Abstract
Epibiotic species and epibiotic communities are heterogeneously distributed among individuals and body parts and habitats. They have a profound ecological impact, since the presence of epibionts affects the fitness of basibionts directly as well as indirectly, by modulating its interactions with the abiotic and biotic environment. In this article we assess the composition, alpha and beta diversity of epizoic macroalgae on crustaceans. Alpha diversity of epizoic macroalgae was determined for each of the basibionts as the effective number of species (qD). We proceeded with the correlation methodology Olmstead-Tukey to determine the hierarchical organization. On the shallow coast of the province of Santiago de Cuba, 24 epizoic macroalgae were found on the exoskeletons of six species of crustaceans. The hierarchical position of epizoic macroalgae in crustaceans is characterized by a predominance of rare species, followed by dominant, occasional and finally constant taxa. The greatest richness and diversity of epizoic macroalgae species was found on majoid crustaceans, which have active a masking behavior. The highest values of species richness, alpha and beta diversity of epizoic macroalgae were found in crustaceans with active camouflage. The fact that beta diversity is determined by the replacement of species reinforces the idea that these crustaceans use macroalgal assemblages to camouflage themselves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In marine ecosystems, it is common for the hard and stable surfaces provided by various organisms to be used as substrata for taxa such as macroalgae (Wahl 2008; Fernandez-Leborans et al. 2013). Epibiotic macroalgae are those that obligately or facultatively adhere to the surface of living organisms during all or part of their life cycle (Harder 2008; Wahl 2015). Worldwide, these algae have been studied on sponges, corals, mollusks, fishes, and turtles (Bretos and Chihuailaf 1990; Ballantine et al. 2001; Connelly and Turner 2009; Levenets et al. 2010; Serio et al. 2011).
In the tropical seas of the North American hemisphere, publications characterizing the composition of the flora of epibiontic macroalgae are species-specific. Within this group, epiphytes are the best known and most widely addressed in the literature (Cabrera et al. 2012; Mateo-Cid et al. 2014; Nava-Olvera et al. 2017; Jover et al. 2020). However, research on epizoic macroalgae is concentrated in Mexico (Sentíes et al. 1999; Alvarez-Cerrillo et al. 2017; Quiroz-González et al. 2020) and Cuba (Ros and Suárez 1980; Reyes de Armas 2016; Cabrera and Jover 2019; Alfonso et al. 2020). In this region, only Alfonso et al. (2020) refer for the first time to the structure of the epizoic macroalgal assemblage in octocorals. These authors limit themselves to analyzing the richness of the species observed and the differences between sampling sites in terms of richness and specific composition.
Currently, there is a conceptual and mathematical framework to estimate and compare species richness in an ecological sense (effective species number or diversity of order q: qD) (Cultid-Medina and Escobar 2019). Effective species number is a useful tool to characterize alpha and beta diversity of ecological communities (Moreno et al. 2011; Cruz et al. 2017; Fontenla 2018). It is advisable to quantify species richness and their representativeness to understand species diversity in its taxonomic dimension (Moreno et al. 2018). These approaches have not yet been used to analyze the distribution patterns of the diversity of epizoic macroalgae, although they are important for analyzing community-level structure.
The richness of epizoic macroalgae for Cuba is 97 species according to published works (Ros and Suárez 1980; Reyes de Armas 2016; Cabrera and Jover 2019; Alfonso et al. 2020). The basibionts studied are four species of crabs of the order Brachiura (Ros and Suárez 1980; Cabrera and Jover 2019), one gastropod (Reyes de Armas 2016) and 13 species of octocorals (Alfonso et al. 2020). Despite the fact that studies of epibiosis by macroalgae in crustaceans began in the early twentieth century (Fernandez-Leborans 2018), the distribution patterns of the composition and diversity of species of this taxonomic group in Cuba are underestimated. The aim of this work is to determine the composition, alpha and beta diversity of epizoic macroalgae in crustaceans of the Santiago de Cuba littoral zone.
Materials and methods
Study area
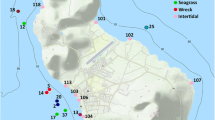
The sampling locations are located in the coastal area of the province of Santiago de Cuba, two in the municipality of Guamá (Guaimaral and La Ceiba) and two in the municipality of Santiago de Cuba (Mar Verde and Siboney). The localities were selected for having rocky soils with abundant macroalgal growth. This type of habitat is typical of crustaceans that have algae on their exoskeleton (Villegas et al. 2006; Costa et al. 2010; Fernandez-Leborans 2018).
