Abstract
This present study was aimed at assessing the toxicological effects of the leaf extracts of Picralima nitida on aspects of the biology of Lanistes varicus. The extraction of the leaf, biochemical parameters and toxicity tests were performed using standard methods.. Acetylcholinesterase’s activity was mostly inhibited in the snail’s intestine and haemolymph by the aqueous extract and in the hepatopancreas by ethanol extract. The total protein level of the snail was mostly decreased in the haemolymph by the ethanol extract while in the hepatopancreas, it was mainly decreased by the aqueous extract. There was decrease in the total protein levels of muscle, haemolymph, hepatopancreas and intestine in comparison with the control groups. There were significant (p < 0.05) decreases in the acid phosphatase activity in all the tissues, especially hepatopancreas, intestine and muscle, of the treated groups, compared to the control. Significant (p < 0.05) decreases were observed in the alkaline phosphatase activity in the haemolymph, hepatopancreas and intestine of all the treated groups. Exposure to increasing concentrations of the extracts, especially the ethanol extract, brought about induction of production of male sex hormone in snails. The study provides a scientific basis for exploiting local and indigenous plant resources in Nigeria for the control of freshwater snails and trematode borne infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many of the prevalent human diseases linked to freshwater gastropods are associated with trematodes that use snails as intermediate hosts, especially for the larval stages. Digenean trematodes, also known as flukes, parasitize animals (both wild and domestic) as well as humans. Opisthorchis viverrini (Poirier, 1886) (liver fluke), for instance, poses a serious public health problem in some parts of Asia, particularly in Thailand and Lao Peoples Democratic Republic (Pichit et al. 2021). These trematodes have a complex life cycle that usually requires an invertebrate host, such as snails, for asexual reproduction of the larval stage of the parasite and a definitive vertebrate host for sexual reproduction of the adult trematodes.
Snails such as Lanistes varicus (Müller, 1774), Bulinus globosus (Morelet, 1866), Biomphalaria pfeifferi (Krauss, 1848), etc., serve as intermediate hosts for helminth parasites that cause diseases such as schistosomiasis, fascioliasis, paragonimiasis and angiostrongylosis among others. Lanistes varicus is among the freshwater snails commonly known as apple snails; these snails act as bio-agents by predating on some medially important snails such as Bulinus spp. and Biophalaria spp. which serve as intermediate hosts for causative agents of human schistosomiasis (Awharitoma and Ehigiator 2012; Sanu et al. 2020).
Schistosomiasis, also known as bilharzia, is a tropical parasitic disease caused by blood flukes (trematodes) of the genus Schistosoma (Weinland, 1858). Humans are being infected by the species S. haematobium (Bilharz, 1852), S. intercalatum (Fisher, 1934), S. japonicum (Katsurada, 1904), S. mansoni (Sambon, 1907) and S. mekongi (Voge, Bruckner and Bruce 1978), and these parasites usually affect vital organs of the body such as the liver, intestine and urethra. The infection ensues when there is contact with cercaria (the infectious stage of Schistosoma) released by intermediate host snails, e.g. Bulinus spp., Oncomelatia spp. and Biomphalaria spp., which penetrate the human skin (McManus et al. 2018; Nelwan 2019).
Globally, Schistosoma species infect up to 250 million people and cause about 280,000 deaths annually. Schistosoma haematobium infection is the most common and has been reported in many countries of the sub-Saharan Africa and the Middle East (Sripa et al. 2010). Schistosoma mansonii infection is mostly found in sub-Saharan Africa, Brazil, Caribbean islands, Puerto Rico, Suriname and Venezuela. In China and Philipines, there have been cases of infection by S. japonicum. The other species with lower prevalence; S. guineensis (Bilharz, 1852), S. intercalatum and S. mekongi have been found to be endemic in West Africa, Central Africa and Lao People’s Republic, respectively (McManus et al. 2018; Nelwan 2019).
