Abstract
Psychological stress and anxiety have been linked to significant impairments of neurobiological functions. There is a wide range of conditions including neurological disorders such as Alzheimer’s disease and use of certain substances or medications that induce stress and anxiety in humans. Confused state of mind, anxiety and psychological stress have been associated with varying degree of disabilities and poor quality of life in humans. Anxiolytic medications are important modulators of CNS that reduce the anxiety and the related psychological effects in patients. Natural product derived drugs such as galantamine have been shown to possess promising neuro-modulatory properties in neurological disorders. Averrhoa carambola is traditionally used for anti-oxidative, anti-inflammatory, anti-microbial, and anti-ulcerative properties. In this study, we have examined the CNS depressant activity of A. carambola leaves extracts. The neuro-modulatory properties were assessed with the standard protocols that are used to identify such CNS depressant activity and included thiopental sodium-induced sleeping time test, hole cross test, hole board test, and open field test. The extract was found to decrease the motor activity and exploratory behaviour of mice in hole cross, hole board and open field tests. The extract also significantly maximized the duration of sleeping time when administered with thiopental sodium, which also demonstrated the CNS depressant activity. The findings of our study suggest that A. carambola extracts have active CNS depressant and hypnotic properties. However, further studies are warranted for isolation of bioactive constituents and understanding the molecular mechanism and modes of action for such pharmacological effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychological stress, anxiety and sleep disorders can lead to short-term and long-term disability and account for the symptoms of major neuro-psychiatric illnesses worldwide (Han et al. 2012). Insomnia has a high prevalence rates across the globe and is associated with severe psychological and cardio-metabolic health issues (Kalmbach et al. 2018). Approximately 20% of the people suffer major psychiatric episodes and manifestations at a certain point in their lives (Suetani et al. 2019). These symptoms become more prevalent in elderly patients, and they have a wide range of negative implications in their personal, social, and professional lives (Creaven et al. 2018). Loss of mental well-being and psychological distress are considered as the most frequent neuropsychiatric condition, similar to stroke (Di Lorenzo et al. 2019). According to the World Health Organization’s Global Burden of Disease report, psychiatric illnesses including depression and anxiety are one of the leading causes of long-term impairment (GBD 2019). Currently, there are a number of pharmacological drugs, which are clinically beneficial for these conditions but are associated with long-term adverse effects. Antidepressant medications require a longer treatment period and have subtle effects on the patients’ depressive symptoms (Lopresti et al. 2019). Antidepressant medications have also been associated with numerous side effects, including dizziness, shivering, fatigue tremor, mental impairment, sexual problems, and urine retention (Milan and Vasiliadis 2020). It has been observed that 70% of patients did not gain complete recovery, and 30% did not make it to the present medication treatment (Nabavi et al. 2017). Studies have also reported the association of anxiolytic and hypnotic drugs with significantly increased risk of mortality (Weich et al. 2014). In the present context, however, several conventional therapies such as acupuncture, physical exercise, and the influence of natural ingredients on depressive-like symptoms are showing promise (Mendelson 2019).
Life and illnesses have been linked from the ancient period and so is the quest for discovering the therapeutic and preventive strategies. Human population is often challenged with new ailments, many of which are difficult to cure with conventional treatments. The changes in the life-style that have contributed to high stress levels in human lives have resulted in a number of disturbed neuro-psychological conditions. Thus, there is a strong desire to find potent, but less hazardous alternatives, both for therapeutic and preventive outcomes. In this context, natural products provide an inexhaustible source for molecules that can interfere with the disease pathogenesis and provide relief. Researchers are always on the search for potential drugs, both synthetic and the derivatives of natural resources including marine, plants, and animals (Egbuna et al. 2018). According to current evidence, nutritional supplements are high in phytochemicals that have a beneficial therapeutic effect in neuro-psychiatric disorders. As plants are easily accessible and are extensively used as foods or for other purposes, bioactive compounds originating from plants are thought to be less harmful, particularly in a long-term treatment regimen (Süntar 2020). Therefore, natural products, particularly those derived from plants, have attracted consderable attention as alternative medicines (Nimgampalle et al. 2021). About 25% of all the prescribed medications are obtained from various plant sources around the world (Krause and Tobin 2013). Galantamine which is a FDA approved drug for the treatment of Alzheimer’s disease as AChEI was isolated as an alkaloid form the bulb of Narcissus species (Ansari and Khodagholi 