The Guaimaral tide pool (19°56′35''N; 76°46′17''W) is located 1.7 km west from the mouth of the Turquino River, while La Ceiba (19°56′42''N; 76°44′48''W) is located 2.3 km east of the mouth of the Turquino River. The mean depth at these locations was 2.7 m and 3.2 m, respectively. In Guaimaral, small patches of Thalassia testudinum K. D. Koenig were also found in the macroalgal bed.
The Aguadores site (19°57′51''N; 75°49′47''W) is located at the western tip of Aguadores beach, about 400 m from the mouth of San Juan River, and has an average depth of 0.7 m. Mar Verde (19°57′32''N; 75°57′23''W) is located 10 km west of Santiago de Cuba Bay. This last locality has an average depth of 2.5 m and among the macroalgae there are patches of T. testudinum and Syringodium filiforme Kützing.
Sampling method
Sampling was conducted from June 2018 to July 2019. At each site crabs with macroalgae attached to their exoskeleton were located by SCUBA diving according to the methodology of Guzmán (1979). In each locality, an active search for crabs was carried out in the macroalgal beds. Once the crabs with algae on the exoskeleton were located, they were placed in nylon bags with seawater. To homogenize the sample size at each locality, an intensive search was conducted for two hours. The collected crustaceans were deposited in nylon bags with seawater and labeled with the local data for later analysis in the laboratory.
In the laboratory, macroalgae were separated from crustaceans by scraping them with a scalpel and stored in vials containing a 70% ethanol solution for later identification (Guzmán 1979). Taxonomic identification based on morphological characters was carried out with the help of specialized literature and identification books. In the identification of the morphological characters of the cells and the thallus of the macroalgae, temporary preparations were made that were observed under an optical microscope. Specialized literature was used in the case of crustaceans (Abele and Kim 1986; Diez and Jover 2015; Diez 2018) and for algae (Littler and Littler 2000; Dawes and Mathieson 2008; Littler et al. 2008). For legitimacy of names and taxonomic classification, the criteria of WoRMS (2020) for crustaceans and Algaebase (Guiry and Guiry 2020) for algae were followed. All crustaceans were deposited in the collections of Museo Historia Natural Charles Ramsden de la Torre and algae in Ficoteca Cubana y Antillana, both at the Universidad de Oriente.
The abundance of epizoic macroalgae was estimated visually, as the percentage of cover of the exoskeleton of the crab (Parapar et al. 1997). All macroalgae were assigned to a morphofunctional group based on the criteria of Steneck and Dethier (1994). In addition, each macroalga was assigned a type of fixation structure according to the literature (León-Alvarez et al. 2007; León-Alvarez and Núñez 2011; León Álvarez et al. 2019; Quiroz-González et al. 2020).
Data analysis
Alpha diversity of epizoic macroalgae was determined for each of the basibionts as the effective number of species, termed qD (Jost 2006) according to the equation:
Observed alpha diversity was determined for q = 0 and used as the observed richness index. For q = 1, the Shannon entropy index was used, and for q = 2, the inverse of the Simpson index (Jost 2006) calculated using the 'hillR' package in R (Li 2020).
For calculating the estimated diversity of order 0 (species richness), the non-parametric estimator ACE (Abundance based coverage estimator) was selected (Chao and Lee 1992). For order 1 diversity (Shannon index exponential), a proposed estimator was used in the absence of complete community knowledge (Bias-corrected Shannon diversity estimator; Chao and Shen 2003). For order 2 diversity (inverse of the Simpson index), the MVUE estimator (minimum variance umbiaset estimator; Chao and Shen 2010) was used. All these estimators were calculated using the SPADE package in R (Chao and Shen 2010).
In the case of basibionts, the index of constancy of occurrence was determined according to the proposal of Román-Valencia et al. (2017). According to these authors, the constancy index is determined by the quotient of the number of recollections where the species is found among the total number of collections made in the study multiplied by 100. Constant basibionts were those that occurred in more than 50% of the sampling sites; accessories those that ranged between 25 and 50%, and random those with a percentage lower than 25%.
