Abstract
This study aimed to investigate the ability of twelve strains of Enterococcus spp. to form biofilms on stainless steel (AISI 316 L and AISI 304 L) and to characterize the physicochemical properties of the bacterial and surface supports. The study also examined the auto-aggregation and extracellular polymeric substances of strains in both planktonic and sessile forms. The results showed that all Enterococcus spp. strains tested were capable of forming biofilms, with varying degrees reaching 7.41 log CFU/cm2 on AISI 316 L for Enterococcus mundtii A1. On AISI 304 L, the highest biofilm recorded was 7.01 log CFU/cm2 for Enterococcus mundtii A13. It was observed that auto-aggregation was influenced by the strains’ hydrophobicity. These results shed light on the possible application of Enterococcus spp. biofilm as a strategy to control pathogenic biofilms in various industries and provide insight into the role of physicochemical properties in biofilm formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, Enterococcus spp. have gained significant attention among the wide group of lactic acid bacteria (LAB) due to their remarkable health-promoting properties. These species occur naturally in a variety of food sources including vegetables, dairy, meat, fish, and plant material. The diverse properties of Enterococcus spp. have led to their application in different food systems. Enterococcus spp. have shown potential application in dairy products due to their ability to withstand harsh conditions, such as temperature, salinity, and pH. Moreover, they positively impact the taste, texture, aroma, and sensory profile of various types of cheese (Giraffa and De Fernando 2022). Additionally, they are known for their ability to produce bacteriocins, specifically enterocins, which play a crucial role in inhibiting the growth of other bacteria (Favaro et al. 2014; Qiao et al. 2020; Du et al. 2022).
The probiotic potential and health-promoting effects of Enterococcus strains have been widely investigated (Zommiti et al. 2018; Nami et al. 2019). These bacteria have been classified as probiotics and are commonly used in the food industry for the production of functional foods, as well as for the prevention and treatment of certain animal and human diseases. Enterococcal strains have been employed in the prevention of certain animal and human diseases, such as chronic intestinal diseases (Ghosh et al. 2013). Some strains, such as Enterococcus durans M4–5, Enterococcus mundtii ST4SA, and E. durans LAB18s have been shown to induce anti-inflammatory effects, contribute to the integrity of the intestinal epithelium, and lower human serum cholesterol levels (Avram-Hananel et al. 2010; Liu et al. 2016; van Zyl et al. 2016).
LAB are known to possess the capacity to adhere to and form biofilms on biotic and abiotic surfaces (Khalil and Oleiwi 2021). In the food industry, species such as Lactobacillus spp. and Lactococci spp. display a tendency to attach to moist surfaces and proliferate to form biofilms on various materials, including stainless steel and polystyrene microtiter plates (Ait Ouali et al. 2014; Pérez Ibarreche et al. 2014). Moreover, the ability of LAB to adhere to food components has been explored in several works. For instance, Ly et al. (2006) demonstrated that lactococci adhere to food components such as lipids and hydrophobic compounds. As a novel approach to preventing biofilm formation, LAB biofilms present an alternative to chemical preservatives. In this regard, several studies suggest that LAB biofilms or their antimicrobial production, such as bacteriocins, biosurfactants, and exopolysaccharides, can serve as barriers to preventing pathogenic biofilms (Nel et al. 2002; Rodrigues et al. 2004; Li et al. 2014; Gómez et al. 2016; Pérez Ibarreche et al. 2016; Srivastava and Bhargava 2016; Zanzan et al. 2023).
Although Enterococcus strains have not yet been officially classified as Generally Recognized as Safe (GRAS), no reports of disease caused by commercialized enterococci, such as E. faecium SF68 and E. faecalis (Franz et al. 2011). Therefore, enterococcal strains are used as starter cultures in the food industry, as well as in probiotics, protective cultures, and/or anti-biofilm agents due to their beneficial effects. Several studies have focused on the ability of Enterococcus spp. to adhere and form biofilms on biotic surfaces, such as intestinal cells (Botes et al. 2008; Amaral et al. 2017). Biofilms are communities of microorganisms attached to biotic or abiotic surfaces and surrounded by a matrix of biopolymers. This phenomenon can be found in various environments and can be used for diverse technological applications (Srivastava and Bhargava 2016). Adhesion of bacteria to suitable surfaces is the first critical step for biofilm formation. This process involves various factors, including the physicochemical properties of the surface and bacterial surface (Azelmad et al. 2017), followed by cell aggregation and, ultimately, the formation of a mature biofilm by producing an extracellular biopolymer matrix. This ability of Enterococcus spp. to form biofilms allows them to survive under harsh environmental conditions, such as high temperatures, ionizing radiation, and antibiotics. However, only a few studies have investigated the adhesion and biofilm formation of Enterococcus spp. on materials commonly used in the food industry, such as stainless steel (Zhao et al. 2013; Zanzan et al. 2023).
Hence, the identification of novel strains of Enterococcus spp. holds paramount significance, due to their distinct mechanisms of adhesion and anti-pathogenic activity against industrial surfaces, as well as their technological traits. The aim of this study is to investigate the biofilm-forming capacity of twelve Enterococcus spp. strains isolated from different types of milk on two common food equipment materials, namely AISI 316 L and AISI 304 L stainless steel. In addition to assessing the physicochemical attributes of cells and substrates, the investigation includes the evaluation of the auto-aggregation phenomenon and extracellular polymeric substance production in both planktonic and sessile states.
Material and methods
Enterococcus spp. strains, culture conditions and bacterial suspension
Enterococcus spp. used in the present study were obtained from diverse milk sources and traditional cheese of Morocco, namely camel’s milk, ewe’s milk, and goat’s Jben cheese (Table 1). The strains were grown in de Man Rogosa and Sharpe (MRS) broth (Biokar Diagnostics, Barcelona, Spain) at 30 °C for 16 hours. Following this, the cells were harvested by centrifugation at 4000×g for 20 minutes at 4 °C, and the resulting pellets were resuspended twice in a sterile saline solution (0.1 M) and standardized to 108 CFU/mL at 620 nm (≈ 0.5 McFarland).