2013). Plants as a resource of pharmacologically active lead compounds are still underexplored, and there are numerous novel avenues for finding the requisite drug compounds (Holanda et al. 2020; Nimgampalle et al. 2021; Najmi et al. 2022). Studies in literature have explored several criteria, which human populations have used in selecting plants according to their therapeutic and preventive significance reported in various ethnopharmacological records (da Silva et al. 2018; Fitzgerald et al. 2020). The major selection criteria used for the current plant included geographical location and abundance; edibility and nutraceutical values of the plant and available literature on the use in traditional medicine. Averrhoa carambola L., often known as ‘Golden Star,’ is a beautiful fruit in the Oxalidaceae family that is grown in tropical and sub-tropical areas around the world (Vargas-Madriz et al. 2021). It is a delicious fruit with a succulent pulp, appealing flesh, and distinct flavour that is mostly yellow greenish in colour. It comes in sweet and sour variations, with both types being appropriate for value - addition. The sweet version, on the other hand, is chosen because of its superior sensory qualities in terms of flavour and texture (Ramadan et al. 2020). Fresh fruit or juice drinks are the most common ways to consume this fruit. Natural antioxidants including vitamin C, carotenoids, and certain phenolic compounds are abundant in the A. carambola fruit (Lakmal et al. 2021). Furthermore, it is shown to be a valuable source of insoluble dietary fibre with anti-diabetic properties. In addition to their use as a fresh fruit and in jelly processing, the distinct star shape and rich golden colour have a significant commercial potential as a salad and drink garnish (Hong-liang et al. 2021). The ripened fruit is also used in digestive ailments whereas the roots of A. carambola are used to treat arthralgia, chronic headaches, epitaxis, and spermatorrhea (Sheth 2005; Herbal Medicine Research Centre 2015; Chung et al. 1996). It is also known in traditional medicine for its anti-inflammatory (Sripanidkulchai et al. 2002), analgesic (Das and Ahmed 2012), hypoglycaemic (Chau et al. 2004a, b), anthelmintic (Shah et al. 2011), anti-ulcer (Goncalves et al. 2006), hypotensive (Soncini et al. 2011), anti-oxidant (Shui and Leong 2006), hypocholesterolaemic, hypolipidemic (Chau et al. 2004a, b), antimicrobial (Masum et al. 2007) as well as anti-tumour properties (Tadros and Sleem 2004). In the current study, we have determined the major chemical groups found in the leaves of A. carambola and examined the neuro-pharmacological properties of this plant in the in vivo mouse model.
Methods and materials
Chemicals and plant material
The standard drugs thiopental sodium and diazepam were bought from Incepta Pharmaceuticals Ltd. and Opsonin Pharma Ltd. Bangladesh, respectively. Different reagents and distilled water were purchased from British drug house (BDH) Chemicals Ltd. A. carambola plant material was collected from koyra, Khulna. The genus and the family of the plant were identified from National Herbarium, Bangladesh. (DACB accession number: 45747).
Preparation of methanol extracts
The leaves collected from A. carambola were separated from undesirable materials and washed with distilled water. The leaves were shade dried and ground into a coarse powder using a processor. The powder was packed in a container and stored in a cool, dark and dry environment before the study was initiated. A quantity of 400 g granulated leaves of A. carambola was soaked in 1000 ml of 95% methanol in a glass flask for 10 days with continuous shaking and mixing. Subsequently, the entire blend was filtered through a fine, white cotton material and Whatman filter paper 3 (pore size: 6 μm) to obtain a clear filtrate. In order to dissipate the solvent, the filtrate was placed in an open room and the extract was obtained. The yield of the methanolic extract from the leaves was 2.19% w/w. Extract was re-dissolved in 5% DMSO for oral administration.
Experimental animals and ethical approval
Ninety Swiss albino mice of both sexes (22–25 g weight/6–7 weeks of age) were purchased from Jahangirnagar University, Dhaka, Bangladesh. All the mice were housed in animal cages under standard conditions (22–25 °C, moisture 60–70%, 12-h light: 12-h dark cycle). The animals were fed with a standard pellet diet which was obtained from Jahangirnagar University, Dhaka. All the protocols in our study including in vivo experiments were approved by the Faculty of Allied Health Sciences Research Ethics Committee, Daffodil International University, Dhaka-1207, Bangladesh (Ref: FAHSREC/DIU/2020/1006).
Phytochemical analysis
The presence of some specific phytochemical groups was investigated in the qualitative preliminary phytochemical study. These included tannins, glycosides, alkaloids, steroids, flavonoids, saponins, gums, terpenoids, phenols and carbohydrates, which were identified by colorimetric methods (Awaludin et al. 2020). Carbohydrates were identified with Molisch test and Fehling’s test; Molisch test also determined the presence of gum in the extract. Dragendroff’s, Mayer’s and Hager’s tests reported the presence of alkaloids whereas flavonoid test identified flavonoids. Potassium dichromate, ferric chloride, and lead acetic acid derivation tests detected the presence of tannins. The glycosides were identified by Keller-Kiliani tests while the standard frothing test was done to report the presence of saponins. Steroids were detected using the sulphuric acid analysis.
