Menghini RP, Schaeffer-Novelli Y, Cintrón G, Dahdouh-Gueba F (2011) Mangrove Forests Submitted to Depositional Processes and Salinity Variation Investigated using satellite images and vegetation structure surveys. J Coastal Res 64:344–348
Dangan-Galon F, Dolorosa R, Sespeñe J, Mendoza N (2016) Diversity and structural complexity of mangrove forest along Puerto Princesa Bay, Palawan Island, Philippines. J Marine Island Cult 5:118–125. https://doi.org/10.1016/j.imic.2016.09.001
De Cáceres M, Jansen F, Dell N (2020) Package ‘indicspecies’. http://www2.uaem.mx/r-mirror/web/packages/indicspecies/indicspecies.pdf. Accessed 17 Mar 2023
Decena SCP, Villacorta-Parilla S, Arribado AO, Macasait DR Jr, Arguelles MS, Salamia SS, Relevo ES (2022) Impact of land use conversion on carbon stocks and selected peat physico-chemical properties in the Leyte Sab-a Basin Peatland Philippines. Wetlands 42(2):1. https://doi.org/10.1007/s13157-021-01520-8
Decena SCP, Avorque CA, Arribado AO, Macasait DR Jr (2023) Aboveground and belowground carbon stocks in mangrove ecosystems along Carigara Bay in Leyte. Manuscript submitted for publication, Philippines
Dharmawan IWE, Pramudji (2020) Mangrove community structure in Papuan small islands, case study in Biak Regency. IOP Conf Series: Earth Environ Sci 550:012002. https://doi.org/10.1088/1755-1315/550/1/012002
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Garcia KB, Malabrigo PL Jr, Gevaña DT (2014) Philippines’ mangrove ecosystem: status, threats and conservation. In: Faridah-Hanum I, Latiff A, Hakeem K, Ozturk M (eds) Mangrove Ecosystems of Asia. Springer, New York, NY, pp 81–94. https://doi.org/10.1007/978-1-4614-8582-7_5
Gevaña DT, Pulhin FB, Pampolina NM (2008) Carbon stock assessment of a mangrove ecosystem in San Juan, Batangas. J Environ Sci Manag 11(1):15–25
Goloran A, Demetillo MT, Betco GL (2020) Mangroves assessment and diversity in coastal area of Barangay Cagdianao, Claver, Surigao Del Norte, Philippines. Int J Environ Sci Nat Resour 26(3):69–77
Irawan A, Chikmawati T, Sulistijorini (2021) Diversity and zonation of mangrove flora in Belitung Island. Indonesia. Biodiversitas 22(5):2981–2992. https://doi.org/10.13057/biodiv/d220563
Islam MA, Idris MH, Bhuiyan MDKA, Ali MS, Abdullah MT, Kamal AHM (2022) Floristic diversity, structure, and carbon stock of mangroves in a tropical lagoon ecosystem at Setiu Malaysia. Biodiversitas 23(7):3685–3696. https://doi.org/10.13057/biodiv/d230746
IUCN (2023) The IUCN Red List of Threatened Species. Version 2022–2. https://www.iucnredlist.org. Accessed 13 Mar 2023
Kathiresan K, Bingam BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Kauffman JB, Bhomia RK (2017) Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: Global and regional comparisons. PLoS ONE 12(11):e0187749. https://doi.org/10.1371/journal.pone.0187749
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC (2011) Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 31:343–352. https://doi.org/10.1007/s13157-011-0148-9
Kauffman JB, Donato DC (2012) Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. Working Paper 86. CIFOR, Bogor, Indonesia
Kiruba-Sankar R, Krishnan P, Roy SD, Angel JRJ, Goutham-Bharathi MP, Kumar KL, Ragavan P, Kaliyamoorthy M, Muruganandam R, Rajakumari S, Purvaja R, Ramesh R (2017) Structural complexity and tree species composition of mangrove forests of the Andaman Islands, India. J Coast Conserv 22:217–234. https://doi.org/10.1007/s11852-017-0588-3
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21:471–477. https://doi.org/10.1017/S0266467405002476
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Z 15(3):259–263. https://doi.org/10.1127/0941-2948/2006/0130
Madeira BG, Espírito-Santo MM, Neto SD, Nunes YRF, Azofeifa GAS, Fernandes GW, Quesada M (2009) Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecology 201:291–304. https://doi.org/10.1007/s11258-009-9580-9
Malabrigo PL Jr, Umali AGA, Replan EL (2016) Damage assessment and recovery monitoring of the mangrove forests in Calauit Island affected by Typhoon Yolanda (Haiyan). J Environ Sci Manag 2:39–46
Marteleira RGS (2019) Improving the resilience of water supply towards climate change impacts in Tacloban, Philippines. PhD dissertation, Universidade Nova de Lisboa, 1–179
Md Isa NN, Suratman MN (2021) Structure and diversity of plants in mangrove ecosystems. In: Rastogi RP, Phulwaria M, Gupta DK (eds) Mangroves: Ecology, biodiversity and management. Springer Nature Singapore Pte Ltd, Singapore, pp 362–369. https://doi.org/10.1007/978-981-16-2494-0_15
Oksanen J (2019) Vegan: an introduction to ordination. https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf. Accessed 16 Mar 2023
Patindol TA, Casas EV Jr (2019) Species diversity and composition of mangroves in Tacloban City Philippines. Ann Trop Res 41(2):67–75
Perera KARS, Amarasinghe MD, Somaratna S (2013) Vegetation structure and species distribution of mangroves along a soil salinity gradient in a micro tidal estuary on the north-western coast of Sri Lanka. Am J Marine Sci (1):7–15. https://doi.org/10.12691/marine-1-1-2