To determine the hierarchical organization (degree of appearance) of the macroalgal species found, we proceeded with the correlation methodology Olmstead-Tukey represented by quadrant graphs (Sokal and Rohlf 2012). This method was based on the graphical representation of the relative abundance and the frequency of occurrence of the epizoic macroalgae. The relative abundance was expressed as a percentage of epizoic macroalgae cover and the frequency in the percentage of occurrence of the species in the exoskeletons of crabs. This allowed classifying taxa as dominant (abundance and relative frequency higher than the average values of abundance and frequency), constant (relative abundance lower than their average but relative frequency higher than their own), occasional (relative abundance greater than their average but relative frequency lower than their average) and rare (abundance and relative frequency lower than their average values).
The difference in species composition was determined according to the criteria of Carvalho et al. (2013), examining the extent to which the difference in species composition between pairs of crabs species was due to species replacement or differences in richness. The metrics used to determine beta diversity and its components were the Podani family of indices because they are easy to interpret in ecological terms (Borcard et al. 2018). The Jaccard index was selected as it is the most robust for qualitative data according to Schroeder and Jenkins (2018). Additionally, the Ružička index was determined from the abundance values of the epizoic macroalgae. Quantitative beta diversity indices, such as the Ružička index, are appropriate when communities differ in the abundances of their species (Legendre 2014). Both indices were calculated using beta diversity functions (beta.multi and beta.par), which calculate partitions of multiple sites and pairs of beta diversity, with the package "betapart" in R (Baselga et al. 2018).
Results
On the shallow coast of the province of Santiago de Cuba, 24 epizoic macroalgae were found in the exoskeletons of crustaceans. They are grouped into 14 Rhodophyta, seven Chlorophyta and four Ochrophyta, belonging to 17 families (Table 1). The families Bryopsidaceae and Gracilariaceae were the most represented with four and three species, respectively. The green alga Chaetomorpha aerea (Dillwyn) Kützing was detected on all crustacean species, whereas the red algae Jania pedunculata var. adhaerens (J.V.Lamouroux) A.S.Harvey, Woelkerling & Reviers, Ceramium cimbricum H.E. Petersen and Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine & Farnham, were detected on three of the six crustaceans sampled. Seven species are recorded for the first time as epizoic macroalgae for the Cuban flora. Of these, three belong to the Rhodophyta, one to the Ochrophyta and three to the Chlorophyta. The dominant morphofunctional groups were filamentous followed by corticated filamentous algae with 50% and 25%, respectively. The most common fixation structure in the algae was rhizoids with 58% followed by a basal disk with 33%.
In the sublittoral macroalgae, 54 individuals grouped in six species of crustaceans with epizoic algae were collected in coastal zone beds. Of these, four belong to the infraorder Brachyura and one to Anomura (Table 2). Only Omalacantha bicornuta (Latreille, 1825) was constant in the coastal area, with a value of the index of constancy of occurrence of 60%. Mithraculus sculptus (Lamarck, 1818) is recorded as an accessory basibiont with a constancy of 40%. The three remaining crustaceans are considered accessory basibionts.
The species richness (q = 0) and specific diversity (q = 1, q = 2) of epizoic macroalgae are consistent with the values obtained for all basibiont crustaceans (Table 3). The highest value of epizoic richness is recorded in O. bicornuta with 19 epizoic macroalgae. In the rest of the crustaceans, the richness of macroalgal species ranged from eight to two. If relative abundance is taken as a measure of diversity (q = 1), the pattern of diversity was similar to richness. The greatest diversity of macroalgae species was also recorded in O. bicornuta with 16.76. The remaining basibionts had values ranging from 1.89 to 6.58 effective species of epizoic macroalgae. Diversity values centered on the most abundant species (q = 2) showed a similar trend to species richness and first order diversity.
The measure of 1st order diversity showed that the species O. bicornuta has a diversity equivalent to that of a theoretical community of 16.76 species of epizoic algae, in which they all have the same abundance. The species M. trispinosum and A. biforns have a diversity equivalent to that of a community of 6.58 and 6.45 effective species, respectively. The species with the lowest diversity was N. angustiforns with 1.89 effective species. Expressing these equivalences, O. bicornuta is 10 times more diverse in epizoic algal species than M. trispinosum, which was the second most diverse species. Moreover, O. bicornuta has more than 9 times the species diversity of N. angustiforns. That is, N. angustiforns has only 11% of the diversity that O. bicornuta has.