Quantitative determination of some phytochemical constituents
Harborne’s method was used to analyse alkaloids: 5 g of the sample was weighed into a 250 ml beaker, and 200 ml of 10% acetic acid in ethanol was added, capped, and set aside for 4 h. This was filtered, and the extract was concentrated to one-quarter of its original volume using a water bath. Dropwise additions of concentrated ammonium hydroxide to the extract were made until the precipitation was accomplished. The entire solution was allowed to settle and rinsed with weak ammonium hydroxide before being filtered. The residual alkaloid content was desiccated and weighed. Van-Burden and Robinson’s method was used to determine tannin: A 50 ml plastic bottle filled with 500 mg of the sample. In a mechanical shaker, 50 ml of distilled water was added and agitated for 1 h. This was then filtered into a 50 ml volumetric flask and brought up to the required concentration. The filtrate was then pipetted into a test tube with 2 ml of 0.1 M FeCl3 in 0.1 N HCl and 0.008 M potassium ferrocyanide. Within 10 min, the absorbance was taken at 120 nm. Bohm and Kocipai Abyazan’s procedure for flavonoid determination: At room temperature, 10 g of the plant sample was extracted many times with 100 ml of 80% aqueous methanol. Whatman filter paper No 42 (pore size: 2.5 μm) was used to filter the entire solution (125 mm). The filtrate was then placed in a beaker and dried over a water bath before being weighed at a consistent weight (Amorim et al. 2008; Kumar et al. 2007; Edeoga et al. 2005).
Acute toxicity test
The methanol extract was administered orally in doses of 100, 200, 400 and 800 mg kg−1 to groups of rats (n = 5) and percentage mortality was noted beginning with 24 h up-to a period of 7 days (Bruce 1985).
Thiopental sodium-induced sleeping time test
The methodology mentioned by Ali et al. (2015) was used to study the effect of the leaves extracts on the thiopental sodium induced sleeping time. Experimental mice were placed into five groups including control, standard and three test groups with each group having five animals (n = 5). Group I (control) - provided distilled water with 5% DMSO; group II (standard) - provided diazepam (0.50 mg kg−1 body weight (b.w.)); group III administered 50 mg kg−1 b.w. extract; group IV administered 100 mg kg−1 b.w. extract; and group V administered extract 200 mg kg−1 b.w. extract. After half an hour, thiopental sodium (20 mg kg−1 b.w.) was given intra-peritoneal to all groups to induce sleep. The length of sleep induced by thiopental sodium in all the groups was examined as a decline in the correct reflex. Percentages of effects when treated with standard drug and the extract were calculated as following:
Hole cross test
The experiment was performed as assayed by Uddin et al. (2006). The following dimension was used to make a frame: 0.35 × 0.25 × 0.20 m. At the centre of the enclosure, a hole with a diameter of 3 cm was created at a height of 0.075 m on the designated segment. After treatment, the mice in each group were paced on one side of the compartment and their movement to the other side was tracked for 3 min at intervals 0, 30, 60, 90, and 120 min. Group I was set as control (provided distilled water with 5% DMSO) and Group II was set as a standard (provided diazepam 1 mg kg−1, b.w., p.o.). Groups III and IV were provided with the extract at doses of 100, and 200 mg kg−1 b.w., respectively. The inhibition was calculated by comparison with the control as following:
Hole board test
This study followed the protocol mentioned by Sheikh et al. (2016) with minor modifications. The test set-up had a level foundation (0.90 m × 0.90 m radius) with 16 equitably separated holes within a frame of 0.05 m height. Mice were grouped into four sets; one control, one standard and two test groups, with five mice (n = 5) in each group. Group 1 as control was given distilled water with 5% DMSO. Diazepam administered group II (1 mg kg−1 b.w., p. o.) was used as a standard. Group III and IV were provided leaves extract in the doses of 100 and 200 mg kg−1 b.w., respectively. Number of head dips into the holes for single mice was monitored for 10 min and inhibition was calculated as follows:
Open field test
The analysis followed the test described by Anisuzzman et al. (2017). An experimental board having a plane with 0.5 m2 field with a square progression was used as a test platform in which all the squares on the other side were painted black and white, giving a chessboard-like appearance. The compartment has a height of 0.1 m. The study included four groups with five (n = 5) mice in each group. Group I as control was given distilled water with 5% DMSO whereas diazepam (1 mg kg−1 b.w., p.o.) administered group was set as a standard. The test groups included group II and IV which were given leaves extract in the doses 100 and 200 mg kg−1 b.w., respectively. For a period of 3 min each at regular intervals (0, 30, 60, 90, and 120 min) after the oral route of test medications, the number of squares moved at any pace by the animals was considered to calculate the inhibition as followed:
Statistical analysis
SPSS statistical software, version 20, was used for all the data analysis. The results are reported as the mean ± SEM value. The statistical analysis was accomplished using one-way analysis of variance (ANOVA) pursued by Dunnett’s test for sleeping time test and hole board test. For the hole cross test and open field test two-way ANOVA persued by Bonferroni’s tests was adopted. *p < 0.05, and **p < 0.01 were reported to be statistically significant.
Results
The extract shows the presence of diverse phytochemical groups
The qualitative assessment of the extract showed the presence of some phytochemical groups that are important constituents of plants and known to possess varied pharmacological properties. These included alkaloids, tannins, steroids, flavonoids, phenols, saponins and terpenoids. Glycosides were reported to be absent in the extract. Phytochemical compounds of A. carambola leaves extract are shown in Table 1.