Preparation of surfaces
For this study, two types of stainless steel, AISI 316 L and AISI 304 L, were used. To prepare the materials, they were cut into small squares with dimensions of 1 cm × 1 cm × 0.2 cm. Prior to use, the materials were thoroughly cleaned by immersing them in 95% ethanol for 15 minutes to remove any debris, followed by rinsing with distilled water three times. Finally, they were dried and autoclaved at 120 °C for 20 minutes, in accordance with previously published protocols (Zanzan et al. 2023).
Atomic force microscopy
In order to obtain high-resolution images and precise quantitative measurements of surface topography and roughness, atomic force microscopy was employed in this study. Statistical analysis was used to analyze the results. The surface topography and roughness of both AISI 304 L and AISI 316 L stainless steel surfaces were investigated using the Easyscan 2 software from Nanosurf. A tapping mode (dynamic) was used for scanning and measurement, and the measurements were taken under ambient conditions. The Ra value, which represents the arithmetic mean deviation of the profile, was used as the primary descriptor of surface roughness, as recommended by Verran et al. (2000). The roughness measurements were obtained from three replicates to ensure accuracy and reproducibility.
Contact angle measurement
The surface properties of stainless steel (AISI 304 L and AISI 316 L) and bacterial cells were evaluated using the contact angle assay. This method, as described by Busscher et al. (1984), involved depositing a thick layer of cells (108 cells/mL) on a cellulose acetate filter (0.45 μm) and air-drying it on a glass support for 30–45 minutes at 25 °C to achieve stable “plateau contact angles”. The sessile drop method was then used with a goniometer (GBX instruments, France) to determine contact angles on the air-dried surfaces of the stainless steel materials. Triplicate independent cultures of bacteria were used, and three measurements were taken on each surface with each of the test liquids: formamide (99%), diiodomethane (99%), and distilled water. The surface free energy of these liquids is presented in Table 2 (Van Oss et al. 1988).
The Lifshitz-van der Waals component of the surface free energy (γLW) and the electron acceptor (γ+) and donor (γ-) surface tension components were utilized to calculate the contact angle of three liquids using the following equation:
Additionally, the Lewis acid-base surface tension component (γSAB) was defined as:
Hydrophobicity was evaluated by contact angle following the approach of Van Oss and colleagues (Van Oss et al. 1988). Hydrophobicity was defined as the free energy of interaction (ΔG iwi) between two entities (i) of the material when immersed in water (mJm −2). The material was classified as hydrophobic if ΔG iwi < 0 and the interaction between two entities was stronger than each entity (i) with water. Conversely, the material was considered hydrophilic if ΔG iwi > 0 and the interaction was weaker. ΔG iwi was calculated using the equation:
Auto-aggregation assay
The auto-aggregation assay for Enterococcus strains studied was performed as per Krausova et al. (2019) with minor modifications. Briefly, 4 mL of bacterial suspension was incubated at 30 °C for different times (3 hours and 24 hours) without vortexing, and the absorbance (At) was measured. The auto-aggregation percentage (AP) was expressed as:
where A0 represents the absorbance before incubation.
EPS production from cell suspension and adhesion and biofilm formation in Enterococcus spp.
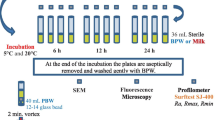
The bacterial suspensions for the determination of total extracellular carbohydrates of planktonic cells were prepared as described above. Planktonic cells were then subjected to centrifugation at 5000 g for 10 minutes to collect the supernatants containing EPS. To quantify the total extracellular carbohydrates involved in adhesion and biofilm formation on stainless steel 316 L, 10 mL of bacterial suspension was deposited onto sterilized Petri dishes containing stainless steel 316 L and incubated for three hours at 30 °C to allow adhesion. The substratu was then washed with sterilized physiological water to remove planktonic cells and ensure that only adhesion and biofilm formation were measured. Next, 10 mL of MRS broth was added and incubated at 30 °C for 24 hours to allow for biofilm formation. To detach the bacterial cells adhered to the substratum, an ultrasonic bath (POWER SONIC 405, Lab Tech, Namyangju-city, Korea) was used by immersing the substratum in a test tube containing 20 mL of physiological saline (NaCl: 9 g/L). The supernatant containing EPS from planktonic cells, adhesion, and biofilm formation was then analysed using the colorimetric method described by Chae et al. (2006).
Colorimetric determination of the total carbohydrate content
EPS quantification was performed using the phenol-sulphuric acid method of DuBois et al. (1956). Two milliliters of EPS suspension obtained from planktonic cells, 3 h adhesion, and 24 h biofilm formation, were pipetted into test tubes. Then, 0.05 mL of 80% phenol solution was added, quickly followed by the addition of 5 mL of 95–98% sulfuric acid. The mixture was allowed to stand for 10 minutes and then incubated at 30 °C for 20–30 minutes in a water bath. The optical density (OD) was measured at a wavelength of 460 nm using a UV-visible spectrophotometer, with glucose used as the standard.
Assessment of Enterococcus spp. biofilm formation on stainless steel surfaces
Ten milliliters of bacterial suspension were deposited onto sterilized substrates in Petri dishes and incubated at 30 °C for 3 hours to facilitate bacterial adhesion. Non-adherent bacteria were carefully removed by rinsing the substrates three times with sterilized distilled water. Next, 10 mL of MRS broth was added, and the substrates were incubated at 30 °C for 24 hours. Following incubation, the substrates were transferred to a test tube containing 20 mL of physiological water and sonicated for 10 minutes using an ultrasonic bath. The resulting bacterial suspension was serially diluted and colony-forming units (CFU) were determined by plating on MRS Agar and incubating for 48 hours at 30 °C (Speranza et al. 2009). Triplicate repetitions were conducted for each strain. The resulting CFU counts were used for subsequent analyses.
Statistical analysis
Statistical analyses were carried out using STATISTICA software version 6 (TIBCO software Inc., California, USA). Bacterial strains were compared using the Tukey test, with a significance level set at p < 0.05. The contribution of significant parameters such as hydrophobicity, electron donor, electron acceptor, and auto-aggregation to biofilm formation on two types of stainless steel was evaluated using principal component analysis (PCA).
