Abstract
In this study we investigated genetic variability of the riffle beetle Elmis maugetii Latreille, 1802 (Coleoptera: Elmidae) in Europe and North Africa. The samples barcoded in the present study cover the following countries: the Netherlands, Croatia, Serbia, and Greece. The analysis also includes additional data from Austria, Germany, France, Spain, Slovakia, France, and North Africa (Morocco). mtDNA sequences of the newly barcoded E. maugetii specimens were compared to all sequences available for this species in the BOLD and NCBI GenBank databases. The present study also provides data on population genetic parameters of the species [haplotype diversity, nucleotide diversity, Theta (per site) from Eta, Tajima’s D value], minimum-spanning networks, phylogeographic structure, and possible corridors of its recolonization of the European continent in the Pleistocene. Genetic diversity of E. maugetii was found to be lower in northern regions of Europe. According to the proposed evolutionary history of E. maugetii, the colonization of northern latitudes most likely started from the Balkan Peninsula (suggested as the species´ main glacial refugium in Europe) and proceeded via Central, Northern, and Western Europe, finally reaching the Pyrenean Peninsula (Spain). The specimens from North Africa (Morocco) are slightly distinct from the European ones. To judge from data obtained on the genetic structure of populations of this riffle beetle species, it is characterized by shallow genetic structure within mitochondrial DNA in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Pleistocene is well known for including periods of glaciation and interglaciation, which were repeated thoroughout the whole epoch. During the periods of glaciation, ice sheets and permafrost extended towards lower latitudes, approximately 52° and 47° N, respectively (Hewitt 2004; Lomolino et al. 2015). The climate during the periods of glaciation was much colder and drier than during interglacial periods, making most of the continent uninhabitable for temperate and boreal taxa. Those taxa were forced to retreat south to refugia on the Iberian Peninsula, the Apennine Peninsula, the Balkan Peninsula, or the area south of the Caucasus Mountains (Hewitt 1999; Schmitt 2007). An important feature of these refugia was their isolation from Central Europe by mountain ranges (the Pyrenees, Alps and Balkan Mountains), which also had their own ice caps. Recent studies showed that extra-Mediterranean refugia are also possible for aquatic insects (e.g., Schmitt and Seitz 2001; Malicky 2006), and could be of great importance for temperate species and not only for alpine and arctic taxa (Schmitt and Varga 2012).
Because of range shrinkage and geographic isolation, during each glacial period subpopulations of species that were once the same started to diverge genetically from others surviving in different refugia. During the interglacial periods, when the glaciers and perm afrost started to recede, different lineages from southern refugia started northward colonization and expanded their ranges. There are several widely accepted scenarios of recolonization of northern regions by southern colonists, which differ in regard to location of the refugia and the directions of dispersal routes (Taberlet et al. 1998; Hewitt 1999, 2000). Beside Pleistocene climate change, which could act as a factor affecting intraspecific population structure, the other potentially relevant factors are the species’ dispersial ability, and life history traits. Latitudinal diversity gradients of lentic and lotic species may also be expected to differ, as lotic species will be more dependent on historical factors and distance to glacial refugia, whereas lentic species will be closer to an equilibrium with current ecological and geographical conditions (Ribera 2008). However, multiple factors influence dispersal ability in addition to habitat stability (Bilton 2001), so macroecological patterns related to habitat type will manifest as statistical trends, exceptions always being possible (e.g., Short and Katerino 2009). Also, the lotic-lentic divide is a simplification of the complexity of freshwater habitats, which could be further subdivided (e.g., Iversen et al. 2016).
The role of high mountains as genetic barriers has been shown to be very important for most of the species (Hewitt 2004), and for the European continent, Balkan refugia provided most of the colonists because the Balkan Mountains and Dinaric Alps are the lowest among other mountain ranges isolating refugial areas. Besides colonists from the Balkans, most of Europe was colonized by colonists from the Iberian Peninsula, while Northern Europe was colonized by the fewest colonists from the Apennine Peninsula (Hewitt 1999, 2000).
New data on highly mobile organisms like birds and flying insects (Wahlberg and Saccheri 2007; Pons et al. 2016; Drag et al. 2018; Drovetski et al. 2018) show different patterns of previously proposed colonization of Northern Europe.
Central Europe (France, Germany and Austria) can harbour Pleistocene refugia as it was recently found for Trichoptera analysed at the community level, i.e., the northern recolonizations following the Pleistocene glaciations originated exclusively from central regions, instead of Mediterranean refugia, as was previously accepted (Grigoropoulou et al. 2022). Also, taxonomic turnover and northward phylogenetic clustering reveal evidence for environmental filtering in structuring Trichoptera communities across Europe (Garcia-Raventós et al. 2021). Overall, latitudinal patterns of taxonomic turnover and variable phylogenetic community structure indicate an important role of contemporary ecological conditions in structuring community composition, probably by environmental filtering. However, the signature of biogeographical history is also relevant to understanding the large-scale distribution of taxa. The permanence of caddisfly communities in temperate regions during Pleistocene glaciations demonstrates the presence of refugia there and, therefore, broadens the spatial extent of refugia beyond Mediterranean areas. This contrasts with theories which placed refugia exclusively in Mediterranean areas (Garcia-Raventós et al. 2021).