Quantitative determination of some phytochemical constituents
Quantitative estimation for certain phyto-constituents such as alkaloids, flavonoids and tannins in A. carambola leaves extract is summarized in Table 2. The estimations found tannins to be present in higher proportion compared to other phytochemicals determined in the study.
Acute toxicity
The mice treated with the methanol extract, 100–800 mg kg−1, b.w., p.o., showed no sign of adverse toxic effects. The extract was found to be safe up to a tested dose of 800 mg kg−1 b.w. in mice.
Oral administration of methanol leaves extract induces neuromuscular sedation in the thiopental Na –sleep model
The methanolic extract of A. carambola leaves at doses of 50, 100 and 200 mg kg−1 demonstrated a substantial reduction (*p < 0.05, **p < 0.01) in the time of onset of sleep in a dose-dependent way in the thiopental-induced hypnosis model. The results showed that the effect of the extract on the onset of sleep were comparable to those of the standard drug diazepam. Doses of the extract, relative to the controls, effectively potentiated the length of thiopental-sodium induced sleeping time in the experimental mice and reduced the latent period (Figs. 1, 2). The extract at 200 mg kg−1 demonstrated the maximum effect of 685.71%, whereas diazepam (0.50 mg kg−1) had a 474.60% effect for lacking resolving reflex (Table 3).
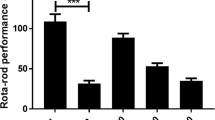
Animals treated with methanol leaves extract demonstrate CNS depressant activity as indicated by hole cross method
In the hole cross test, a substantial decrease was reported in the movement from 0 to 120 min in the test groups treated with the extract and the standard when compared to the control. This was assessed by recording the number of holes crossed between the compartments by the test mice compared to the control group. At 120 min the extract demonstrated about 33.5% suppression in locomotor functions at a dose of 200 mg kg−1 while the suppression resulting from the standard drug diazepam was 41.7% (Table 4).
CNS depressant activity in treated animals induced by methanol leaves extract as indicated by hole-board method
The hole-board test displayed the dose dependent inhibition of head-dips in the treated animals with doses of the extract at 100 mg kg−1 and 200 mg kg−1 b.w., significantly reducing the number of head-dips resulting in 31.95% and 44.38% inhibition, respectively (*p < 0.05, **p < 0.01), indicating CNS depressant activity and substantial suppression of the movement (Table 5).
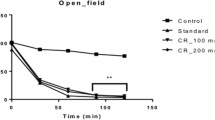
CNS depressant activity observed in animals treated with methanol leaves extracts as indicated by the open-field method
In the same experimental set-up the standard drug induced an inhibition of 51.48%. At the dosage amount of 100 mg kg−1 and 200 mg kg−1 b.w. (*p < 0.05, **p < 0.01) of leaves extract, the number of squares covered by mice was substantially decreased from its base value at 0 to 120 min, when compared with the control (Table 6). The test reported 60.7% inhibition of the locomotor activity for 100 mg kg−1 b.w. dose of the extract, which was similar to the standard drug. Interestingly, the higher dose of 200 mg kg−1 b.w. resulted in 77.8% inhibition showing significant suppressing effect of the extract on the CNS activity.
Discussion
Traditional medicines have been considered an original form of therapeutic approach that was inexpensive and effective since ancient times. Recent efforts have focused on utilizing naturally derived molecules with influence on neurobehavioral function and efficiency to complement modern medication with different ethnomedicinal products. The sedative effects of A. carambola were tested by monitoring the naturalistic locomotor behaviour of mice in open-field and hole-cross studies. In these experiments, the duration and frequency of motion can be mitigated by agents with sedative properties, illustrated as a reduction in the inquisitiveness of the unfamiliar setting. The hole board test was developed to assess mice reactions to a new environment designed to check anxiolytic-like behaviour. Nonetheless, other research has found that an animal’s head dipping movement is linked to their mental condition. The anxiolytic condition was discovered to correspond with mice anxiolytic properties that promote head poking depending on this discovery (Ebert et al. 2006). At higher concentrations, 200 mg kg−1 b.w. p.o., leaves extracts induced a stronger proclivity for head dipping in these mice. The outcomes of leaves extract on sleep time induced by thiopental-Na in mice showed significant sleep modulatory properties. The leaves extract’s centrally acting impact is indicated by a diminution in latency time and an extension of sleep duration. According to the study (Castañeda et al. 2021), the lengthening of barbiturate-induced (thiopental sodium) sleeping time indicates CNS depression-like behaviour. With increasing doses of the leaves extract, which might be a rich source of sleep-inducing elements, sleep duration was significantly extended. The muscle relaxant, anxiolytic, and sedative-hypnotic actions of benzodiazepines like diazepam are primarily due to the enhancement of gamma aminobutyric acid (GABAA) activity (Doyno and White, 2019). GABA is a crucial inhibitory neurotransmitter in the CNS that has been linked to a wide range of physiological processes as well as psychological and neurological diseases (Abruzzo et al. 2021). Since benzodiazepines attach to the GABAA receptor’s gamma subunit, a structural change in the receptor leads to an increase in the GABAA receptor activity. Benzodiazepines do not supplant GABA, which binds to the alpha subunit, but they do increase the frequency of channel opening processes, which results in higher chloride ion conductivity and action potential suppression (Ham et al. 2020; Bruni et al. 2021). The direct stimulation of glycine synapses in the brain may be responsible for benzodiazepine’s anxiolytic effect (Chopade et al. 2021). This could also explain the mode of action of our leaves extract, because the data show that the extract had an impact similar to diazepam. Anxiety and dread have been linked to the presence of alpha-2 receptors. Drugs that raise noradrenaline levels in the limbic forebrain have been demonstrated to reduce fear and anxiety (Bektas et al. 2020; Zangrossi et al. 2020). It was revealed that the animals’ head-dipping behaviour is clearly relevant to their mental state, and this test is used to investigate anxiety-related activities. Based on the research findings, it was hypothesized that an increase in head-dipping behaviour could indicate an anxiolytic state in animals. At the same time, a decrease in the number of head dips was related to a depressive impact (Aziz et al. 2020). Thus, the reduced head dipping behaviour and improved sleeping time of mice in our study may relate to the inhibitory effect of the extract on neuronal activity, similar to the inhibition induced by GABA. The therapeutic benefits of traditional medications might amalgamate a combination of constituents that could serve as an adjuvant to the conventional regimen. It is believed that in case of extracts, formulations and concoctions, the observed pharmacological effects might be due to a principal constituent or due to a synergistic/additive effect by a cooperative interaction of several constituents in the therapeutic and preventive pathways (Roell et al. 2017). Multiple studies have reported that phytochemical compounds including tannins possess sedative and hypnotic effects, which can cause nonspecific CNS depression (Emon et al. 2020; Emon et al. 2021). Hydrolysable tannins have been shown to possess anti-depressant like effects by modulating the hippocampal plasticity and altering monoamine neurotransmitters (Chandrasekhar et al. 2017). Natural products of different phytochemical classes are emerging as a source of lead compounds for the synthesis of drugs in neuro-pharmacology with anti-depressant, anxiolytic and hypnotic effects (Horowitz 2017). Flavonoids, which are a broad class of secondary metabolites and functional constituents of human diet, have also been reported to have significant neuro-modulatory properties (Hritcu et al. 2017). Several such neuro-modulatory action mechanisms have been proposed for alkaloids, flavonoids, steroids, and terpenoids, including neuroprotection against oxidative and metabolic insults; enhancing nicotinic receptors that elevate the sensation and memory; energizing and improving nervous function; activating the transient receptor calcium channels in the membrane of the nerve cell, which also have neuro-pharmacological influence (Welcome 2020; Alzobaidi et al. 2021). The leaves of A. carambola are the main constituent of lawar, a Balinese cuisine, which is served in Balinese traditional events and festivities (Astiti et al. 2019). Moreover, the plant has been known to have traditional uses and possess significant pharmacological properties and potential for the development of nutraceuticals (Luan et al. 2021).
Conclusions
The findings on the in vivo model were all dose-dependent and statistically significant. It can be concluded, based on the evaluation of the current analysis, that the extract of A. carambola possess significant neuro-modulatory properties. It shows potential to satisfy the medicinal need for the neuropsychiatric conditions including anxiety. However, further investigations are warranted to establish the specific phyto-constituents responsible for the neuro-pharmacology activities and the related mechanisms of action.
References