Some medicinal plant extracts have been tested for molluscicidal property against the intermediate hosts of schistosomiasis and fascioliasis agents (Abdullahi et al. 2018; Mandefro et al. 2017). Aqueous extract of Achyranthes aspera was found to be potent against B. pfeifferi and Lymnea natalensis (Krauss, 1848), the intermediate hosts of S. mansoni and Fasciola hepatica (Linnaeus, 1758), respectively. Also, aqueous extracts of parts (leaves, stem bark and roots) of Balanite egyptiaca showed good and promising molluscicidal effect against F. hepatica with the leaf extract having the highest activity.
Aqueous extract of Vernonia amygdalina has been found to potent against Bulinus truncatus (Audouin, 1827), the gastropod vector of schistosomiamis agents, thus, it affected the total protein concentration and acid phosphatase, alkaline phosphatase and acetylcholinesterase activities in the muscles, haemolymph and hepatopancreas of the snail (Eze et al. 2020).
The control of trematode borne infections will relieve the sufferings and economic costs of many people in the endemic regions of the world as it alleviates the reduced quality of life, low productivity and mortality. Snail control is an indirect way of tampering with the life cycle of parasites that may have caused serious trematode infections. Synthetic agents that eliminate molluscs have always been in use for the control of certain vectors of diseases such as snails (Wang et al. 2018). Nevertheless, these chemical agents are normally toxic and over time may cause serious hazards to other non-target organisms in the aquatic environment (Massoud and Habib 2003; Mearns et al. 2019). Thus the use of biological method to control snail populations has been considered a better alternative to the use of chemical control methods.
The search for organic, biodegradable and affordable agents with reduced deleterious effects in the non-target organisms remains a major focus for researchers involved in the development of suitable molluscicides. These qualities are found more in the medicinal plants which have for long been the form of medication known to man; compared to the man made drugs, they have less deleterious effects to the non-target organisms and the environment and are more affordable (Jamshidi-Kia et al. 2018; WHO 1985).
From literature search and to the best of our knowledge, there has been no documented scientific study on the toxicological effects of Picralima nitida on the freshwater snail Lanistes varicus. Therefore, this study was aimed at assessing the toxicological effects of the aqueous and ethanol leaf extracts of P. nitida on aspects of the biology of L. varicus.
Materials and methods
Collection of plant materials
Fresh leaves (Fig. 1) of Picralima nitida (Stapf. and Durand.) were obtained from the Botanical Garden, University of Nigeria, Nsukka. Mr. Alfred Ozioko, a taxonomist of the Bioresource Development and Conservation Programme (BDCP) Research Centre, Nsukka, Nigeria, authenticated the leaves. Voucher specimen of the plant with No. INTERCEED/096 was deposited at the InterCEED Herbarium.
Preparation of leaf extracts
Dried leaves of P. nitida were prepared by blending them into fine powder using a blender. The powdered leaves weighed 800 g after blending and were divided into two equal parts for the aqueous and ethanol extractions. Four hundred (400) g of the powder was macerated in 2 L of cold distilled water and it was filtered with the aid of a muslin cloth and filter paper after 48 h. The filtrate was poured into a tray and dried at room temperature with the aid of an industrial fan. The aqueous extract yielded 38 g on drying. The remainder of the grounded leaves was also soaked and macerated in absolute ethanol and was equally filtered with the aid of muslin cloth and filter paper 48 h. The resulting filtrate was poured into a clean tray and dried at 25 °C. The ethanolic extract yielded 49 g on drying. The extracts were stored in labelled sterile airtight containers until use.
Collection of experimental animals
Non-infected adult fresh water snails (L. varicus) (Fig. 2) with average weight of 9.8 g were collected from water canals at Over-rail, Niger-Cem, Ebonyi State, Nigeria. Acclimatization of the snails was done for two weeks under good laboratory conditions. The snails were housed in plastic aquaria containing tap water and the temperature maintained at 25 °C. The experimental animals were fed with fresh Carica papaya leaves.
Experimental design
Determination of the median lethal concentration (LC50) of the aqueous and ethanol leaf extracts of P. nitida on L. varicus
Fifty (50) sexually mature snails, of average weight 12.5 g, were divided into ten groups, A, B, C, D, E, F, G, H, I and J of five animals in each aquarium.