Results and discussion
Physicochemical characterization of bacterial and stainless steel surfaces
Contact angle measurements were conducted to assess the surface free energy characteristics of twelve Enterococcus spp. strains and two types of stainless steel (AISI 304 L, AISI 316 L). The results, presented in Table 3, showed significant differences in hydrophobicity among the strains, with values ranging from ΔG iwi = −12.2 mJ/m2 for A8 to ΔG iwi = 14.5 mJ/m2 for E. mundtii A13. Approximately 66.6% of the tested strains displayed hydrophobic characteristics, consistent with previous findings by Kiani et al. (2021), who reported hydrophobic values for E. hirae and E. mundtii. Additionally, E. mundtii A13, E. mundtii A1, E. hirae F420, and E. mundtii A12 (33.3%) exhibited hydrophilic characteristics, which corresponding to the findings of Asria et al. (2023) and Li et al. (2021) for E. mundtii.
The results suggest that Enterococcus species exhibit significant diversity in hydrophobicity, which may be strain-dependent and influenced by various factors such as media culture composition and surface chemistry of the strains (Puniya et al. 2016). Previous studies by Giaouris et al. (2009) showed that different strains of Lactococcus lactis, even within the same species, may exhibit varying degrees of hydrophobicity due to mobile genetic elements such as genes encoding surface proteins and enzymes responsible for polysaccharide biosynthesis. For instance, in the same study of Giaouris et al. (2009) the hydrophobic strain TIL672 was obtained through the transfer of a lactose-protease plasmid from NCDO763 into a hydrophilic strain MG1363. The current study found that AM2-C exhibited higher hydrophobicity than AM2, possibly due to prophage curing. Thus, lactose-protease plasmids and prophages may be genetic determinants for hydrophobicity. In a related study, Li et al. (2015) showed that E. faecalis J2 exhibited higher hydrophobicity than E. faecalis 5–5, which was hydrophilic.
In addition, all Enterococcus strains tested in this study were predominantly electron donors, with values exceeding 45 mJ/m2 for (γ−). This finding is in accordance with Faten et al. (2016), who reported a higher electron donor parameter for lactic acid bacteria. All Enterococcus spp. strains, with the notable exceptions of E. mundtii A13 and E. hirae F420, demonstrated a pronounced electron-acceptor character, as shown by significant variations in surface tension parameters (p < 0.05) (Table 3). These findings indicate that hydrophobicity, along with electron-acceptor and electron-donor properties, are distinctive and varied among Enterococcus spp., confirming the species-specific nature of these surface properties.
The results of Table 3 indicate that AISI 316 L and AISI 304 L exhibited hydrophilic behavior, with ΔG iwi values of 26.5 mJ/m2 and 28.1 mJ/m2, respectively. Moreover, AISI 304 L showed a high electron donor character (γ−) of 51.5 mJ/m2, whereas AISI 316 L had a slightly higher electron donor character of 55 mJ/m2. These findings are consistent with those of Hamadi et al. (2014), who reported that stainless steel 304 L was hydrophilic with a ΔG iwi value of 11.5 mJ/m2 and an electron donor character of 32.5 mJ/m2. In contrast, Al-Hamarneh et al. (2012) reported that AISI 316 L exhibited a hydrophobic property before treatment by plasma corona streamer, as determined by contact angle measurements. Similarly, Casarin et al. (2016) found that AISI 316 L and AISI 304 L were hydrophobic with a weak electron acceptor character based on contact angle measurements.
Surface roughness
The surface topography and roughness (Ra) of the two stainless steel types were analysed and visualized in Figs. 1 and 2 using Easyscan 2 software. The results indicated that there were significant differences in Ra values between the two materials on the nanometer scale, with AISI 316 L exhibiting a high Ra value of 343.23 nm, while AISI 304 L had a low Ra value of 28.42 nm (Fig. 2). This finding is in line with the results of other studies that have shown AISI 316 L to be rougher than AISI 304 L (Azelmad et al. 2017; Tantratian et al. 2022).
However, it should be noted that the study conducted by Casarin et al. (2016) found that AISI 304 L had a Ra value of 32 nm, while AISI 316 L had a Ra value of 21 nm. In general, a Ra value of ≤0.8 μm is considered indicative of a hygienic material in terms of cleanability and reduced bacterial adhesion particularly for stainless steel surfaces (Flint et al. 1997). Based on this criterion, it can be concluded that both types of stainless steel analysed in this study have Ra values that are appropriate for use in industrial settings.
Auto-aggregation activity
Auto-aggregation ability of twelve Enterococcus spp. strains was examined following incubation at 30 °C for 3 hours. Except for E. mundtii A14 strains, which displayed a moderate auto-aggregation ability of 12% (Fig. 3), all strains exhibited low auto-aggregation. At 24 hours, a significant variation in auto-aggregation was noted among the tested strains, ranging from 13.1% for A13 to 29.8% for E. hirae F420 (Fig. 3). All strains indicated an auto-aggregation except for E. mundtii A10, A12, A13, and A15, which exhibited the lowest auto-aggregation ability. This finding is similar to that of Li et al. (2015), who reported auto-aggregation levels of 16.46% and 22.80% for E. faecalis J2 and E. faecalis 5–5, respectively. Kiani et al. (2021) reported auto-aggregation rates of 54% and 44% for E. hirae and E. mundtii, respectively. The observation of an increase in auto-aggregation over time in all strains (Fig. 3) is consistent with the findings of Angmo et al. (2016), who reported that the auto-aggregation ability of LAB was enhanced with time, and was higher after 24 hours compared to 3 hours of incubation. Similarly, Nikolic et al. (2010) reported two types of strains based on auto-aggregation capacity, with some exhibiting faster auto-aggregation ability and others with the lowest. Conversely, Ayyash et al. (2018) stated lower auto-aggregation percentages for Enterococcus faecium isolates during 3 hours and 24 hours of incubation at 37 °C. According to the results obtained, the Enterococcus spp. strains tested did not demonstrate a fast auto-aggregation ability, indicating that they require more than 24 hours to exhibit high levels of auto-aggregation.