Abruzzo PM, Panisi C, Marini M (2021) The alteration of chloride homeostasis/GABAergic signaling in brain disorders: could oxidative stress play a role? Antioxidants 10(8):1316. https://doi.org/10.3390/antiox10081316
Ali MS, Dash PR, Nasrin M (2015) Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complement Altern Med 15:158. https://doi.org/10.1186/s12906-015-0670-z
Alzobaidi N, Quasimi H, Emad NA, Alhalmi A, Naqvi M (2021) Bioactive compounds and traditional herbal medicine: promising approaches for the treatment of dementia. Degener Neurol Neuromuscul Dis 11:1–14. https://doi.org/10.2147/DNND.S299589
Amorim EL, Nascimento JE, Monteiro JM, Peixoto Sobrinho TJ, Araújo TA, Albuquerque UP (2008) A simple and accurate procedure for the determination of tannin and flavonoid levels and some applications in ethnobotany and ethnopharmacology. Funct Ecosys Commun 2(1):88–94
Anisuzzman M, Hasan MM, Acharzo AK, Das AK, Rahman S (2017) In vivo and in vitro evaluation of pharmacological potentials of secondary bioactive metabolites of Dalbergia candenatensis leaves. Evid Based Complement Altern Med 2017:5034827. https://doi.org/10.1155/2017/5034827
Ansari N, Khodagholi F (2013) Natural products as promising drug candidates for the treatment of Alzheimer's disease: molecular mechanism aspect. Curr Neuropharmacol 11(4):414–429. https://doi.org/10.2174/1570159X11311040005
Astiti NPA, Susdirga SK, Ramona Y (2019) Analysis of phenolic and tannin contents in the methanol extract of sweet and sour star fruit plants (Averrhoa carambola L) leaves commonly used as raw materials of lawar (a Balinese traditional food). Adv Trop Biodivers Environ Sci 3(1):5–7. https://doi.org/10.24843/ATBES.2019.v03.i01.p02
Awaludin A, Kartina K, Maulianawati D, Manalu W, Andriyanto A, Septiana R, Arfandi A, Lalang Y (2020) Phytochemical screening and toxicity of ethanol extract of Sauropus androgynus. Biodiversitas 21:2966–2970. https://doi.org/10.13057/biodiv/d210712
Aziz MA, Barua N, Tareq AM, Alam N, Prova RJ, Mamun MN, Sayeed MA, Chowdhury MA, Emran TB (2020) Possible neuropharmacological effects of Adenia trilobata (Roxb.) in the Swiss albino mice model. Future J Pharm Sci 6(1):1–8. https://doi.org/10.1186/s43094-020-00102-5
Bektas N, Arslan R, Alyu F (2020) The anxiolytic effect of perampanel and possible mechanisms mediating its anxiolytic effect in mice. Life Sci 261:118359. https://doi.org/10.1016/j.lfs.2020.118359
Bruce RD (1985) An up-and-down procedure for acute toxicity testing. Fundam Appl Toxicol 5(1):151–157. https://doi.org/10.1016/0272-0590(85)90059-4
Bruni O, Ferini-Strambi L, Giacomoni E, Pellegrino P (2021) Herbal remedies and their possible effect on the GABAergic system and sleep. Nutrients 13(2):530. https://doi.org/10.3390/nu13020530
Castañeda R, Cáceres A, Velasquez D, Rodriguez C, Morales D, Castillo A (2021) Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: an overview. J Ethnopharmacol 283:114746. https://doi.org/10.1016/j.jep.2021.114746
Chandrasekhar Y, Ramya EM, Navya K, Phani Kumar G, Anilakumar KR (2017) Antidepressant like effects of hydrolysable tannins of Terminalia catappa leaf extract via modulation of hippocampal plasticity and regulation of monoamine neurotransmitters subjected to chronic mild stress (CMS). Biomed Pharmacother 86:414–425. https://doi.org/10.1016/j.biopha.2016.12.031
Chau CF, Chen CH, Lee MH (2004a) Characterization and physiochemical properties of some potential fibers derived from Averrhoa carambola. Nahrung 48(1):43–46. https://doi.org/10.1002/food.200300354
Chau CF, Huang YL, Lee MH (2004b) Effect of novel pomace fiber on lipid and cholesterol metabolism in the hamster. Nutr Res 24:337–345. https://doi.org/10.1016/j.nutres.2004.01.003
Chopade AR, Pol RP, Patil PA, Dharanguttikar VR, Naikwade NS, Dias RJ, Mali SN (2021) Pharmacological and in-silico investigations of anxiolytic-like effects of Phyllanthus fraternus: a probable involvement of GABA-A receptor. Curr Enzym Inhib 17(1):42–48. https://doi.org/10.2174/1573408016999201026200650
Chung KS, Paul PH, Kimura T (1996) International collation of traditional and folk medicine: Northeast Asia. World Scientific Publishing Company, Singapore
Creaven AM, Healy A, Howard S (2018) Social connectedness and depression: is there added value in volunteering? J Soc Person Relation 35(10):1400–1417. https://doi.org/10.1177/0265407517716786
da Silva TC, da Silva JM, Ramos MA (2018) What factors guide the selection of medicinal plants in a local pharmacopoeia? A case study in a rural community from a historically transformed Atlantic forest landscape. Evid Based Complement Alternat Med 2018:2519212. https://doi.org/10.1155/2018/2519212
Das BN, Ahmed M (2012) Analgesic activity of fruit extract of Averrhoa carambola. Int J Life Sci Biotech Pharm Res 1(3):22–26