Group A was administered 10 g of the aqueous extract in 1 L of water, while groups B, C, D, and E received 20, 30, 40 and 50 g L−1 of the aqueous extract, respectively. Groups F-J received similar doses as groups A-E but with ethanolic extract. After 24 h, the animals were inspected for mortality (acute toxicity), chronic toxicity was also determined after 96 h. Death of the animals was authenticated by absence of irritation and sensitivity to needle probe. Probit analysis (Finney 1971) was employed in the determination of LC50 of the extracts.
Molluscicidal evaluation of the leaf extracts of P. nitida
The freshwater snails were treated with aqueous and ethanol leaf extracts of P. nitida according standard procedures for molluscicidal potency of extracts. Nine aquaria with five snails in each, having an average weight of 7.6 g, were usually set up using tap water. The first aquarium contained the control snails while the remaining eight contained different concentrations (5, 6, 8 and 10 g L−1) of the two plant’s extracts. Each aquarium containing a concentration of the extracts was made up to 1 L by diluting with tap water. The aquarium for the control snails only contained clean tap water. The snails were exposed to substance concentrations for 48 h and were allowed to recover for another 48 h in clean water. Death of the experimental animals was validated by absence of reaction to irritation of the foot after a probe.
The experiments were performed in triplicates.
Experimental groups
Group 1: Normal snails (control)
Group 2: 5 g of aqueous extract in 1 L of water
Group 3: 5 g of ethanolic extract in 1 L of water
Group 4: 6 g of aqueous extract in 1 L of water
Group 5: 6 g of ethanolic extract in 1 L of water
Group 6: 8 g of aqueous extract in 1 L of water
Group 7: 8 g of ethanolic extract in 1 L of water
Group 8: 10 g of aqueous extract in 1 L of water
Group 9: 10 g of ethanolic extract in 1 L of water
Homogenate processing
After the period of recovery, the snails’ shells were broken. Using forceps and scissors, the snails’ tissues were dissected. These tissues were separately collected. At room temperature and under the revolution of 3000 rpm for 5 min, the haemolymph was separated using a bench centrifuge. The resultant supernatant was transferred into bottles and kept frozen until use. The other tissues of the snail (muscle, hepatopancreas and ovotestis) were homogenized, subsequently washed with fine sand and normal saline. Centrifuged at 3000 rpm for 5 min, the supernatant was collected into labeled containers and kept frozen until use.
Determination of biochemical parameters in snail homogenates
Absorbances were measured using digital photo colorimeter (E1,312 Model, Japan).
Acetylcholinesterase activity
The assay was performed according to the method of Ellman et al. (1961). Twenty five (25) μl of haemolymph and 50 μl of the other tissue homogenates were made into triplicates. Into each of the test tubes 50 μl of 5, 5’-dithiobis-2-nitrobenzoic acid (DNTB) in 5 mM sodium phosphate buffer containing 17.74 mM NaHCO3, pH of 8.0, was added. After mixing properly, the mixture was transferred into a cuvette with the addition of 1.45 ml of 5 mM sodium phosphate buffer. The reading for the initial absorbance was taken at 412 nm. The initiation of the reaction was done by adding 50 μl of the substrate (12.5 mM acetylcholine iodide) and stirring. The absorbance reading was taken at 30 s interval for 4 min. The change in absorbance per time for each experimental data was determined. The following expression was used in determining the acetylcholinesterase’s activity:
Enzyme activity (μmol SH hydrolyzed min/mg protein) = ΔA × TV/ εDTNB × ℓ × SV,
where ΔA = change in absorbance/min;
TV = total volume of the assay;
SV = sample volume (25 μl/50 μl);
ℓ = path length of cuvette (1 cm);
εDTNB = molar extinction co-efficient of DTNB (1.36 × 104 M−1cm−1).
Estimation of total protein
This is based on the principle that a coloured complex is formed when cupric ions in alkaline medium interact with peptide bonds.