In general, extra-Mediterranean refugia were apparently often located in the vicinity of water donating mountains systems as the glaciated Alps, Carpathians or Balkan Mountains which may have received more precipitation during the kryoxerotic LGM than the adjacent lowland less steppe areas (Schmitt and Seitz 2001). The southern refugia of temperate species were often surrounded by extended cold-arid steppe areas, e.g., in the central part of the Balkan Peninsula and in the Carpathian Basin (Petit et al. 2003).
Additionally, depending on many different factors, the speed of recolonization of northern parts can vary significantly between species and populations from different refugial areas. In general, there are three differ ent modes of expansion (Hewitt 2004). The first is rapid expansion, where northern populations of colonists have reduced allele diversity and there are huge areas of genetic homogeneity (Ibrahim et al. 1996). The rapid pattern is reported for many species from the Northern Hemisphere (Hewitt 1996; Schmitt et al. 2002). This phenomenon is also known as leading-edge colonization because it is more difficult for populations from other southern refugia to expand once space has been filled (Hewitt 1993). The second mode of recolonization is slow expansion to the north by species which have limited dispersal abilities. In their populations, genetic diversity is high in the north and they have larger effective population sizes. These two modes of recolonization are at opposite ends of the spectrum, and the third mode of recolonization, viz., stepping-stone recolonization, is situated between them. In deciphering the phylogeographic signals of a species, it is necessary to consider its natural history and how it expanded and contracted in its range (Hewitt 2004).
Recent study on population structure and genetic diversity of freshwater insect lineages from temperate to tropical streams that differ in dispersal propensity revealed positive relationship between intraspecific nucleotide diversity (π) and population structure (ΦST) at a global scale (Salinas-Ivanenko and Múrria 2021). Across Europe, the low estimates of π and the wide array of ΦST values and haplotype networks found across species, lineages and latitude were contrary to the biogeographical and dispersal paradigms. Beyond the macroecological trend found, genetic trajectories of co-distributed temperate species were disassociated from their functional traits and probably caused by persistent demographic fluctuations associated with local-scale habitat instability. Overall, the idiosyncratic relationship between π and ΦST across species prevents the establishment of conclusive global patterns and questions the phylogeographical patterns established when studying a reduced number of co- distributed species (Salinas-Ivanenko and Múrria 2021). For aquatic lineages including Elmis, opposite patterns of intraspecific genetic structuring were found when several species were compared: habitat stability was positively associated with population genetic diversity which means that there is not a generalizable model and northern recolonization, and it is most likely to be idiosyncratic (Salinas-Ivanenko and Múrria 2021). Also, for some plant taxa the low levels of genetic diversity for northern populations were not the result of founder effects following interglacial recolonizations: the low levels of genetic diversity for northern populations could have been caused by small effective population sizes in response to fluctuations in distribution ranges following paleoclimatic change (Peter and Slatkin 2015).
To date, there have been many studies on the phylogeography of insects and their modes of recolonization of Northern Europe (e.g., Schmitt et al. 2002; Wahlberg and Saccheri 2007; Drag et al. 2018), but they are mostly restricted to highly mobile species. Some elmid genera are known to have non-macropterous wings in at least some species (Shepard 2019), i.e., the following European genera: Stenelmis Dufour, 1835, Macronychus Müller, 1806 (Segal 1933), Elmis Latreille, 1798, Limnius Illiger, 1802 and Oulimnius Gozis, 1886 (Elliot 2008). The Elmidae, commonly known as riffle beetles, are predominantly found in freshwater streams. There are about 1500 species known world-wide (Jäch et al. 2016), but probably many more exist which have not yet been discovered. Riffle beetles are small (0.8–11mm long) aquatic beetles that are most often found crawling on various substrates in fast-flowing streams. Only a few species are found in slow streams or still water. They have relatively long legs with well-developed claws, and both adults and larvae are well-sclerotized (Kodada et al. 2016).
Elmis maugetii Latreille, 1802 is a species of the western Palaearctic with two subspecies (Jäch et al. 2016); the nominotypical one, Elmis maugetii maugetii, is distributed in 32 European countries including continental Europe (except northern Fennoscandia) and is also recorded in Armenia and Turkey (Kodada et al. 2016). Elmis m. velutina Reiche, 1879 is distributed in Algeria and Morocco. Previously known as subspecies, E. m. fossulata, distributed in France (Corsica) and Italy (Sardinia) has meanwhile turned out to be a distinct species, E. fossulata (Bruvo Mađarić et al. in prep), which was already indicated in Jäch et al. (2016). In this paper data on E. m. maugetii and E. m. velutina are included.