Di Lorenzo A, Sobolev AP, Nabavi SF, Sureda A, Moghaddam AH, Khanjani S, Di Giovanni C, Xiao J, Shirooie S, Sokeng AJ, Baldi A (2019) Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (Molina) Stuntz) in a mouse model of post-stroke depression. Food Chem Toxicol 129:434–443. https://doi.org/10.1016/j.fct.2019.04.023
Doyno CR, White CM (2019) Sedative-hypnotic agents that impact gamma-aminobutyric acid receptors: focus on flunitrazepam, gamma-hydroxybutyric acid, phenibut, and selank. J Clin Pharmacol 61:S114–S128. https://doi.org/10.1002/jcph.1922
Ebert B, Wafford KA, Deacon S (2006) Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther 112(3):612–629. https://doi.org/10.1016/j.pharmthera.2005.04.014
Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4(7):685–688. https://doi.org/10.5897/AJB2005.000-3127
Egbuna C, Nadia S, Jabeen NS (2018) Pharmacognosy and prehistoric uses of medicinal plants. In: Egbuna C, Kumar S, Ifemeje JC, Kurhekar JV (eds) Phytochemistry. Apple Academic Press, New York, pp 3–16
Emon NU, Alam S, Rudra S, Chowdhury S, Rajbangshi JC, Ganguly A (2020) Evaluation of pharmacological potentials of the aerial part of Achyranthes aspera L.: in vivo, in vitro and in silico approaches. Adv Trad Med 25:1–4. https://doi.org/10.1007/s13596-020-00528-5
Emon NU, Alam S, Rudra S, Riya SR, Paul A, Hossen SM, Kulsum U, Ganguly A (2021) Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: in vivo, in vitro, and in silico approaches. Food Sci Nutr 9(2):833–846. https://doi.org/10.1002/fsn3.2047
Fitzgerald M, Heinrich M, Booker A (2020) Medicinal plant analysis: a historical and regional discussion of emergent complex techniques. Front Pharmacol 10:1480. https://doi.org/10.3389/fphar.2019.01480
GBD 2019 Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396(10258):1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Goncalves ST, Baroni S, Fernando A, Cortez DAG, Melo Gessilda AN (2006) Preliminary studies on gastric anti-ulcerogenic effects of Averrhoa carambola in rats. Acta Farm Bonaer 25(2):245–247
Ham HJ, Lee YS, Yun J, Han SB, Son DJ, Hong JT (2020) Anxiolytic-like effects of the ethanol extract of Magnolia obovata leaves through its effects on GABA-benzodiazepine receptor and neuroinflammation. Behav Brain Res 383:112518. https://doi.org/10.1016/j.bbr.2020.112518
Han KS, Kim L, Shim I (2012) Stress and sleep disorder. Exp Neurobiol 21(4):141–150
Holanda DK, Wurlitzer NJ, Dionisio AP, Campos AR, Moreira RA, de Sousa PH, de Brito ES, Ribeiro PR, Iunes MF, Costa AM (2020) Garlic passion fruit (Passiflora tenuifila Killip): assessment of eventual acute toxicity, anxiolytic, sedative, and anticonvulsant effects using in vivo assays. Food Res Int 128:108813. https://doi.org/10.1016/j.foodres.2019.108813
Hong-liang ZH, Xia JI, Tian-min HU, Yue QI, Guang-ming HU, Ren-bin HU, Zhen-guang HU (2021) Study on the mechanism of the root of Averrhoa carambola L. in the treatment of diabetic kidney disease based on network pharmacology. Nat Prod Res Dev (NPRD) 33(4):647. https://doi.org/10.16333/j.1001-6880.2021.4.015
Horowitz M (2017) Antidepressant and anxiolytic-like, sedation and hypnosis. J Basic Clin Physiol Pharmacol 28(2):91–92. https://doi.org/10.1515/jbcpp-2017-0022
Hritcu L, Ionita R, Postu PA et al (2017) Antidepressant flavonoids and their relationship with oxidative stress. Oxidative Med Cell Longev 2017:5762172. https://doi.org/10.1155/2017/5762172
Kalmbach DA, Anderson JR, Drake CL (2018) The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res 27(6):e12710. https://doi.org/10.1111/jsr.12710
Krause J, Tobin G (2013) Discovery, development, and regulation of natural products. In Kulka M (ed) Using old solutions to new problems-natural drug discovery in the 21st century. IntechOpen, London, pp 1-35. https://doi.org/10.5772/56424
Kumar AR, Subburathinam KM, Prabakar G (2007) Phytochemical screening of selected medicinal plants of Asclepiadaceae family. Asian J Microbiol Biotechnol Environ Sci 9(1):177–180
Lakmal K, Yasawardene P, Jayarajah U, Seneviratne SL (2021) Nutritional and medicinal properties of star fruit (Averrhoa carambola): a review. Food Sci Nutr 9(3):1810–1823. https://doi.org/10.1002/fsn3.2135
Lopresti AL, Smith SJ, Hood SD, Drummond PD (2019) Efficacy of a standardised saffron extract (affron®) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: a randomised, double-blind, placebo-controlled study. J Psychopharmacol 33(11):1415–1427. https://doi.org/10.1177/0269881119867703
Luan F, Peng L, Lei Z, Jia X, Zou J, Yang Y, He X, Zeng N (2021) Traditional uses, phytochemical constituents and pharmacological properties of Averrhoa carambola L.: a review. Front Pharmacol 12:699899. https://doi.org/10.3389/fphar.2021.699899
Masum M, Rahman S, Begum K, Begum B, Rashid A (2007) Phytochemical and biological studies of Averrhoa carambola. J Pharm Sci 6(2):125–128. https://doi.org/10.3329/dujps.v6i2.688
Mendelson S (2019) Herbal treatment of major depression: scientific basis and practical use. CRC Press, Boca Raton