Pototan BL, Capin NC, Delima AD, Novero AU (2021) Assessment of mangrove species diversity in Banaybanay, Davao Oriental, Philippines. Biodiversitas 22:144–153. https://doi.org/10.13057/biodiv/d220120
Prasanna MGM, Ranawana KB, Jayasuriya KMGG (2019) Species composition, abundance and diversity of mangroves in selected sites in Amprara District in the east coast of Sri Lanka. Ceylon J Sci 48(2):169–175. https://doi.org/10.4038/cjs.v48i2.7621
Primavera JH (2000) Development and conservation of Philippine mangroves: institutional issues. Ecol Econ 35(1):91–106. https://doi.org/10.1016/S0921-8009(00)00170-1
Primavera JH (2009) Field guide to Philippine mangroves. Zoological Society of London-Philippines, Iloilo City, Philippines
Primavera JH, Sadaba RB, Lebata MJHL, Altamirano JP (2004) Handbook of mangroves in the Philippines – Panay. SEAFDEC Aquaculture Department, Tigbauan, Iloilo, Philippines
Quevedo J, Uchiyama Y, Kohsaka R (2020) Perceptions of local communities on mangrove forests, their services and management: implications for Eco-DRR and blue carbon management for Eastern Samar, Philippines. J Forest Res 25(1):1–11. https://doi.org/10.1080/13416979.2019.1696441
Quiñones CMO, Asio VB (2015) Soils derived from ophiolitic rocks in northeastern Leyte: morphological, physical, and chemical properties. Ann Trop Res 37:36–56
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/. Accessed 17 Mar 2023
Raganas AFM, Magcale-Macandog DB (2020) Physicochemical factors influencing zonation patterns, niche width and tolerances of dominant mangroves in southern Oriental Mindoro, Philippines. Ocean Life 4(2):51–62. https://doi.org/10.13057/oceanlife/o040201
Sebidos RF, Galinato MI (1996) Mangrove floral composition and zonation in Western Leyte. Ann Trop Res 18:35–48
Sinfuego KS, Buot IE Jr (2014) Mangrove zonation and utilization by the local people in Ajuy and Pedada Bays, Panay Island, Philippines. J Marine Island Cult 3:1–8. https://doi.org/10.1016/j.imic.2013.11.002
Singh JK (2020) Structural characteristics of mangrove forest in different coastal habitats of Gulf of Khambhat arid region of Gujarat, west coast of India. Heliyon 6:e04685. https://doi.org/10.1016/j.heliyon.2020.e04685
Sreelekshmi S, Preethy C, Varghese R, Joseph P, Asha C, Nandan S, Radhakrishnan C (2018) Diversity, stand structure, and zonation pattern of mangroves in southwest coast of India. J Asia-Pac Biodivers 11:573–582. https://doi.org/10.1016/j.japb.2018.08.001
Trettin CC, Stringer CE, Zarnoch SJ (2015) Composition, biomass and structure of mangroves within the Zambezi River Delta. Wetlands Ecol Manage 24:173–186. https://doi.org/10.1007/s11273-015-9465-8
Trettin CC, Dai Z, Tang W, Lagomasino D, Thomas N, Lee SK, Simard M, Ebanega MO, Stoval A, Fatoyinbo TE (2021) Mangrove carbon stocks in Pongara National Park, Gabon. Estuar Coast Shelf Sci 259:107432. https://doi.org/10.1016/j.ecss.2021.107432
Utawale AG, Dwivedi SN, Singbal SYS (1973) Ecology of mangroves in Mandovi and Zuari estuaries and the interconnecting Cumbarja canal of Goa Ind. J Mar Sci 2:47–55
Vilarrúbia TV (2000) Zonation pattern of an isolated mangrove community at Playa Medina, Venezuela. Wetlands Ecol Manage 8:9–17. https://doi.org/10.1023/A:1008458409143
Wood S (2019) Mgcv: mixed GAM computation vehicle with automatic smoothness estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed 15 Mar 2023
Yuliana E, Hewindati YT, Winata A, Djatmiko WA, Rahadiati A (2019) Diversity and characteristics of mangrove vegetation in Pulau Rimau Protection Forest, Banyuasin District, South Sumatra Indonesia. Biodiversitas 20(4):1215–1221. https://doi.org/10.13057/biodiv/d200438
Acknowledgements
We thank the Municipal Mayors, Hon. Norman D. Sabdao of San Miguel, and Hon. Aron C. Balais of Barugo, Leyte, as well as the Barangay Captains, Hon. Leonardo T. Cadorna (Brgy. Mawodpawod), Hon. Pacita S. Abril (Brgy. Malpag), Hon. Lyndon C. Viñas (Brgy. Bagacay), and Hon. Felipe L. Cirera (Brgy. Minuhang) for granting the permission in the access of the study area, and accommodation. We also acknowledge the Department of Environment and Natural Resources (DENR), Region VIII, Tacloban City, Leyte, for granting the Wildlife Gratuitous Permit (DENR-GP No. 2022-39).
Author information
Authors and Affiliations
Contributions
All the authors contributed significantly to the development of the manuscript. SCPD and CAA designed the study. The field data collection and analyses were performed by all the authors (SCPD, CAA, DRM, and AOA). The initial draft of the manuscript was prepared by SCPD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval not applicable.
Conflicts of interest
There are no conflicts of interest to disclose.
Consent for publication
All authors have read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Decena, S.C.P., Avorque, C.A., Requioma, D.M. et al. Diversity and assemblage of mangroves along the carigara bay in Leyte, Philippines. Biologia (2024). https://doi.org/10.1007/s11756-024-01765-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11756-024-01765-8