Elmis maugetii occurs in the middle parts of streams and rivers, approximately in the metarhithral to epipotamal zone, and also in crenal parts of sufficiently warm lowland streams. It always occurs farther downstream than Elmis aenea (Müller, 1806) and E. rioloides (Kuwert, 1890). It is usually found in gravel and on moss and submerged wood, larvae and adults seem to tolerate even slow current and increased saprobity (Boukal et al. 2007).
Already a common method of taxonomic research, DNA barcoding is primarily used for specimen identification. This technique is based on sequencing of a standardized fragment of the mitochondrial (mt) cytochrome c oxidase gene subunit I (COI), which bears high interspecific and low intraspecific variability, thus making possible reliable species assignation based on a complete DNA barcode reference library (Hebert et al. 2003; Ratnasingham and Hebert 2007). The method of DNA barcoding has also been found to be very useful in Elmidae research, especially in cases of morphologically similar species (Čiampor et al. 2009; Čiampor and Kodada 2010). Although barcoding data on Elmidae are increasing, riffle beetles of the Balkans are still scarcely covered.
In the present study, we build two main hypotheses concerning the species’ evolutionary history. The first one is that the Balkan Peninsula is the species´ main glacial refugium in Europe, and the second one is that the species’ main glacial refugium is situated in Central Europe, respectively. We tested both hypotheses using the intraspecific population genetic parameter metrics.
The main goal of this study was to determine genetic parameters and phylogeographic structure of the studied species for understanding potential corridors of the recolonisation following Pleistocene glaciations. The study also provides new data on Elmis maugetii DNA barcoding.
Materials and methods
Sample collection and taxa identification
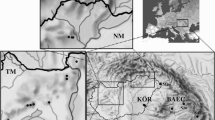
Samples were gathered using a hand net (25 × 25cm, 500μm mesh size) or specimens were collected by hand. The multi-habitat sampling procedure (Hering et al. 2004) was employed, and samples were preserved in pure ethanol (99.8%) for further processing in the laboratory. Identification was done using a Leica MS 5 stereomicroscope, a Carl Zeiss StereoDiscovery V8 instrument with an AxioCam ICc5 camera attached, and appropriate taxonomic keys (Olmi 1976; Friday 1988). A map of the distribution of E. maugetii specimens analysed is provided in Fig.1.
Map of analysed E. maugetii individuals in Europe and North Africa (circle sizes are proportional to the number of specimens). Blue and yellow circles represent individuals from potential refugial areas for the species, red and white ones represent specimens from areas presumably recolonized after the last glacial maximum. Blue- and red-coloured individuals represent a COI barcoding fragment (including our individuals) with 25 individuals (557 base pairs), yellow- and white-coloured samples represent 42 specimens genotyped for the 3’ part of the COI gene (822 base pairs)
Molecular analyses
Samples from ethanol were dried, and DNA was extracted using a commercial kit (innuPREP DNA Mini Kit, Jena) according to the manufacturer’s protocol. Primers LCO 1490 and HCO 2198 (Folmer et al. 1994) were used to amplify the barcoding COI fragment, and PCR was conducted in a 25µl volume of reaction mixture containing 1 x GoTaq™ reaction buffer (Promega), 2 mM MgCl2, 0.2 mM dNTP mix (BioLabs), 0.2 µM of each primer, 0.75 units of GoTaq polymerase (Promega), and 3–5µl of template DNA, the mixture being filled to the final volume with doubly distilled H2O. The PCR cycling conditions were as follows: initial denaturation at 95°C for 2min; 38 cycles of 95°C for 40s; annealing at 46°C for 40s; elongation at 72°C for 50s; and final elongation at 72°C for 10min. The obtained PCR products were checked for quality and quantity on 1% agarose gel purified using exonuclease I and the FastAP™ thermosensitive alkaline phosphatase enzymatic system (ThermoFischer Scientific) according to the manufacturer’s specifications and sent for sequencing to Macrogen Europe Inc. (Amsterdam). Contigs of forward and reverse sequences were created, and the final editing was performed in Sequencher v. 5.1, Gene Codes Corporation, Ann Arbor, MI USA http://www.genecodes.com.
Assessment of genetic diversity
Additionally, we downloaded available sequences from the NCBI GenBank nucleotide database (www.ncbi.nlm.nih.gov/Genbank) and BOLD database (www.barcodinglife.com). For analyses, we used two data sets with sequences of E. maugetii: (1) the COI barcoding fragment (including our samples) with 25 samples (557 base pairs) (Fig.1: blue- and red-coloured circles; Table1); and (2) 42 specimens genotyped for the 3’ part of the COI gene (822 base pairs) using different primers (Múrria et al. 2017) (Fig.1: yellow- and white-coloured circles).