Milan R, Vasiliadis HM (2020) The association between side effects and adherence to antidepressants among primary care community-dwelling older adults. Aging Mental Health 24(8):1229–1236. https://doi.org/10.1080/13607863.2019.1594165
Nabavi SM, Daglia M, Braidy N, Nabavi SF (2017) Natural products, micronutrients, and nutraceuticals for the treatment of depression: a short review. Nutr Neurosci 20(3):180–194. https://doi.org/10.1080/1028415X.2015.1103461
Najmi A, Javed SA, Al Bratty M, Alhazmi HA (2022) Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 27(2):349. https://doi.org/10.3390/molecules27020349
Nimgampalle M, Devanathan V, Saxena A (2021) Importance of in silico studies on the design of novel drugs from medicinal plants against 21st-century pandemics: past, present, and future. In: Viswanath B (ed) Pandemic outbreaks in the 21st century 2021. Academic Press, Cambridge, pp 211–223
Ramadan NS, Wessjohann LA, Mocan A, Vodnar DC, El-Sayed NH, El-Toumy SA, Abdou Mohamed D, Abdel Aziz Z, Ehrlich A, Farag MA (2020) Nutrient and sensory metabolites profiling of Averrhoa carambola L.(starfruit) in the context of its origin and ripening stage by GC/MS and chemometric analysis. Molecules 25(10):2423. https://doi.org/10.3390/molecules25102423
Roell KR, Reif DM, Motsinger-Reif AA (2017) An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol 8:158. https://doi.org/10.3389/fphar.2017.00158
Shah NA, Raut BA, Baheti A, Kuchekar BS (2011) In-vitro anthelmintic activity of leaf extract of Averrhoa carambola against Pheretima posthuma. Pharmacogyonline 1:524527
Sheikh BY, Zihad SMNK, Sifat N et al (2016) Comparative study of neuropharmacological, analgesic properties and phenolic profile of Ajwah, Safawy and Sukkari cultivars of date palm (Phoenix dactylifera). Orient Pharm Exp Med 16:175–183. https://doi.org/10.1007/s13596-016-0239-5
Sheth A (2005) The Herbs of Ayurveda. Vol. 1. Sheth Publisher, Gujrat
Shui G, Leong LP (2006) Residue from starfruit as valuable source for functional food ingredients and nutraceuticals. Food Chem 97:277–284. https://doi.org/10.1016/j.foodchem.2005.03.048
Soncini R, Santiago MB, Orlandi L, Moraes GO, Peloso AL, dos Santos MH, Alves-da-Silva G, Paffaro VA Jr, Bento AC, Giusti-Paiva A (2011) Hypotensive effect of aqueous extract of Averrhoa carambola L. (Oxiladaceae) in rats: an in-vivo and in-vitro approach. J Ethnopharmacol 133(2):3537. https://doi.org/10.1016/j.jep.2010.10.001
Sripanidkulchai B, Tattawasart U, Laupattarakasem P, Wongpanich V (2002) Antiinflammatory and bactericidal properties of elected indigenous medicinal plants used for dysuria. Thai J Pharm Sci 26(12):33–38
Suetani S, Stubbs B, McGrath JJ, Scott JG (2019) Physical activity of people with mental disorders compared to the general population: a systematic review of longitudinal cohort studies. Soc Psychiatr Epidemiol 1:1–5. https://doi.org/10.1007/s00127-019-01760-4
Süntar I (2020) Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev 19(5):1199–1209. https://doi.org/10.1007/s11101-019-09629-9
Tadros SH, Sleem AA (2004) Pharmacognostical and biological study of the stem and leaf of Avehrroa carambola L. Bull Fac Pharm 42:225–246
Uddin SJ, Shilpi JA, Rahman MT, Ferdous M, Rouf R, Sarker SD (2006) Assessment of neuropharmacological activities of Pandanus foetidus (Pandanaceae) in mice. Pharmazie 61:362–364
Vargas-Madriz AF, Kuri-García A, Vargas-Madriz H, Chávez-Servín JL, Ayala-Tirado RA (2021) Phenolic profile and antioxidant capacity of fruit Averrhoa carambola L.: a review. Food Sci Technol. https://doi.org/10.1590/fst.69920
Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J (2014) Frisher M (2014) effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ 348:g1996. https://doi.org/10.1136/bmj.g1996
Welcome MO (2020) Neuroinflammation in CNS diseases: molecular mechanisms and the therapeutic potential of plant derived bioactive molecules. Pharma Nutrition 11:100176. https://doi.org/10.1016/j.phanu.2020.100176
Zangrossi H Jr, Del Ben CM, Graeff FG, Guimarães FS (2020) Serotonin in panic and anxiety disorders. In: Müller CP, Cunningham KA (eds) Handbook of behavioural neuroscience, Vol 31. Elsevier, Amsterdam, pp 611–633
Acknowledgments
The authors are grateful to the department of pharmacy, Daffodil International University for providing necessary support to conduct the research.
Funding
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia under grant no. (KEP-1-141-41). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akter, A., Islam, F., Bepary, S. et al. CNS depressant activities of Averrhoa carambola leaves extract in thiopental-sodium model of Swiss albino mice: implication for neuro-modulatory properties. Biologia 77, 1337–1346 (2022). https://doi.org/10.1007/s11756-022-01057-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01057-z