The hierarchical position of epizoic macroalgae in crustaceans is characterized by a predominance of rare species with 56%, followed by dominant 28%, occasional 12% and finally constant 4% (Fig. 1). Of the 14 rare species, 29% were characterized by being filamentous with basal disc and rhizoid as fixation structures. Of these, 21% were corticated filamentous with basal disc; 14% were calcareous and their fixation form could not be determined, and 7% were foliose with basal disc. Seven dominant species were recorded in which 57% had filamentous morphology with a basal disc as structure for attachment to a basibiont. The rest of the dominant species were calcareous and filamentous corticated with basal disc. Occasional species are Dictyota ciliolata Sonder ex Kützing (corticated thallus with rhizoids), Bryopsis plumosa (Hudson) C. Agardh (filamentous thallus with rhizoids) and Ulva flexuosa Wulfen (foliose with basal disc). Finally, only one constant species was recorded, Sphacelaria tribuloides Meneghini, which has a filamentous thallus with rhizoids.
Hierarchical classification of the epizoic macroalgae assemblage on the coast of Santiago de Cuba. The dotted line represents the mean value of frequency and abundance respectively. The numbers correspond to the identifier in Table 1
The similarity indices of Jaccard (0.151) and Ružička (0.107) were close to zero (Fig. 2), which demonstrates the dissimilarity between the structure of the epizoic macroalgae assemblages among the five crustaceans studied. For both indices, replacement is the component of beta diversity that contributed the most to dissimilarity, with an average value of 0.458 (Jaccard) and 0.465 (Ružička), whereas in the second component, the difference in richness was 0.392 and the difference in abundance was 0.429 between the pairs of crustaceans species.
Triangular plots of beta diversity comparisons using the Jaccard (A) and Ružička (B) dissimilarity index for epizoic macroalgal assemblages on the Santiago de Cuba littoral. Each point represents a pair of crabs. Its position is determined by a triplet of values from the S (similarity), Repl (replacement), and RichDiff/AbDiff (richness/abundance difference) matrices; each triplet sums to one
Discussion
All macroalgae reported as epizoic on shallow littoral crustaceans on the southeastern coast of Cuba were recorded with their epibiont habit, at least as epiphytes (Ortega et al. 2001; Fredericq et al. 2009; Suárez et al. 2015; Jover et al. 2020). This suggests that the exoskeleton of crustaceans on the coast of Santiago de Cuba is not a unique habitat for macroalgae. The families with the highest specific richness and the best distributed species among the collected crustaceans correspond to macroalgae, which often develop on living organisms (Nava-Olvera et al. 2017; Jover et al. 2020). The insufficient information on the composition, abundance and structure of epizoic macroalgal assemblages in the Cuban marine platform, limited to four papers, does not allow us to determine the families with the highest species richness, although genera such as Chaetomorpha, Jania and Ceramium are among those that contribute most to the species richness of epizoic macroalgae (Reyes de Armas 2016; Cabrera and Jover 2019; Alfonso et al. 2020).
The 29% of species are recorded as epizoic on the Cuban shelf for the first time, although all species are common in the flora of the region (Ortega et al. 2001; Fredericq et al. 2009; Suárez and Martínez-Daranas 2020). 29% of the species are recorded for the first time as epizoic on the Cuban shelf, although all species are common in the flora of the region (Suárez et al. 2015). In addition, the small size of these algae and the fact that there are immature stages that make taxonomic identification difficult, cause them to be underestimated during sampling (Quiroz-González et al. 2020).
The highest richness of Rhodophyta followed by Chlorophyta is consistent with the pattern that exists in other tropical ecosystems (Wynne 1986; John et al. 2003; Phang et al. 2016) and in the study area (Jover et al. 2009, 2012). Moreover, it is consistent with the pattern of richness by phyla observed by Reyes de Armas (2016) in the mollusk Lobatus gigas (Linnaeus, 1758) and by Cabrera and Jover (Cabrera and Jover 2019) in Brachiura. However, Alfonso et al. (2020) noted in octocorals that Rhodophyta had the highest species richness, followed by Ochrophyta and finally Chlorophyta. Whether the species and habitus (sessile or mobile) of the basibiont determine the richness of epizoic macroalgae is not well established in the scientific literature.