Both datasets we used cannot be directly compared; we have no sequences of the fragment used in Múrria et al. (2017).
Population genetic analyses
For E. maugetii populations, we estimated nucleotide and haplotype diversity (Nei 1987), Theta per site from the number of polymorphic sites per nucleotide (Tajima 1996), and Tajima’s D value (Tajima 1989) using DnaSP software v. 5.10 (Librado and Rozas 2009) for both datasets. We used TCS v. 1.21 (Clement et al. 2000) to construct haplotype networks of two different datasets of the partial COI gene.
In the first dataset, samples of E. maugetii from the Netherlands, France, Austria, and Germany belong to the northern population group, while samples from Croatia, Serbia, and Greece belong to the southern population group. The second dataset, used from Múrria et al. (2017), is represented by populations from Central and Western Europe (Slovakia and France), Spain (South and North) and Morocco.
Results
The first dataset composed of 25 sequences yielded six haplotypes with two parsimony-informative sites. The minimum spanning network (Fig.2) indicates a weak phylogeographic structure among different haplotypes.
Minimum-spanning network among six COI haplotypes (557bp) of E. maugetii (see Table1). Size of the circles represents relative frequency of the given haplotypes, while each line between nodes (black nodes-undetected haplotypes) indicates a single mutational step
Based on the E. maugetii sequences of the barcoding fragment, the minimum-spanning network revealed six different COI haplotypes (557bp) distributed in seven countries (the Netherlands, France, Germany, Austria, Croatia, Serbia, and Greece). The most distant haplotype is found in the population from Serbia, while a common haplotype was shared in all populations but the Serbian. It is most likely the Serbian haplotype revealed that just in those areas there will be higher diversity whereas in Germany there are the most recent haplotypes. Genetic diversity indices within populations (using the barcoding fragment) differed among the populations (Table2). Haplotype diversity, nucleotide diversity, and Theta per site were significantly higher in the southern population group than in the northern population group. For both population groups, Tajima’s D values were negative, and these values were significant in the case of the northern population group, whereas for the southern population group they were not significant.
The second dataset (822 base pairs of the 3’ COI region) yielded 23 haplotypes with 15 parsimony-informative sites. The minimum spanning network (Fig.3) indicated a moderate phylogeographic structure among different haplotypes, with some segregation among different populations (North Spain and Slovakia, as well as South Spain and Morocco). These populations have similar genetic structure, with slight differences in base pairs. Members of the Moroccan population possess private haplotypes, not found in other (European) populations. Otherwise, there are haplotypes shared between populations from North Spain, South Spain, and Slovakia. There are also some private haplotypes corresponding to these geographical areas. The genetic diversity indices within populations in the second dataset differed among the populations (Table3). Haplotype diversity was lowest in Morocco (0.857), slightly higher in Spain (0.896), and highest in Slovakia and France (0.939), while nucleotide diversity was lowest in Spain (0.0036), somewhat higher in Morocco (0.0045) and highest in Slovakia and France (0.0050). The second region of COI shows the highest diversity in Slovakia and France but that from Morroco seems not to be much lower (Fig.3). Theta per site was lowest in Spain (0.0043), higher in Slovakia (0.0048), and highest in Morocco (0.0065). Tajima’s D values were negative for the populations from Morocco and Spain and positive for the population in Slovakia. Additionally, the population from Morocco had significant Tajima’s D values, while the other two populations had no statistically significant Tajima’s D values.
Minimum-spanning network of 23 COI haplotypes (822-bp 3’ part of COI) of E. maugetii: the data were taken from Múrria et al. (2017). Size of the circles represents relative frequency of the given haplotypes, while each line between nodes (black nodes-undetected haplotypes) indicates a single mutational step
Discussion
Using both studied COI fragments, E. maugetii showed shallow genetic structure within mitochondrial DNA. This is considered rare since the great majority of phylogeographic analyses in various animal and plant taxa revealed the existence of several mitochondrial lineages in Europe (Comes and Kadereit 1998; Hewitt 1999; Drag et al. 2018; Drovetski et al. 2018; Raković et al. 2019). For less mobile taxa like some other aquatic insects, such as Dinaric endemic caddisflies Drusus spp. Stephens, 1837, even in one refugial area (the Balkan Peninsula) there are several mitochondrial lineages, and this pattern is known as a ‘refugium within a refugium’, which underlines the complex history of this refugial region (Previšić et al. 2009). One possible cause of the shallow genetic structure of E. maugetii is recolonization of Northern Europe from one refugium in a relatively short time. This seems to be the case for this species in Europe. However, even though the present study showed uniform genetic structure within this species, we have to highlight the fact that we did not cover all refugial areas with sampling. Also, in the Greek population we found a widespread haplotype. But even if the above-mentioned populations are hypothetically different and potentially represent independent evolutionary lineages, these populations did not contribute to the gene pool of Central and Northern Europe.