Reagent 1: Biuret reagent composed of NaOH, Na–K-tartrate, potassium iodide and cupric sulphate; Reagent 2: blank reagent, which contained NaOH and Na–K-tartrate.
Reagents 1 and 2 were diluted with 400 ml of distilled water, before use.
Standard solution contains a calculated standard protein concentration.
Procedure: Into three test tubes labelled homogenate, standard and blank were added 20 µl of homogenate (supernatant), 10 µl of the standard and 10 µl of distilled water respectively. Thereafter, 1000 μl of reagent 1 was added to all the test tubes and incubated for 3 min at 25 °C. The absorbance was measured at 500 nm.
The concentration of the protein was calculated as follows:
Concentration of protein (g/dl) = Abs. of sample/Abs. of standard × df × concentration of standard protein, where; Abs = absorbance at 650 nm and df = dilution factor.
Assay of alkaline and acid phosphatases
The alkaline and acid phosphatases’ assays were performed following the method of Sanni and Van Etten (1978) with slight modification by Oyedapo (1996).
A quantity (50 μl) of the snail’s haemolymph and other tissue homogenates were incubated for 3 min at 37 °C. For the blank, water was used in place of tissue homogenates. With the addition of 1.0 ml of 5 mM p-nitrophenyl phosphate (the substrate) in appropriate buffer, the reaction was initiated. After the incubation of the mixtures for 15 min, 2.0 ml of 0.02 M NaOH was added for the termination of the reaction. Allowing the reaction mixtures to cool at room temperature, the absorbance reading was taken at 410 nm against the blank.
The activities of the both acid and alkaline phosphatases were calculated as:
where ε = molar extinction co-efficient (1.88 × 104 M−1cm−1);
l = path length of cuvette (1.0 cm);
TV = total assay volume;
SV = sample volume (25 μl/50 μl) and
t = incubation time (15 min)
Gonadotropin determination
The absorbance was taken using BIO TRUST microplate reader (MR-9602A Model, USA).
Gonadotropin levels in the ovotestis of the animals were assayed using standard Enzyme Linked Immunosorbent Assay (ELISA) kits for gonadotropin releasing hormone. The kit was a product of MyBioSource.com, USA (catalogue number MBS2515759).
This is based on the principle of enzyme-linked immunosorbent assay for antigen detection. All reagents, samples and standards were prepared. Fifty (50) µL of the sample was added to each well followed by immediate addition of 50 µL prepared detection reagent A immediately. They were shaken and mixed properly and incubated for 1 h at 37 °C. The wells were aspirated and washed 3 times and then 100 µL of prepared detection reagent B was added. Incubation was carried out for 30 min at 37 °C and the wells were aspirated and washed 5 times. Ninety (90) µL of the substrate solution was added and incubation was done for 20 min at 37 °C. Finally, 50 µl stop solution was added and absorbance was read at 450 nm immediately. The concentration of GnRH in the samples was determined by comparing the O.D. of the samples to the standard curve.
Statistical analysis
Data obtained from the results were analyzed using one-way ANOVA. All analysis was performed using IBM Statistical Packages for Social Sciences (SPSS), (Version 20). A p-value less than 0.05 was considered statistically significant and the results were expressed as mean ± standard deviation of mean.
Results and discussion
Mortality
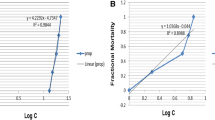
There was no mortality recorded in the animals exposed to 0 to 30 g of the aqueous and ethanol extracts in 1 L of water within 24 h. Ten (10) % and 30% mortality occurred in the animals exposed to 40 and 50 g of the aqueous and ethanolic extracts in 1 L of water, respectively. During 96 h chronic toxicity exposure to the extracts, there was no mortality in animals exposed to 10 g of the aqueous extract in 1 L of water; 10%, 30%, 50% and 60% mortality was recorded in the 20, 30, 40 and 50 g L−1 of the aqueous extract, respectively. Mortality occurred in the animals exposed to the ethanolic extract in a dose dependent manner, 30%, 40%, 70%, 90% and 100% mortality was recorded on exposure to 10, 20, 30, 40 and 50 g of extract in 1 L of water. Using Probit analysis, there was no mortality estimated for the aqueous extract within 24 h exposure. LC50 for the aqueous extract within 96 h of exposure was estimated to be 42.23 g L−1 of water while LC50 for the ethanolic extract within 24 and 96 h of exposure was estimated to be 55.01 and 21.94 g L−1 of water, respectively, as shown in Table 1.