The predominance of corticated filamentous species and filamentous species is consistent with what has been found for epiphytic (Széchy et al. 2008; Soares and Fujii 2012; Diez et al. 2013; Jover et al. 2020) and epizoic macroalgae (Alfonso et al. 2020; Quiroz-González et al. 2020). These morphofunctional groups are adapted to live on substrata that are of short duration and exposed to different levels of disturbance (Széchy et al. 2008). The high growth and reproduction rate of filamentous algae lead to the opportunistic behavior of these species and rapid colonization of the substratum (Steneck and Dethier 1994). The richness of filamentous macroalgae has been noted among the mollusk L. gigas (Reyes de Armas 2016), the crabs O. bicornutus (Guzmán 1979), octocorals (Alfonso et al. 2020) and sea turtles (Sentíes et al. 1999; Loza 2011).
Fixation structures play an important role in the establishment and development of benthic algae. The presence of rhizoids, discs and haptera is typical of macroalgae that attach to solidified substrata (Santelices 1977). The predominance of rhizoids as a fixation structure of epiphytic macroalgae on crustaceans of the southeast coast of Cuba is consistent with that found in various basibionts of the Mexican Pacific (Quiroz-González et al. 2020). Rhizoids exhibit a number of adaptive properties, including apical growth, frequent branching, negative phototropism, positive geotropism, positive thigmotaxis, and morphological plasticity, which allow macroalgae to increase the surface-substratum contact area and to form a strong adhesive bond with the substratum (Fletcher and Callow 1992). According to these authors, the properties of the substratum such as topography and chemical composition favor the development and establishment of rhizoids. Therefore, the characteristics of the exoskeletons of crustaceans, like those of molluscs, are characterized by their hardness, the presence of appendages that increase the contact area and the chemical composition which favor the adhesion of macroalgae with rhizoids and fixation discs.
The superfamily Majoidea was the most represented with five species and the superfamily Galatheoidea with one. These crustaceans use algae for camouflage and feeding (Martinelli et al. 2011; Machado et al. 2013; Greco et al. 2014). All basibiont crustaceans have been recorded in macroalgae beds on the shallow littoral of the southeast coast of Cuba. (Diez and Jover 2015; Diez 2018). The majoid crustaceans are recorded in the study area as representing constant and accessory species. These species have a wide distribution in rocky substrata dominated by macroalgal beds due to their behavior as decorative crustaceans. (Hultgren and Stachowicz 2009; Martinelli et al. 2011). In addition, in areas of the Caribbean with beds dominated by seagrasses and macroalgae, the most common macrocrustaceans are majoids (Garcia et al. 1998; Carmona-Suárez 2000).
The greatest richness and diversity of epizoic macroalgal species was found on majoid crustaceans, which have an active masking behavior. Majoid crustaceans have structures in their exoskeleton that allow them to cover themselves with materials from their environment for camouflage (Hultgren and Stachowicz 2011). In addition, unlike other brachyurans, these majoids have a terminal that may favor the growth of macroalgae in their exoskeleton (Hinsch 1972). The greater diversity of epizoic algae responds to the camouflage strategy employed by majoids to create a mask resembling a group of attached algae (McLay 2020). However, N. angustifrons has the lowest species richness and diversity of epizoan algae because it does not exhibit active decorating behavior (Wahl et al. 1997).
The assemblage of epizoic macroalgae on the exoskeleton of the studied crustaceans is characterized by the dominance of rare species, a characteristic phenomenon of ecological communities (Magurran and Henderson 2011). In addition, the abundance of rare species could be due to habitat availability (crustaceans) and structure (exoskeleton morphology). Habitat availability and architectural structure influence the abundance of epibionts present in the camouflage (Stevens and Ruxton 2019). However, with the information available in this study, it is not possible to analyze whether there are macroalgal preferences for crustacean species. The information analyzed also makes it impossible to analyze the relationship between the abundance of epizoic macroalgae and the abundance of macroalgae present in the habitat.
The evaluation of the beta diversity of epizoic macroalgae in Cuba is limited to a single work in octocorals (Alfonso et al. 2020). These authors do not analyze the components of beta diversity and the analysis is between communities and not between basibionts. However, it ecognizes the importance of identifying the components of beta diversity to determine the processes that dominate between sampling sites in a study (Legendre 2014). In Cuban marine epibiont communities, analysis of the components of beta diversity has been limited to only one study in epiphytic macroalgae (Jover et al. 2020). These authors found that difference in richness was the component that contributed most to beta diversity in epiphytic macroalgae. However, in assemblages of epizoic algae on crustaceans, the replacement of species is the component that determines low similarity in these assemblages. It is known that the change in species composition may reflect environmental classification of species or dispersal constraints that lead to selective differentiation of species groups among assemblages (Victorero et al. 2018). The fact that beta diversity is determined by the replacement of species reinforces the idea that these crustaceans use macroalgal assemblages to camouflage themselves.