It is important to highlight the role of extra-Mediterranean refugia for some aquatic insect groups, such as caddisflies. At least 13 Central European regions (outside the Mediterranean area) were found for caddisfly species, with accumulations or presence of stenendemic species, which are interpreted as areal cores and as possible refuges during the last (Würm) glacial period for these and other species (Malicky 2006). Also for montane caddisfly Drusus discolor (Rambur, 1842) that past fragmentation is the prominent process structuring the populations across Europe. The high level of the species’ genetic differentiation between mountain ranges and estimates of demographic history provide evidence for the existence of multiple glacial refugia including several in Central Europe showing that these aquatic organisms reacted differently to Pleistocene cooling than many terrestrial species. They persisted in numerous refugia over multiple glacial cycles (Pauls et al. 2006) which could also be one of the phylogeographic scenario for E. maugetii, with existence of more extra-Mediterranean refugia, particularly in the southern part of Central Europe. It is also possible the species’ western lineage could contribute to the colonization of Central Europe and the relatively higher variability in Slovakia and France as a consequence of several lineage colonization.
One of the main results of this study is the finding of high genetic diversity of E. maugetii in the Balkans, and lower genetic diversity in Central and Northern Europe. The usual pattern to justify a recolonization of central and northern Europe by southern refugia is a higher haplotype diversity in the south, with a rarefaction of the southern diversity towards the north. Several other species show lower genetic diversity in northern populations that expanded rapidly from the Balkans, e.g., beech Fagus sp. L. and crested newt Triturus sp. Rafinesque, 1815 (Hewitt 1999). In these species, southern populations show considerably higher genetic diversity than in the north, where genetic diversity is low. Such southern richness can be considered as a product of repeated major climatic oscillations, in the presence of which genomes were able to survive on the southern mountainous peninsulas, where suitable habitats could be continuously ensured by small range changes (Hewitt 1999). Hence, Northern Europe was probably inhabited by individuals from the Balkan Peninsula, as in the case of other taxa, e.g., the meadow grasshopper Chorthippus parallelus (Zetterstedt, 1821) (Hewitt 2000) or the dunnock Prunella modularis (Linnaeus, 1758) (Drovetski et al. 2018). The existence of many distinct species haplotypes suggests that phylogeographic divergence was maintained in the investigated areas during at least the latest glacial period. Among the Mediterranean peninsulas, the Balkan Peninsula has been postulated to be the primary source of post-glacial expansions because the Alps and the Pyrenees acted as migration barriers for northward movements of species from other European refugia (Hewitt 2000). Furthermore, the Balkans served as a bridge for movements of species from Asia Minor during the Pleistocene glaciation (Hewitt 1999) because the Balkan region of Europe and Asia Minor were linked by a land connection during the last glaciation (Aksu et al. 1999; Tzedakis 2004). Nowadays the Balkans are also a biodiversity “hotspot” with a great number of species. High biodiversity in the Balkans can be explained by the long-term persistence of favourable environmental conditions within the refugium that allowed for the maintenance of stable and genetically variable populations (Canestrelli et al. 2010).
All available data on the 822-bp fragment of the 3’ end of COI for Elmis maugetii from Europe revealed that nucleotide diversity is lowest in Morocco and Spain and highest in Slovakia, indicating the potential existence of a species expansion corridor from Central Europe to the Iberian Peninsula. A pattern of colonization of the Iberian Peninsula from the Balkans was found for the tawny owl Strix aluco Linnaeus, 1758 (Brito 2005). The species Elmis maugetii probably colonized Morocco from the Iberian Peninsula. The lowest diversity in Moroccan specimens can be attributed to geographical barriers (Gibraltar, the Atlas Mountains), which slowed the rate of the species´ southward expansion. It is also possible that once established, the population in Morocco diverged from the mainland population, since all haplotypes found there were private. Exclusive haplotypes in some areas supposed to be refuge suggesting long term isolation without subsequent recolonisation. It is known that the Strait of Gibraltar acted as an important geographical barrier for some taxa (Escudero et al. 2010; Terrab et al. 2008).
With respect to the dispersion ability of riffle beetle species, it can be assumed that flying adults, i.e., macropterous forms, could have successfully overcome geographical barriers (e.g., mountain ranges) in the past by flying over them, presumably on a long-time scale, thereby reaching the most suitable habitats situated in river gorges and valleys with optimal conditions for their survival. Mixed populations also contain brachypterous forms, which are characterized by a low dispersal ability, as in the case of larval instars, and can only drift downstream or, occasionally, be carried with other benthic species to the nearest neighbouring water body during periods with high water levels and torrents. For the studied species it is most likely that ecological factors affect its “local” longitudinal distribution, particularly in the light of recent climate change and water pollution.