The results of the study showed that there was no discernible correlation between auto-aggregation and physicochemical properties (namely hydrophobicity, electron donor and electron acceptor) for all strains examined. Previous studies by Li et al. (2015) and Xu et al. (2009) also reported no significant correlation between auto-aggregation and these physicochemical properties. However, a positive correlation (R2 = 0.51) was observed for eight strains with a hydrophobic character, indicating that auto-aggregation increased with the increasing hydrophobicity of cells (Fig. 4). This finding is in agreement with the results of Nikolic et al. (2010) and Tuo et al. (2013), who reported that strains with auto-aggregation ability were highly hydrophobic and this property may be species-specific. Bacterial cells typically have a variety of macromolecules on their surfaces, including proteins, lipids, and other biomolecules such as bacteriocins, some of which may exhibit hydrophobic characteristics. These regions can interact with each other, promoting self-aggregation or auto-aggregation. The outer surfaces of bacterial cells play an important biological role, as they are involved in constant interactions between the cell (Sleytr 1978). However, for the strains with hydrophilic character, no correlation was found with auto-aggregation, which emphasizes the complexity of the interactions between bacterial strains and their surface properties.
Quantifying exopolysaccharide production by Enterococcus spp. strains
The quantity of extracellular polymeric substances (EPS) was determined using the phenol-sulphuric acid method. For planktonic cells, EPS production varied widely across strains, ranging from 12.82 to 65.84 mg/L, with a significant distinction observed between enterococci (p < 0.05). Notably, E. faecium F58 exhibited the highest EPS yield at 65.84 mg/L, followed by E. mundtii A14 with 39.70 mg/L EPS yield, while the remaining ten enterococci displayed relatively low EPS production, averaging approximately 16.85 mg/L (Fig. 5). At 3 hours and 24 hours of incubation, the EPS content of sessile cells produced by Enterococcus spp. strains ranged from 28.118 to 72.73 mg/L and 20.94 to 75.44 mg/L, respectively (Fig. 6), surpassing the levels observed in planktonic cells after a 3 hours incubation. These findings align with prior research that has demonstrated attachment cells’ capability to generate considerably greater amounts of EPS than their planktonic counterparts (Chae et al. 2006; Harimawan and Ting 2016), while also revealing the positive association between planktonic cells and biofilm formation. E. faecium F58, in particular, demonstrated significantly greater carbohydrate content in both planktonic culture and after 24 hours of attachment relative to other enterococci (p < 0.05). For some of the strains tested, EPS production from planktonic and attachment cells increased significantly (p < 0.05) from 3 hours to 24 hours. Notably, EPS production serves as a protective mechanism for bacterial cells against harsh environmental conditions, rather than a source of energy (Yin et al. 2019; Jiang et al. 2021). These findings suggest that EPS production under biofilm growth is significantly greater than under planktonic growth conditions.
Adhesion and biofilm formation of Enterococcus spp. on stainless steel surfaces
Figures 7 and 8 display the results of biofilm formation by Enterococcus spp. on AISI 316 L and 304 L stainless steel, respectively. Among the strains tested on AISI 316 L, the highest biofilm-forming capacity was observed in E. mundtii A1, with a concentration of 7.41 log CFU/cm2. Conversely, the lowest quantity of biofilm was observed for E. mundtii A11 strain, with 5.81 log CFU/cm2 (Fig. 7). Regarding AISI 304 L, the biofilm formation varied from one bacterium to another (Fig. 8). The quantity of biofilm formed ranged from 4.82 log CFU/cm2 to 7.01 log CFU/cm2, with E. mundtii A13 strain exhibiting the maximum (7.01 log CFU/cm2), while a low quantity was detected for E. mundtii A10 (4.82 log CFU/cm2). Previous studies have highlighted the significant difference in biofilm formation on AISI 316 L and AISI 304 L substrates by LAB (Ait Ouali et al. 2014; Pérez Ibarreche et al. 2014; Faten et al. 2016). These findings align with the study of this work, indicating that Enterococcus spp. can form biofilms on both types of substrates, with a notable distinction.
The findings of the present investigation clearly indicate that Enterococcus spp. strains exhibit a greater tendency to form biofilms on AISI 316 L compared to AISI 304 L. This observation aligns with the report of Tran et al. (2021), who suggest that different types of stainless steels (SS 410, SS 420, SS 316, DSS 2206) show varying levels of bacterial attachment to the surface, which could be attributed to the different chemical compositions and surface properties of each steel. Recent studies have reported that the implantation of certain components can affect bacterial adhesion to stainless steel surfaces (Różańska et al. 2017; Shuai et al. 2018; Meroufel 2020). Zhao et al. (2007) suggested that ion implantation of N+, O+, and SiF3+ on the surface of stainless steel 316 L has a significant effect on reducing bacterial attachment of Pseudomonas aeruginosa, Staphylococcus epidermidis, and Staphylococcus aureus.
Furthermore, the difference in adhesion can also be attributed to surface roughness, as observed in the results, where AISI 316 L was rougher than AISI 304 L. Many studies have reported that the number of adhesions increases with increasing surface roughness (Kouider et al. 2010; Bohinc et al. 2014; Azelmad et al. 2017), which can be attributed to increased surface area and more irregularities on the surface that offer more attachment sites. The irregularities and microstructures on a rough surface can trap and hold substances that serve as a nutrient source for microorganisms. However, Casarin et al. (2016) found no correlation between adhesion and roughness. The formation of biofilm, particularly the key step of adhesion, can also be explained by physicochemical interactions between the bacterial cell surface and the substrate. Numerous studies have shown a positive correlation between the adhesion ability and hydrophobicity of strains (Ehrmann et al. 2002; Pompilio et al. 2008; Xu et al. 2009; Elfazazi et al. 2021). The electron donor and electron acceptor of strains and substrates are also among the factors that mediate bacterial adhesion (Hamadi et al. 2005; Amaral et al. 2017). Conversely, Krausova et al. (2019) found no relationship between hydrophobicity and adhesion of isolated potential probiotic strains. These disparate results may be explained by differences in methodology and the strains used.