Acetylcholinesterase (AChE) activity in tissues of Lanistes varicus
In the snail’s tissue (haemolymph, hepatopancreas, intestine and muscle), the activity of AChE was noticed to be highest in the hepatopancreas of the group treated with 10 g L−1 of aqueous extract of P. nitida (Group 8) while the least activity of the enzyme was observed in the intestine of the group treated with 8 g L−1 of aqueous extract (Group 6) (Table 2).
Groups 3 and 4 (animals treated with 5 g L−1 of ethanol extract and 6 g L−1 of aqueous extract, respectively) caused significant (p < 0.05) reductions in the AChE activity in the haemolymph. In the intestine of the snail, Groups 4, 6 and 8 (animals treated with 6 g L−1 of aqueous extract, 8 g L−1 of aqueous extract and 10 g L−1 of aqueous extract) caused significant (p < 0.05) reductions in AChE activity.
AChE plays a role in the co-ordination of impulses by inactivating acetylcholine during the processes of synaptic transmission, thus, its inhibition leads to uncontrolled muscular spasm and paralysis and eventually death (Singh and Singh 2003).
In this present study, exposure of L. varicus to P. nitida aqueous and ethanolic leaf extracts caused a significant inhibition in AChE activity in their tissues, particularly in the haemolymph and intestine. These responses might be useful indicators of the toxicity of the extracts on freshwater snails. Thus, it might be deduced that the extracts posed some toxic effects to the tissues of fresh water snails by tampering with the normal muscular activity mechanism. The result is in consonance with that of Akinpelu et al. (2012) in which exposure of a plant’s (E. suaveolens) stem bark saponins to tissues of Lanistes lybicus (Morelet 1848) produced a significant inhibition in AChE activity.
Total protein concentration in tissues of Lanistes varicus
In the snail’s tissue (haemolymph, hepatopancreas, intestine and muscle), the total protein concentration was noticed to be highest in the haemolymph of the group treated with 5 g L−1 of aqueous extract of P. nitida (Group 2) while the least activity of the enzyme was observed in the intestine of the group treated with 8 g L−1 of ethanol extract (Group 7) (Table 3).
The extracts at all concentrations caused significant (p < 0.05) reductions in the total protein level in all the tissues of the experimental animal. However, Group 2 showed increase in total protein in the haemolymph, and Groups 2 and 3 showed increase in total protein in the intestine.
Both extracts caused decreases in the total protein concentration in all the tissues, especially haemolymph and muscle. The decreases in the total protein concentration could be traced to the inability of protein synthesizing machinery to function properly which suggests a gradual loss of function and this is an indicative of toxicity. This result is supported by a study conducted by Eze et al. (2020) where extract of V. amygdalina caused a significant reduction in the total protein concentration of tissues of B. truncatus.
Acid phosphatase (ACP) activity in tissues of Lanistes varicus
In the snail’s tissue (haemolymph, hepatopancreas, intestine and muscle), the ACP activity was noticed to be highest in the muscle of the group treated with 8 g L−1 of ethanol extract of P. nitida (Group 7) while the least activity of the enzyme was observed in the intestine of the group treated with 6 g L−1 of aqueous extract (Group 4) (Table 4).
The extracts at all concentrations caused significant (p < 0.05) increase in ACP activity in all the tissues, especially hepatopancreas and muscle.
When ACP is released by the lysosomes, it is involved in the hydrolysis of certain harmful foreign materials in the cells, thus, catalyze reactions at the onset of intracellular digestion (Lodish et al. 2000).