As a conclusion, it can be asserted that the colonization of newly established habitats of Elmis maugetii in northern parts of its range in Europe could have started from the Balkan Peninsula, proposed as likely the species´ main glacial refugium in Europe, and proceeded via Central, Northern, and Western Europe, finally reaching the Pyrenean Peninsula (Spain) and Morocco (North Africa). However, to support this claim, considerably more material will be needed in the future, covering in more detail the range of the species.
References
Aksu AE, Hiscot RN, Yaşar D (1999) Oscillating Quartenary water levels of the Marmara Sea and vigorous outflow into the Aegean Sea from the Marmara Sea-Black Sea drainage corridor. Mar Geol 153(1–4):275–302
Bilton DT, Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates. Annu Rev Ecol Syst 32:159–181. https://doi.org/10.1146/annurev.ecolsys.32.081501.114016
Boukal DS, Boukal M, Fikáček M, Hájek J, Klečka J, Skalický S, Šťastný J, Trávníček D (2007) Catalogue of water beetles of the Czech Republic (Coleoptera: Sphaeriusidae, Gyrinidae, Haliplidae, Noteridae, Hygrobiidae, Dytiscidae, Helophoridae, Georissidae. Klapalekiana 43(Suppl):1–289 [in Czech and English]. https://doi.org/10.1111/j.1365-294X.2005.02663.x
Brito PH (2005) The influence of Pleistocene glacial refugia on tawny owl genetic diversity and phylogeography in western Europe. Mol Ecol 14:3077–3094. https://doi.org/10.1111/j.1365-294X.2005.02663.x
Canestrelli D, Aloise G, Cecchetti S, Nascetti G (2010) Birth of a hotspot of intraspecific genetic diversity: notes from the underground. Mol Ecol 19:5432–5451. https://doi.org/10.1111/j.1365-294X.2010.04900.x
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660. https://doi.org/10.1046/j.1365-294x.2000.01020.x
Comes HP, Kadereit JW (1998) The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci 3(11):432–438. https://doi.org/10.1016/S1360-1385(98)01327-2
Čiampor F Jr, Čiamporová-Zaťovičová Z, Kodada J (2009) Description of the larva of Oulimnius echinatus Berthélemy (Coleoptera: Elmidae: Elminae). Zootaxa 1984:57–60. https://doi.org/10.5281/zenodo.185385
Čiampor F Jr, Kodada J (2010) Taxonomy of the Oulimnius tuberculatus species group (Coleoptera: Elmidae) based on molecular and morphological data. Zootaxa 2670:59–68. https://doi.org/10.11646/zootaxa.2670.1.4
Drag L, Hauck D, Říčan O, Schmit T, Shovkoon D, Godunko R, Curletti G, Cižek L (2018) Phylogeography of the endangered saproxylic beetle Rosalia longicorn, Rosalia alpina (Coleoptera, Cerambycidae), corresponds with its main host, the European beech (Fagus sylvatica, Fagaceae). J Biogeogr 45(12):2631–2644. https://doi.org/10.1111/jbi.13429
Drovetski SV, Fadeevm IV, Rakovic M, Lopes RJ, Boano G, Pavia M, Koblik EA, Lohman YV, Red’kin YA, Aghayan SA, Reis SS, Drovetskaya SS, Voelker G (2018) A test of the European Pleistocene refugial paradigm, using a Western Palaearctic endemic bird species. Proc Biol Sci 285. https://doi.org/10.1098/rspb.2018.1606
Elliott JM (2008) The ecology of riffle beetles (Coleoptera: Elmidae). Freshw Rev 1:189 – 203. https://doi.org/10.1608/FRJ-1.2.4
Escudero M, Vargas P, Arens P, Ouborg NJ, Luceño M (2010) The east-west-north colonization history of the Mediterranean and Europe by the coastal plant Carex extensa (Cyperaceae). Mol Ecol 19(2):352–370. https://doi.org/10.1111/j.1365-294X.2009.04449.x
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Friday LE (1988) A key to the adults of British water beetles. Field Studies Council, Darwin College, Cambridge, p 88
Garcia-Raventós A, Viza A, Múrria C (2021) Taxonomic turnover and northward phylogenetic clustering reveal evidence for environmental filtering in structuring Trichoptera communities across Europe. Freshw Biol 66(6):1060–1073. https://doi.org/10.1111/fwb.13699
Grigoropoulou A, Schmidt-Kloiber A, Múrria C (2022) Incongruent latitudinal patterns of taxonomic, phylogenetic and functional diversity reveal different drivers of caddisfly community assembly across spatial scales. Glob Ecol Biogeogr 00:1–15. https://doi.org/10.1111/geb.13479
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc Biol Sci 270:313–322. https://doi.org/10.1098/rspb.2002.2218
Hering D, Verdonschot PFM, Moog O, Sandin L (2004) Overview and application of the AQEM assessment system. Hydrobiologia 516:1–20