Conclusions
The assemblage of epizoic macroalgae on six species of crustaceans on the southeast coast of Cuba consists of 25 macroalgae, dominated by rhodophytes and filamentous algae. The highest values of species richness, alpha and beta diversity of epizoic macroalgae were found on crustaceans with active camouflage. These epizoic macroalgae assemblages are characterized by the richness of rare species, followed by the dominant species. Additionally, the richness and diversity values of the epizoic macroalgal species found to confirm that the crustaceans studied represent key species in the structure of benthic macroalgal assemblages.
References
Abele LG, Kim W (1986) An illustrated guide to the marine decapod crustaceans of Florida. State of Florida, Department of Environmental Regulation
Alfonso Y, Rey-Villiers N, Martínez-Daranas B (2020) Macroalgas epizoicas en octocorales en el litoral de La Habana, Cuba. Rev Investig Mar 40:1–21
Alvarez-Cerrillo LR, Valentich-Scott P, Newman WA (2017) A remarkable infestation of epibionts and endobionts of an edible chiton (Polyplacophora: Chitonidae) from the Mexican tropical Pacific. Nautilus (philadelphia) 131:87–96
Ballantine DL, Navarro JN, Hensley DA (2001) Algal colonization of Caribbean scorpionfishes. Bull Mar Sci 69:1089–1094
Baselga A, Orme A, Villerger S et al (2018) Partitioning Beta Diversity into Turnover and Nestedness Components. https://cran.r-project.org/web/packages/betapart/index.html. Accessed 21 Jan 2020
Borcard D, Gillet F, Legendre P (2018) Numerical Ecology with R, 2nd edn. Springer International Publishing
Bretos M, Chihuailaf R (1990) Biometría y otros aspectos biológicos de Fissurella pulchra (Mollusca: Prosobranquia). Biol Mar 25:1–14
Cabrera A, Jover A (2019) Algas epibiontes en braquiuros (Crustacea) de dos pocetas intermareales en la costa suroriental de Cuba. Novit Caribaea 13:13–21
Cabrera R, Alfonso Y, Martínez-Daranas B, Suárez AM (2012) Estructuras reproductoras y epifitas del género Avrainvillea en aguas cubanas. Rev Investig Mar 31:3–10
Carmona-Suárez CA (2000) Differences between Mithraculus spp. communities in exposed and sheltered shallow-water Thalassia beds in Venezuela. Crustac Issues 12:419–430
Carvalho JC, Cardoso P, Borges PAV et al (2013) Measuring fractions of beta diversity and their relationships to nestedness: a theoretical and empirical comparison of novel approaches. Oikos 122:825–834. https://doi.org/10.1111/j.1600-0706.2012.20980.x
Chao A, Lee S-M (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87:210–217. https://doi.org/10.1080/01621459.1992.10475194
Chao A, Shen T-J (2003) Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ Ecol Stat 10:429–443. https://doi.org/10.1023/A:1026096204727
Chao A, Shen TJ (2010) User’s guide for program SPADE (Species prediction and diversity estimation). Taiwan Natl. Tsing Hua Univ. https://chao.shinyapps.io/SpadeR/
Connelly PW, Turner RL (2009) Epibionts of the eastern surf chiton, Ceratozona squalida (Polyplacophora: Mopaliidae), from the Atlantic coast of Florida. Bull Mar Sci 85:187–202
Costa TM, Christofoletti RA, Pinheiro MAA (2010) Epibionts on Arenaeus cribrarius (Brachyura: Portunidae) from Brazil. Zoologia 27:387–394. https://doi.org/10.1590/S1984-46702010000300010
Cruz D, Martínez D, Fontanela J, Mancina C (2017) Inventarios y estimaciones de la biodiversidad. In: AMA E (ed) Diversidad biológica de Cuba: métodos de inventario, monitoreo y colecciones biológicas. La Habana, pp 22–43