Because the activity of ACP goes up as it begins to counteract any toxic impact in the tissues of animals, it is known as inducible enzyme.
In this particular study, the activity of ACP in the tissues, particularly in the hepatopancreas of the snail, was observed to oscillate as the extract’s concentration increase; the extract caused increase in the enzyme’s activity in the muscle and haemolymph of the snail. Conversely, lower doses were found to cause increase in the enzyme’s activity in the intestine of the experimental animal. The observed increase in the activity of this enzyme in the snails administered with the extract may be due to serious damage or destruction done to the vital organs or tissues of the snails.
Alkaline phosphatase (ALP) activity in tissues of Lanistes varicus
In the snail’s tissue (haemolymph, hepatopancreas, intestine and muscle), the ALP activity was noticed to be highest in the hepatopancreas of the group treated with 8 g L−1 of aqueous extract of P. nitida (Group 6) while the least activity of the enzyme was observed in the haemolymph of the group treated with 10 g L−1 of aqueous extract (Group 8) (Table 5).
The extracts at all concentrations caused significant (p < 0.05) increase in ALP activity in the hepatopancreas, intestine and haemolymph. Only Group 3 showed a significant (p < 0.05) increase in ALP in the muscle.
ALP helps in the transport of materials across cell membranes and also plays a role in shell formation and glycogen metabolism in the molluscs (Gupta and Rao 1974). This bioassay result showed increase in ALP activity mostly in the hepatopancreas and intestine; these two organ systems are responsible for digestion and excretion in snail. Higher ALP activity in the haemolymph and muscle was caused mainly by ethanol extract while in the hepatopancreas, it was caused mainly by the aqueous extract. Enhanced ALP activity in the hepatopancreas is like to be due to the breakdown of ATP to ADP and inorganic phosphate making free energy available for metabolic processes (Bothman and Mayes 2003). In this study, the observed increase in the activity of liver ALP could be pinned to cellular damages caused by the extracts. Group 7 had reduction in the ALP activity in the hepatopancreas and this could be attributable to inability of the enzyme to catalyze the above-mentioned reaction due to toxicity impacted by high concentration of the extract. This finding of this study is in tandem with that of Eze et al. (2020) who observed that ethanol extract of V. amygdalina caused increase in ALP in freshwater snail.
Gonadotropin levels in ovotestis homogenates of Lanistes varicus
In the snail’s ovotestis, gonadotropin level was noticed to be highest in the group treated with 8 g L−1 of ethanol extract of P. nitida (Group 7) while the least level was observed in the group treated with 10 g L−1 of aqueous extract (Group 8) (Table 6).
The extracts significantly (p < 0.05) increased the gonadotropin level of the experimental animals. Group 8, however, showed a non-significant increase in gonadotropin level (Table 6).
Gonadotropins such as follicle stimulating hormone (FSH) and luteinizing hormone (LH) act on testis and ovaries leading to the production of testosterone and oestrogen (Sarieh et al. 2014). In this assay, both extracts, especially ethanolic, caused significant (p < 0.05) increases in the gonadotropin level in the ovotestis of the snail. This is in accordance with the work of Otoo et al. (2015) in which a 7-day treatment with P. nitida extract on broilers significantly (p ≤ 0.01–0.001) increased testosterone level. Despite the toxicity caused by the plant extracts to the animal tissues, they have enhancing effects on reproductive hormones. Increased level of gonadotropin is suggested to be due to the plant extract directly inducing hypothalamic-pituitary–testicular axis, which in turn influences the gonads (Sharma et al. 2013).
Picralima nitida extracts have been asserted to induce reversible sex change (from female to male) in snails over long period of exposure (Otoo et al. 2015). This implies decreased egg-laying and in turn population decrease of the host snails. Assessment of ethanol seed extract of P. nitida on reproductive and developmental indices arrived at a conclusion that acute administration of P. nitida extract enhances sexual behaviours (aphrodisiac effect) in both males and females, possibly by affecting reproductive hormones. Its chronic administration in females, however, reduces the chances of fertility, i.e., caused contraception but had no teratogenic or abortifacient effect during pregnancy.