Hewitt GM (1993) After the ice: parallelus meets erythropus in the Pyrenees. In: Harrison RG (ed) Hybrid zones and the evolutionary process. Oxford University Press, New York, pp 140–156
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc Lond 58:247–276
Hewitt GM (1999) Post-glacial re-colonization of European biota. Biol J Linn Soc Lond 68:87–112. https://doi.org/10.1006/bijl.1999.0332
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913. https://doi.org/10.1038/35016000
Hewitt G (2004) The structure of biodiversity - Insights from molecular phylogeography. Front Zool 1(1):4. https://doi.org/10.1186/1742-9994-1-4
Ibrahim K, Nichols R, Hewitt GM (1996) Spatial patterns of genetic variation generated by different forms of dispersal. Heredity (Edinb) 77(3):282–291. https://doi.org/10.1038/hdy.1996.142
Iversen LL, Jacobsen D, Sand-Jensen K (2016) Are latitudinal richness gradients in European freshwater species only structured according to dispersal and time? Ecography 39:1247–1249. https://doi.org/10.1111/ecog.02183
Jäch MA, Kodada J, Brojer M, Shepard WD, Čiampor F, Jr (2016) Coleoptera: Elmidae and Protelmidae. World Catalogue of Insects 14:318. Brill, Leiden XXI + pp. https://doi.org/10.1163/9789004291775
Kodada J, Jäch MA, Čiampor F, Jr (2016) Elmidae Curtis, 1830. In: Beutel RG, Leschen RAB (eds) Handbook of Zoology Vol 4, Part 38, 2nd edn. Coleoptera, Beetles, Volume 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), Walter de de Gruyter, Berlin, pp 561–589
Librado P, Rozas J (2009) DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Lomolino MV, Pijanowski BC, Gasc A (2015) The silence of biogeography. J Biogeogr 42:1187-1196. https://doi.org/10.1111/jbi.12525
Malicky H (2006) Mitteleuropäische (extra - mediterrane) Arealkerne des Dinodal am Beispiel von Köcherfliegen (Trichoptera). Beitr Entomol 56(2):347–359. https://doi.org/10.21248/contrib.entomol.56.2.347-359
Múrria C, Bonada N, Vellend M, Zamora-Muñoz C, Alba-Tercedor J, Sainz-Cantero CE, Garrido J, Acosta R, El Alami M, Barquín J, Derka T, Álvarez-Cabria M, Sáinz-Bariain M, Filipe FA, Vogler AP (2017) Local environment rather than past climate determines community composition of mountain stream macroinvertebrates across Europe. Mol Ecol 26(21):6085–6099
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York, 512 pp
Novaković BB (2020) Morfometrijska i filogenetska analiza vrsta familije Elmidae (Insecta: Coleoptera) Balkanskog poluostrva. Dissertation, University of Belgrade, Faculty of Biology, 192 pp [in Serbian]
Olmi M (1976) Coleoptera, Dryopidae, Elminthidae. Fauna d’ Italia, vol 12. Edizioni Calderini, Bologna, 280 pp. [in Italian]
Pauls SU, Lumbsch HT, Haase P (2006) Phylogeography of the montane caddisfly Drusus discolor: Evidence for multiple refugia and periglacial survival. Mol Ecol 15:2153–2169. https://doi.org/10.1111/j.1365-294X.2006.02916.x
Peter BM, Slatkin M (2015) The effective founder effect in a spatially expanding population. Evolution 69:721–734. https://doi.org/10.1111/evo.12609
Petit RJ, Aguinagalde I, de Beaulieu J-L, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohnty A, Müller-Starck G, Demesure-Musch B, Palmé A, Pedro Martin J, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565. https://doi.org/10.1126/science.1083264
Pons JM, Thibault JC, Aymí R, Grussu M, Muntaner J, Olioso G, Sunyer JR, Touihri M, Fuchs J (2016) The role of western Mediterranean islands in the evolutionary diversification of the spotted flycatcher Muscicapa striata, a long-distance migratory passerine species. J Avian Biol 47:386–398. https://doi.org/10.1111/jav.00859
Previšić A, Walton C, Kučinić M, Mitrikeski PT, Kerovec M (2009) Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Mol Ecol 18:634–647. https://doi.org/10.1111/j.1365-294X.2008.04046.x
Raković M, Neto JM, Lopes RJ, Koblik EA, Fadeev IV, Lohman YV, Aghayan SA, Boano G, Pavia M, Perlman Y, Kiat Y, Ben Dov A, Martin Collinson J, Voelker G, Drovetski SV (2019) Geographic patterns of mtDNA and Z-linked sequence variation in the Common Chiffchaff and the ‘chiffchaff complex’. PLoS ONE 14(1). https://doi.org/10.1371/journal.pone.0210268
Ratnasingham S, Hebert PDN (2007) BOLD: The Barcode of Life Data System. Mol Ecol Notes 7:355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Ribera I (2008) Habitat constraints and the generation of diversity in freshwater macroinvertebrates. In: Lancaster J, Briers RA (eds) Aquatic Insects: Challenges to Populations. CAB International, Wallingford, pp 289–311