As previously established, the formation of biofilms involves the attachment of microorganisms to a surface, forming a cohesive structure through the production of EPS. The amount and composition of EPS may play a crucial role in understanding the mechanisms of biofilm formation. In a study by Castillo Pedraza et al. (2017), an increase in the amount of insoluble EPS was observed in streptococcal strains tested in single and mixed species, which was directly related to an increase in total biomass of biofilms. Polysaccharides have been identified as one of the most important compounds that confer adherence properties to biofilm matrices compared to other organic macromolecules (Costa et al. 2018). Harimawan and Ting (2016) found that polysaccharides promote the adhesion strength of EPS on stainless steel SS-316 more effectively than proteins, which showed less adherence effects. Moreover, biofilm growth was associated with higher EPS production and cellular adhesion. Despite previous studies suggesting a positive correlation between EPS production and biofilm formation in Enterococcus spp., this study did not find such a correlation. The amount of EPS produced by the enterococci strains did not seem to have a significant effect on biofilm formation. This suggests that the role of EPS in biofilm formation and surface colonization may be more complex than previously thought, and may have both positive and negative effects on the process. An increase in EPS production reduced auto-aggregation which is important for colonization and adhesion. Auto-aggregation is directly mediated by specific interactions between proteins or organelles on the surfaces of interacting cells or indirectly by the presence of secreted macromolecules such as eDNA and exopolysaccharides (Nwoko and Okeke 2021). A comparison between Lactobacillus johnsonii FI9785, which produces an EPS layer and its mutants indicated that auto-aggregation was significantly higher in mutants that have reduced EPS, indicating that EPS can mask surface structures responsible for cell-cell interactions. These results suggest that an increase in EPS gives a more effective masking of hydrophobic cell surface moieties, while a decrease in EPS content gives greater exposure to these components (Dertli et al. 2015).
Principal Component Analysis (PCA) was used to examine the relationship between physicochemical properties, auto-aggregation, and biofilm formation. The first PCA plane accounted for 64.43% of the total variation and provided sufficient information to interpret the results. The first coordinate was positively associated with hydrophobicity, electron acceptor, and biofilm formation on AISI 304 L, while the second coordinate was positively associated with auto-aggregation after 24 hours. The third coordinate was positively associated with electron donor and biofilm formation on AISI 316 L (Table 4). The projection of Enterococcus spp. isolates onto the plane formed by the first two dimensions of PCA showed a clear separation of isolates (Fig. 9). The results indicated that E. hirae F420 and E. mundtii A13 exhibited a hydrophilic character with high biofilm formation on AISI 304 L and a negative relationship with electron acceptor (Table 5).
Multivariate variation among 12 Enterococcus strains analysed for auto-aggregation ability at 24 hours (AB 24 h), auto-aggregation ability at 3 hours (AB 3 h), hydrophobicity (ΔG iwi), electron acceptor (ˠ (+)), electron donor (ˠ (−)), biofilm formation on AISI 316 L (AISI 316 L), biofilm formation on AISI 304 L (AISI 304 L), visualized using Principal Component Analysis
The correlation between biofilm formation and hydrophobicity was in consistent with previous research (Gómez et al. 2016). However, certain hydrophobic strains adhered to AISI 316 L and AISI 304 L, which were hydrophilic. Similarly, Casarin et al. (2016) indicated that hydrophilic strains of Listeria monocytogenes and Salmonella enteritidis adhered to AISI 316 L and AISI 304 L, even though those surfaces were hydrophobic. The relationship between electron acceptor and electron donor with biofilm formation explained some of our results. Some strains with higher electron donor and acceptor adhered significantly to AISI 304 and AISI 316 L, while other strains with a higher electron donor and acceptor character exhibited moderate biofilm formation. Additionally, the ability of auto-aggregation after 24 hours played an important role in biofilm formation for some strains (E. mundtii A12 and E. faecium F58) (Table 5). Indeed, several studies have reported that auto-aggregation ability is crucial in biofilm formation to prevent pathogenic biofilm formation (Trunk et al. 2018; Nwoko and Okeke 2021). Botes et al. (2008) also demonstrated that E. mundtii ST4SA with strong auto-aggregation exhibited a strong adhesion to epithelial cells. According to Tuo et al. (2013), there was a positive correlation between auto-aggregation and adhesion of the strains belonging to the same species of lactobacilli. Similarly, Sorroche et al. (2012) found a significant positive correlation between auto-aggregation and biofilm formation of some isolates.
Based on PCA analysis, the biofilm formation of LAB strains on substrata differs from one bacterium to another. Some strains’ adhesion and biofilm formation are controlled by physicochemical properties, while others are influenced by auto-aggregation ability. The findings will contribute to developing strategies to improve adhesion and biofilm formation of Enterococcus spp. on substrata (AISI 316 L and AISI304L) to prevent pathogenic biofilm formation (Zanzan et al. 2023).
Conclusion
The study investigated the ability of Enterococcus spp. to form biofilms on commonly used stainless steel substrates in the food processing environment. The results showed that biofilm formation varied among the strains tested. Greater biofilm formation was observed on AISI 316 L, possibly due to its increased surface roughness compared to AISI 304 L. Additionally, the surface topography around a critical size close to the diameter of the bacterial cells may trap bacteria. The roughness of AISI 316 L was 0.343 μm, which approached the size of the cells (1 μm), while AISI 304 L, with a roughness of 0.028 μm, had a smoother surface. The chemical composition of AISI 316 L can affect the adhesion of bacteria compared to AISI 304 L, which can influence their surface characteristics, including roughness. A thorough understanding of the mechanisms by which Enterococcus spp. form biofilms is crucial for developing effective strategies to control pathogenic biofilms. The findings also emphasize the importance of physicochemical properties and auto-aggregation as critical factors in biofilm formation, but not the only determinants. A positive correlation was observed between auto-aggregation and strains with hydrophobic characteristics, whereas no correlation was found between EPS production and biofilm formation. These findings underscore the need for further investigations to fully understand the biofilm formation process in lactic acid bacteria.