Conclusion
It was evident that extracts of P. nitida exerted toxic effects on acetylcholinesterase, acid and alkaline phosphatases, total protein but did not have toxic effect on the gonadotropin hormone in the examined tissues of the L. varicus. The aqueous extract is as effective as the ethanolic extract and it could be the preferred form for use in freshwaters to avoid contamination with ethanol, however, ethanol extract is encouraged for laboratory purposes.
In this study, however, high concentrations P. nitida extracts were used, and for it to be considered a very potent molluscidal agent, lesser concentrations should be tried. Efforts are being made to further purify the extract so that it can be considered efficacious and safe for the population control of freshwater gastropods and trematodes-borne infections. It is also imperative to isolate and characterize the very active ingredient(s) responsible for molluscicidal activity of the plant’s extract.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdullahi Y, Muhammad I, Yerima MI (2018) Molluscicidal activity of aqueous extract of leaves, stem back and roots of Desert date (Balanite egyptiaca Del.) against common liver fluke (Fasciola hepatica) found in the snail (Lymnea natalensis). J Appl Sci Environ Manage 22(3):409–413. https://doi.org/10.4314/jasem.v22i3.21
Akinpelu BA, Dare CA, Adebesin FI, Iwalewa EO, Oyedapo OO (2012) Effects of stem-bark of Erythrophleum suaveolens (Guill. and Perri.) saponin on freshwater snail (Lanistes lybicus) tissues. AJEST 6(11):446–451. https://doi.org/10.5897/AJEST12.007
Awharitoma AO, Ehigiator FAR (2012) Parasitic infection in the freshwater snails, Pila ovata and Lanistes varicus (Gastropoda: Ampullariidae). NISEB J 12(1):15–20
Botham KM, Mayes PA (2003) Bioenergetics: The role of ATP. In: Botham KM, Mayes PA (eds) Harper’s illustrated biochemistry, 26th edn. McGraw-Hill, New York, pp 80–85
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetricdetermination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Eze JC, Okafor F, Nwankwo NE, Okeke ES, Onwudiwe NN (2020) Schistosomiasis prevention option: toxicological evaluation of Vernonia amygdalina on the tissues of Bulinus truncatus at different pH conditions. Heliyon 6:e04796. https://doi.org/10.1016/j.heliyon.2020.e04796
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge
Gupta V, Rao G (1974) Histochemical studies on the choroid plexus of the goat embryos. Histochemical distribution of acid and alkaline phosphatases. Acta Trop 499:60–63
Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H (2018) Medicinal plants: Past history and future perspective. J Herbmed Pharmacol 7(1):1–7. https://doi.org/10.15171/jhp.2018.01
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Organelles of the eukaryotic cell. In: Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (eds) Molecular and Cell Biology, 4th edn. WH Freeman, New York, pp 730–745
Mandefro B, Mereta ST, Tariku Y, Ambelu A (2017) Molluscicidal effect of Achyranthes aspera L. (Amaranthaceae) aqueous extract on adult snails of Biomphalaria pfeifferi and Lymnaea natalensis. Infect Dis Poverty 6:133. https://doi.org/10.1186/s40249-017-0349-4
Massoud AM, Habib FS (2003) The effects of Myrrh (Commiphora molmol) on infected snails of Schistosoma spp. and their egg masses: Effect on shedding of cercariae and on snail fecundity. J Egypt Soc Parasitol 33(2):585–596
McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN (2018) Schistosomiasis. Nat Rev Dis Primers 2018(4):13. https://doi.org/10.1038/s41572-018-0013-8
Mearns AJ, Bissell M, Morrison AM, Rempel-Hester MA, Arthur C, Rutherford N (2019) Effects of pollution on marine organisms. Water Environ Res 91(10):1229–1252. https://doi.org/10.1002/wer.1218
Nelwan ML (2019) Schistosomiasis: Life cycle, diagnosis, and control. Curr Ther Res 91:5–9. https://doi.org/10.1016/j.curtheres.2019.06.001