Schmitt T (2007) Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front Zool 4:11. https://doi.org/10.1186/1742-9994-4-11
Salinas-Ivanenko S, Múrria C (2021) Macroecological trend ofincreasing values of intraspecific genetic diversity and populationstructure from temperate to tropical streams. Glob Ecol Biogeogr 30:1685–1697. https://doi.org/10.1111/geb.13344
Schmitt T, Seitz A (2001) Intraspecific allozymatic differentiation reveals the glacial refugia and the postglacial expansions of European Erebia medusa (Lepidoptera: Nymphalidae). Biol J Linn Soc 74:429–458. https://doi.org/10.1111/j.1095-8312.2001.tb01404.x
Schmitt T, Gießl A, Seitz A (2002) Postglacial colonisation of western Central Europe by Polyommatus coridon (Poda 1761) (Lepidoptera: Lycaenidae): evidence from population genetics. Heredity 88:26–34. https://doi.org/10.1038/sj.hdy.6800003
Schmitt T, Varga Z (2012) Extra-Mediterranean refugia: The rule and not the exception? Front Zool 9:22. https://doi.org/10.1186/1742-9994-9-22
Segal B (1933) The hind wings of some Dryopidae in relation to habitat (Coleoptera). Entomol News 44:85–88
Shepard W (2019) Flight wing polymorphisms in Elmidae and Dryopidae (Coleoptera: Byrrhoidea). Coleopt Bull 73(1):27–44. https://doi.org/10.1649/0010-065X-73.1.27
Short AEZ, Caterino MS (2009) On the validity of habitat as a predictor of genetic structure in aquatic systems: a comparative study using California water beetles. Mol Ecol 18:403–414. https://doi.org/10.1111/j.1365-294X.2008.04039.x
Taberlet P, Fumagalli L, Wust-Saucy AG, Cosson JF (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464. https://doi.org/10.1046/j.1365-294x.1998.00289.x.
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tajima F (1996) The amount of DNA polymorphism maintained in a finite population when the neutral mutation rate varies among sites. Genetics 143:1457–1465
Terrab A, Schönswetter P, Talavera S, Vela E, Stuessy TF (2008) Range-wide phylogeography of Juniperus thurifera L., a presumptive keystone species of western Mediterranean vegetation during cold stages of the Pleistocene. Mol Phylogenet Evol 48:94–102
Tzedakis PC (2004) The Balkans as prime glacial refugial territory of European temperate trees. In: Griffiths HI, Kryštufek B, Reed JM (eds) Balkan Biodiversity. Dordrecht, Boston, London, pp 49–68
Wahlberg N, Saccheri I (2007) The effects of Pleistocene glaciations on the phylogeography of Melitaea cinxia (Lepidoptera: Nymphalidae). Eur J Entomol 104:675–684. https://doi.org/10.14411/eje.2007.085
Acknowledgements
The authors are deeply grateful to Mr. Cesc Múrria (University of Barcelona, Spain) for permission to publish data from Múrria et al. (2017); Mr. Arjen Speksnijder, Mr. Oscar Vorst (Naturalis Biodiversity Center, the Netherlands) and Mr. Vincent Dubut (Aix-Marseille Université, France) for permission to publish their data; Mrs. Vlatka Mičetić Stanković (Croatian Natural History Museum, Zagreb, Croatia), Mrs. Branka Bruvo Mađarić (“Ruđer Bošković“ Institute, Zagreb, Croatia) and Ms. Jelena Đuknić (“Siniša Stanković” Institute for Biological Research, Belgrade, Serbia) for suggestions during writing of the manuscript, and Mr. Raymond W. Dooley for linguistic review of the manuscript.
Funding
This study was partly supported by VEGA project 2/0042/20.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Boris Novaković collected some of specimens in the field. Fedor Čiampor worked on barcoding and molecular taxa analyses. Marko Raković worked on population genetic analyses. Boris Novaković and Marko Raković wrote the manuscript. Teodora Teofilova and Ivana Živić reviewed several drafts of the manuscript. The first draft of the manuscript was written by Boris Novaković and Marko Raković and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The present study is a part of the PhD research of the riffle beetles from the Balkan Peninsula (Novaković 2020).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Novaković, B.B., Raković, M.B., Čiampor Jr, F. et al. Genetic variability of the riffle beetle Elmis maugetii Latreille, 1802 (Coleoptera: Elmidae) in Europe and North Africa. Biologia 77, 3173–3183 (2022). https://doi.org/10.1007/s11756-022-01206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01206-4