Abbreviations
- LAB:
-
Lactic acid bacteria
- GRAS:
-
Generally recognized as safe
- MRS:
-
Man Rogosa and Sharpe
- CFU:
-
Colony forming units
- EPS:
-
Extracellular polymeric substances
- PCA:
-
Principal component analysis
References
Ait Ouali F, Al Kassaa I, Cudennec B, Abdallah M, Bendali F, Sadoun D, Chihib N-E, Drider D (2014) Identification of lactobacilli with inhibitory effect on biofilm formation by pathogenic bacteria on stainless steel surfaces. Int J Food Microbiol 191:116–124. https://doi.org/10.1016/j.ijfoodmicro.2014.09.011
Al-Hamarneh I, Pedrow P, Eskhan A, Abu-Lail N (2012) Hydrophilic property of 316L stainless steel after treatment by atmospheric pressure corona streamer plasma using surface-sensitive analyses. Appl Surf Sci 259:424–432. https://doi.org/10.1016/j.apsusc.2012.07.061
Amaral DMF, Silva LF, Casarotti SN, Nascimento LCS, Penna ALB (2017) Enterococcus faecium and Enterococcus durans isolated from cheese: survival in the presence of medications under simulated gastrointestinal conditions and adhesion properties. J Dairy Sci 100(2):933–949. https://doi.org/10.3168/jds.2016-11513
Angmo K, Kumari A, Savitri, Bhalla TC (2016) Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT 66:428–435. https://doi.org/10.1016/j.lwt.2015.10.057
Asria M, Ouafib R, Bahafida W, Elabeda S, Koraichia SI, Costad F, Tavaresd T, El Ghachtoulia N (2023) Chromium removal by newly developed microbial consortia supported on wood husk. Desalination Water Treat 289:80–91. https://doi.org/10.5004/dwt.2023.29342
Avram-Hananel L, Stock J, Parlesak A, Bode C, Schwartz B (2010) E Durans strain M4–5 isolated from human colonic Flora attenuates intestinal inflammation. Dis Colon Rectum 53(12):1676–1686. https://doi.org/10.1007/DCR.0b013e3181f4b148
Ayyash M, Abushelaibi A, Al-Mahadin S, Enan M, El-Tarabily K, Shah N (2018) In-vitro investigation into probiotic characterisation of Streptococcus and Enterococcus isolated from camel milk. LWT 87:478–487. https://doi.org/10.1016/j.lwt.2017.09.019
Azelmad K, Hamadi F, Mimouni R, Amzil K, Latrache H, Mabrouki M, El Boulani A (2017) Adhesion of Staphylococcus aureus and staphylococcus xylosus to materials commonly found in catering and domestic kitchens. Food Control 73:156–163. https://doi.org/10.1016/j.foodcont.2016.07.044
Bohinc K, Dražić G, Fink R, Oder M, Jevšnik M, Nipič D, Godič-Torkar K, Raspor P (2014) Available surface dictates microbial adhesion capacity. Int J Adhes Adhes 50:265–272. https://doi.org/10.1016/j.ijadhadh.2014.01.027
Botes M, Loos B, van Reenen CA, Dicks LMT (2008) Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch Microbiol 190(5):573–584. https://doi.org/10.1007/s00203-008-0408-0
Busscher HJ, Weerkamp AH, van der Mei HC, van Pelt AW, de Jong HP, Arends J (1984) Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol 48(5):980–983. https://doi.org/10.1128/aem.48.5.980-983.1984
Casarin LS, Casarin FO, Brandelli A, Novello J, Ferreira SO, Tondo EC (2016) Influence of free energy on the attachment of Salmonella enteritidis and Listeria monocytogenes on stainless steels AISI 304 and AISI 316. LWT 69:131–138. https://doi.org/10.1016/j.lwt.2016.01.035
Castillo Pedraza MC, Novais TF, Faustoferri RC, Quivey RG, Terekhov A, Hamaker BR, Klein MI (2017) Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms. Biofouling 33(9):722–740. https://doi.org/10.1080/08927014.2017.1361412
Chae MS, Schraft H, Truelstrup Hansen L, Mackereth R (2006) Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiol 23(3):250–259. https://doi.org/10.1016/j.fm.2005.04.004
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01636
Dertli E, Mayer MJ, Narbad A (2015) Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol 15(1):8. https://doi.org/10.1186/s12866-015-0347-2
Du R, Ping W, Ge J (2022) Purification, characterization and mechanism of action of enterocin HDX-2, a novel class IIa bacteriocin produced by Enterococcus faecium HDX-2. LWT 153:112451. https://doi.org/10.1016/j.lwt.2021.112451
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Ehrmann MA, Kurzak P, Bauer J, Vogel RF (2002) Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J Appl Microbiol 92(5):966–975. https://doi.org/10.1046/j.1365-2672.2002.01608.x
Elfazazi K, Zahir H, Tankiouine S, Mayoussi B, Zanane C, Lekchiri S, Ellouali M, Mliji EM, Latrache H (2021) Adhesion behavior of Escherichia coli strains on glass: role of cell surface qualitative and quantitative hydrophobicity in their attachment ability. Int J Microbiol 5580274. https://doi.org/10.1155/2021/5580274
Faten K, Hamida K, Soumya EA, Saad ISK, Hasna M, Hassan L, Moktar H (2016) Lactobacillus plantarum: effect of a protective biofilm on the surface of olives during storage. Braz J Microbiol 47. https://doi.org/10.1016/j.bjm.2015.11.028
Favaro L, Basaglia M, Casella S, Hue I, Dousset X, de Melo Franco BDG, Todorov SD (2014) Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from home made white brine cheese. Food Microbiol 38:228–239. https://doi.org/10.1016/j.fm.2013.09.008
Flint SH, Bremer PJ, Brooks JD (1997) Biofilms in dairy manufacturing plant-description, current concerns and methods of control. Biofouling 11(1):81–97. https://doi.org/10.1080/08927019709378321
Franz CMAP, Huch M, Abriouel H, Holzapfel W, Gálvez A (2011) Enterococci as probiotics and their implications in food safety. Int J Food Microbiol 151(2):125–140. https://doi.org/10.1016/j.ijfoodmicro.2011.08.014