Otoo LF, George AK, Ansah C, Mensah BK, Benneh CC (2015) Assessment of an ethanolic seed extract of Picralima nitida ([Stapf.] Th. and H. Durand) on reproductive hormones and its safety for use. J Intercult Ethnopharmacol 4(4):293–301. https://doi.org/10.5455/jice.20151030085004
Oyedapo OO (1996) Studies on bioactivity of the root extract of Plumbago zeylanica. Int J Pharmacogen 34:365–369. https://doi.org/10.1076/phbi.34.5.365.13249
Pichit W, Thapana C, Watchariya P (2021) Cercarial trematodes in freshwater snails from Bangkok, Thailand: Prevalence, morphological and molecular studies, and human parasite perspective. Parasitol 148(3):366–383. https://doi.org/10.1017/S0031182020002073
Sanni MS, Van-Etten RL (1978) An essential carboxylic acid group in human prostrate acid phosphatase. Biochem Biophysiol Acta 568:370–376. https://doi.org/10.1016/0005-2744(79)90305-x
Sanu KM, Istifanus WA, Musa MS, Mao PS (2020) The diversity of fresh water snail fauna in Kiri dam, Adamawa State, North Eastern Nigeria. GSC Biol Pharm Sci 11(02):099–104. https://doi.org/10.30574/gscbps.2020.11.2.0118
Sarieh S, Javad SR, Farzaneh MR, Mohammad RS, Mohammad RA (2014) Effects of aqueous root extracts of Anacyclus pyrethrum on gonadotropins and testosterone serum in adult male rats. AJPCT 2(6):767–772
Sharma V, Boonen J, Spiegleer BD, Dixit VK (2013) Androgenic and spermatogenic activity of alkylamide-rich ethanol solution extract of Anacyclus pyrethrum. Phytother Res 27(1):99–106. https://doi.org/10.1002/ptr.4697
Singh SK, Singh A (2003) Molluscicidal and anticholinesterase activity of Alstoniascholaris plant against fresh water snail Lymnaea acuminata. PJBS 6:1442–1446. https://doi.org/10.3923/pjbs.2003.1442.1446
Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ (2010) Food-bornetrematodiases in Southeast Asia: epidemiology, pathology, clinical manifestation and control. J Adv Parasitol 72:305–350. https://doi.org/10.1016/S0065-308X(10)72011-X
Wang W, Mao Q, Yao J, Yang W, Zhang Q, Lu W, Deng Z, Duan L (2018) Discovery of the pyridylphenylureas as novel molluscicides against the invasive snail Biomphalaria straminea, intermediate host of Schistosoma mansoni. Parasit Vectors 11:291. https://doi.org/10.1186/s13071-018-2868-7
World Health Organization (WHO) (1985) Control of foodborne trematode infections. Report of a WHO study group. World Health Organ Tech Rep Ser 849:1–157
Acknowledgements
We sincerely wish to acknowledge all the staff members of the Department of Zoology and Environmental Biology both academic and non-academic, University of Nigeria, Nsukka, for their assistance during the period of this study.
Funding
The research did not receive funding from any organization or institution.
Author information
Authors and Affiliations
Contributions
CUA carried out the laboratory study of the research. ALE helped in the designing and supervision of the research. JCE helped out in the laboratory experiments and also statistically analyzed the data. NEN helped out in designing the work and wrote the manuscript for publication. FCO supervised and contributed financially to the execution of the project.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Clearance for humane handling of experimental animals and for conducive experimental conditions for laboratory practice was given by the ethical committee of the Department of Zoology and Environmental Biology, University of Nigeria Nsukka.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abonyi, C.U., Ezugwu, A.L., Eze, J.C. et al. Toxicological impact of Picralima nitida (pile plant) extracts on the gastropod Lanistes varicus (freshwater snail), as a control measure against trematodes infections. Biologia 77, 2093–2101 (2022). https://doi.org/10.1007/s11756-022-01075-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01075-x