Ghosh A, Borst L, Stephen SH, Suyemoto M, Moisan P, Zurek L, Jody GL (2013) Mortality in kittens is associated with a shift in ileum mucosa-associated enterococci from Enterococcus hirae to biofilm-forming Enterococcus faecalis and adherent Escherichia coli. J Clin Microbiol 51(11):3567–3578. https://doi.org/10.1128/JCM.00481-13
Giaouris E, Chapot-Chartier MP, Briandet R (2009) Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int J Food Microbiol 131(1):2–9. https://doi.org/10.1016/j.ijfoodmicro.2008.09.006
Giraffa G, De Fernando GG (2022) Lactic acid bacteria: Enterococcus in milk and dairy products. In Reference Module in Food Science Elsevier BV. Amsterdam, The Netherlands, pp. 1–7
Gómez NC, Ramiro JMP, Quecan BXV, de Melo Franco BDG (2016) Use of potential probiotic lactic acid Bacteria (LAB) biofilms for the control of Listeria monocytogenes, salmonella typhimurium, and Escherichia coli O157:H7 biofilms formation. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.00863
Hamadi F, Latrache H, Mabrrouki M, Elghmari A, Outzourhit A, Ellouali M, Chtaini A (2005) Effect of pH on distribution and adhesion of Staphylococcus aureus to glass. J Adhes Sci Technol 19(1):73–85. https://doi.org/10.1163/1568561053066891
Hamadi F, Asserne F, Elabed S, Bensouda S, Mabrouki M, Latrache H (2014) Adhesion of Staphylococcus aureus on stainless steel treated with three types of milk. Food Control 38:104–108. https://doi.org/10.1016/j.foodcont.2013.10.006
Harimawan A, Ting Y-P (2016) Investigation of extracellular polymeric substances (EPS) properties of P. Aeruginosa and B. Subtilis and their role in bacterial adhesion. Colloids Surf B 146:459–467. https://doi.org/10.1016/j.colsurfb.2016.06.039
Jiang Z, Nero T, Mukherjee S, Olson R, Yan J (2021) Searching for the secret of stickiness: how biofilms adhere to surfaces. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.686793
Khalil AL, Oleiwi SR (2021) Effect of Lactobacillus fermentum filtrate on Pseudomonas aeruginosa adhesion at biotic and abiotic surfaces. Sys Rev Pharm 11(8):713–720
Kiani A, Nami Y, Hedayati S, Jaymand M, Samadian H, Haghshenas B (2021) Tarkhineh as a new microencapsulation matrix improves the quality and sensory characteristics of probiotic Lactococcus lactis KUMS-T18 enriched potato chips. Sci Rep 11(1):12599. https://doi.org/10.1038/s41598-021-92095-1
Kouider N, Hamadi F, Mallouki B, Bengourram J, Mabrouki M, Zekraoui M, Ellouali M, Latrache H (2010) Effect of stainless steel surface roughness on Staphylococcus aureus adhesion. Int j pure appl sci 4(1):1–7
Krausova G, Hyrslova I, Hynstova I (2019) In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Ferment 5(4):100. https://doi.org/10.3390/fermentation5040100
Li W, Ji J, Rui X, Yu J, Tang W, Chen X, Jiang M, Dong M (2014) Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT 59(2, Part 1):732–739. https://doi.org/10.1016/j.lwt.2014.06.063
Li Q, Liu X, Dong M, Zhou J, Wang Y (2015) Aggregation and adhesion abilities of 18 lactic acid bacteria strains isolated from traditional fermented food. Int J Agric Policy Res 3(2):84–92. https://doi.org/10.15739/IJAPR.030
Li W, Zhang J, Zhu Y, Li F (2021) Probiotic characterization of Enterococcus mundtii isolated from larval gut of the diamondback moth, Plutella xylostella. J Kans Entomol Soc 93(3):196–210. https://doi.org/10.2317/0022-8567-93.3.196
Liu H, Zhang L, Yi H, Han X, Gao W, Chi C, Song W, Li H, Liu C (2016) A novel enterocin T1 with anti-Pseudomonas activity produced by Enterococcus faecium T1 from Chinese Tibet cheese. World J Microbiol Biotechnol 32(2):21. https://doi.org/10.1007/s11274-015-1973-4
Ly MH, Vo NH, Le TM, Belin JM, Waché Y (2006) Diversity of the surface properties of Lactococci and consequences on adhesion to food components. Colloids and Surf B 52(2):149–153. https://doi.org/10.1016/j.colsurfb.2006.04.015
Meroufel AA (2020) Corrosion in reverse osmosis desalination processes: forms and mitigation practices. In: (eds) Corrosion and fouling control in desalination industry. Springer International Publishing, Cham, pp 131–151
Nami Y, Bakhshayesh RV, Jalaly HM, Lotfi H, Eslami S, Hejazi MA (2019) Probiotic Properties of Enterococcus Isolated From Artisanal Dairy Products Front Microbiol:10. https://doi.org/10.3389/fmicb.2019.00300
Nel HA, Bauer R, Wolfaardt GM, Dicks LMT (2002) Effect of Bacteriocins Pediocin PD-1, Plantaricin 423, and Nisin on biofilms of Oenococcus oeni on a stainless steel surface. Am J Enol Vitic 53(3):191. https://doi.org/10.5344/ajev.2002.53.3.191
Nikolic M, Jovcic B, Kojic M, Topisirovic L (2010) Surface properties of Lactobacillus and Leuconostoc isolates from homemade cheeses showing auto-aggregation ability. Eur Food Res Technol 231(6):925–931. https://doi.org/10.1007/s00217-010-1344-1
Nwoko EQA, Okeke IN (2021) Bacteria autoaggregation: how and why bacteria stick together. Biochem Soc Trans 49(3):1147–1157. https://doi.org/10.1042/BST20200718
Pérez Ibarreche M, Castellano P, Vignolo G (2014) Evaluation of anti-Listeria meat borne Lactobacillus for biofilm formation on selected abiotic surfaces. Meat Sci 96(1):295–303. https://doi.org/10.1016/j.meatsci.2013.07.010
Pérez Ibarreche M, Castellano P, Leclercq A, Vignolo G (2016) Control of Listeria monocytogenes biofilms on industrial surfaces by the bacteriocin-producing Lactobacillus sakei CRL1862. FEMS Microbiol Lett 363(12):118. https://doi.org/10.1093/femsle/fnw118
Pompilio A, Piccolomini R, Picciani C, D'Antonio D, Savini V, Di Bonaventura G (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287(1):41–47. https://doi.org/10.1111/j.1574-6968.2008.01292.x
Puniya M, Ravinder Kumar M, Panwar H, Kumar N, Ramneek AKP (2016) Screening of lactic acid bacteria of different origin for their probiotic potential. J Food Process Technol 7(1):545
Qiao X, Du R, Wang Y, Han Y, Zhou Z (2020) Purification, characterization and mode of action of enterocin, a novel bacteriocin produced by Enterococcus faecium TJUQ1. Int J Biol Macromol 144:151–159. https://doi.org/10.1016/j.ijbiomac.2019.12.090
Rodrigues L, van der Mei H, Teixeira JA, Oliveira R (2004) Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Appl Microbiol Biotechnol 66(3):306–311. https://doi.org/10.1007/s00253-004-1674-7
Różańska A, Chmielarczyk A, Romaniszyn D, Sroka-Oleksiak A, Bulanda M, Walkowicz M, Osuch P, Knych T (2017) Antimicrobial properties of selected copper alloys on Staphylococcus aureus and Escherichia coli in different simulations of environmental conditions: with vs. without organic contamination. Int J Environ Res Public Health 14(7):813. https://doi.org/10.3390/ijerph14070813
Shuai C, Zhou Y, Yang Y, Gao C, Peng S, Wang G (2018) Ag-introduced antibacterial ability and corrosion resistance for bio-mg alloys. Biomed Res Int 6023460. https://doi.org/10.1155/2018/6023460
Sleytr UB (1978) Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol 53:1–64. https://doi.org/10.1016/S0074-7696(08)62240-8
Sorroche FG, Spesia MB, Zorreguieta Á, Giordano W (2012) A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl Environ Microbiol 78(12):4092–4101. https://doi.org/10.1128/AEM.07826-11
Speranza B, Sinigaglia M, Corbo MR (2009) Non starter lactic acid bacteria biofilms: a means to control the growth of Listeria monocytogenes in soft cheese. Food Control 20(11):1063–1067. https://doi.org/10.1016/j.foodcont.2009.01.006
Srivastava S, Bhargava A (2016) Biofilms and human health. Biotechnol Lett 38(1):1–22. https://doi.org/10.1007/s10529-015-1960-8
Tantratian S, Srimangkornkaew N, Prakitchaiwattana C, Sanguandeekul R (2022) Effect of different stainless steel surfaces on the formation and control of Vibrio parahaemolyticus biofilm. LWT 166:113788. https://doi.org/10.1016/j.lwt.2022.113788
Tran TTT, Kannoorpatti K, Padovan A, Thennadil S (2021) A study of bacteria adhesion and microbial corrosion on different stainless steels in environment containing Desulfovibrio vulgaris. R Soc Open Sci 8(1):201577. https://doi.org/10.1098/rsos.201577
Trunk T, Khalil HS, Leo JC (2018) Bacterial autoaggregation. AIMS Microbiol 4(1):140–164. https://doi.org/10.3934/microbiol.2018.1.140
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci 96(7):4252–4257. https://doi.org/10.3168/jds.2013-6547
Van Oss CJ, Chaudhury MK, Good RJ (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88(6):927–941. https://doi.org/10.1021/cr00088a006
van Zyl WF, Deane SM, Dicks LMT (2016) Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 excludes Listeria monocytogenes from the GIT, as shown by bioluminescent studies in mice. Benef Microbes 7(2):227–235. https://doi.org/10.3920/BM2015.0082
Verran J, Rowe DL, Cole D, Boyd RD (2000) The use of the atomic force microscope to visualise and measure wear of food contact surfaces. Int Biodeterior Biodegrad 46(2):99–105. https://doi.org/10.1016/S0964-8305(00)00070-6
Xu H, Jeong HS, Lee HY, Ahn J (2009) Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett Appl Microbiol 49(4):434–442. https://doi.org/10.1111/j.1472-765X.2009.02684.x
Yin W, Wang Y, Liu L, He J (2019) Biofilms: the microbial “protective clothing” in extreme environments. Int J Mol Sci 20(14):3423. https://doi.org/10.3390/ijms20143423
Zanzan M, Achemchem F, Hamadi F, Latrache H, Elmoslih A, Mimouni R (2023) Anti-adherence activity of monomicrobial and polymicrobial food-derived Enterococcus spp. biofilms against pathogenic bacteria. Curr Microbiol 80:216. https://doi.org/10.1007/s00284-023-03326-9
Zhao Q, Liu Y, Wang C, Wang S, Peng N, Jeynes C (2007) Bacterial adhesion on ion-implanted stainless steel surfaces. Appl Surf Sci 253(21):8674–8681. https://doi.org/10.1016/j.apsusc.2007.04.044
Zhao T, Podtburg TC, Zhao P, Chen D, Baker DA, Cords B, Doyle MP (2013) Reduction by competitive bacteria of Listeria monocytogenes in biofilms and Listeria bacteria in floor drains in a ready-to-eat poultry processing plant. J Food Prot 76(4):601–607. https://doi.org/10.4315/0362-028X.JFP-12-323
Zommiti M, Cambronel M, Maillot O, Barreau M, Sebei K, Feuilloley M, Ferchichi M, Connil N (2018) Evaluation of probiotic properties and safety of Enterococcus faecium isolated from artisanal Tunisian meat “Dried Ossban”. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.01685
Acknowledgments
The authors would like to express their gratitude to Mr. Mouhamed BOUROUACH from the Faculty of Science in Agadir for his valuable assistance with principal component analysis. We would also like to thank El Mostafa Hamadi for his meticulous proofreading and editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanzan, M., Ezzaky, Y., Achemchem, F. et al. Bacterial biofilm formation on stainless steel: exploring the capacity of Enterococcus spp. isolated from dairy products on AISI 316 L and AISI 304 L surfaces. Biologia 79, 1539–1551 (2024). https://doi.org/10.1007/s11756-024-01630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01630-8