Cultid-Medina C, Escobar F (2019) Pautas para la estimación y comparación estadística de la diversidad biológica (qD). In: Moreno C (ed) La biodiversidad en un mundo cambiante: Fundamentos teóricos y metodológicos para su estudio. Universidad Autónoma del Estado de Hidalgo/Libermex, Ciudad de México, pp 175–202
Dawes CJ, Mathieson AC (2008) The seaweeds of Florida. University Press of Florida, Gainesville
Diez YL (2018) New data on the distribution of crabs (Decapoda: Anomura and Brachyura) in Cuba. Rev Investig Mar 38:139–158
Diez Y, Jover A (2015) List of marine crabs (Decapoda: Anomura and Brachyura) of shallow littoral of Santiago de Cuba, Cuba. Check List 11:1601. https://doi.org/10.15569/11.2.1601
Diez YL, Jover A, Suárez AM et al (2013) Distribution of epiphytic macroalgae on the thalli of their hosts in Cuba. Acta Bot Brasilica 27:815–826. https://doi.org/10.1590/S0102-33062013000400022
Fernandez-Leborans G (2018) Epibiosis in Crustacea: an overview. Crustaceana 83:549–640. https://doi.org/10.1163/001121610X491059
Fernandez-Leborans G, Dávila P, Cerezo E, Contreras C (2013) Epibiosis and hyperepibiosis on Pagurus bernhardus (Crustacea: Decapoda) from the west Coast of Scotland. J Mar Biol Assoc United Kingdom 93:1351–1362. https://doi.org/10.1017/S0025315412001610
Fletcher RL, Callow ME (1992) The settlement, attachment and establishment of marine algal spores. Br Phycol J 27:303–329
Fontenla J (2018) Entre Diversidades Ecológicas. Poeyana 507:23–39
Fredericq S, Oh T, Earle S et al (2009) Seaweeds of the Gulf of Mexico. In: Felder D, Camp D (eds) Gulf of Mexico Origin, Waters, and Biota: Biodiversity. Texas A&M University Press, pp 187–260
Garcia L, Hemández G, Bolaños J (1998) Anomura y Brachyura de isla de Aves. Saber 10:5–10
Greco G, Faimali M, Davenport J (2014) Factors influencing the deterioration of the carapace surface during the moult cycle of Carcinus maenas (Linnaeus, 1758). Contrib to Zool 83:167–175
Guiry MD, Guiry GM (2020) AlgaBase. In: World-wide Electron. Publ. Natl. Univ. Ireland, Galway. http://www.algaebase.org/. Accessed 2 Jan 2020
Guzmán H (1979) Comportamiento decorador con algas en el cangrejo Microphyrys bicornutus Latreille (Majidae; Decapoda). Rev Biol Trop 27:321–327
Harder T (2008) Marine epibiosis: concepts, ecological consequences and host defence. In: Flemming H, Sriyutha P, Venkatesan R, Cooksey K (eds) Marine and industrial biofouling. Springer, Berlin, pp 219–231
Hinsch G (1972) Some factors controlling reproduction in the spider crab, Libinia emarginata. Biol Bull 143:358–366. https://doi.org/10.2307/1540059
Hultgren KM, Stachowicz JJ (2009) Evolution of decoration in majoid crabs: a comparative phylogenetic analysis of the role of body size and alternative defensive strategies. Am Nat 173:566–578. https://doi.org/10.1086/597797
Hultgren KM, Stachowicz JJ (2011) Camouflage in decorator crabs. In: Stevens M, Merilaita S (eds) Animal camouflage: mechanisms and function. Cambridge University Press, Cambridge, pp 212–236
John DM, Lawson GW, Ameka GK (2003) The marine macroalgae of the tropical West Africa sub-region. Nova Hedwigia, Beih 125:1–217
Jost L (2006) Entropy and diversity. Oikos 113:363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Jover A, Llorente G, Viña N (2009) Variación espacio-temporal de la composición de macroalgas del mesolitoral rocoso del sector Aguadores, plataforma suroriental, Cuba. Rev Investig Mar 30:3–9
Jover A, Reyes de Armas L, Gómez L, Suárez AM (2012) Variación espacial y temporal de las macroalgas del mesolitoral rocoso en Aguadores-Baconao, Cuba I: composición. Rev Investig Mar 32:38–49
Jover A, Ramos A, Cabrera A et al (2020) Epiphytic macroalgae and hosts of the marine shelf of Cuba: Current status, composition and diversity. Reg Stud Mar Sci 34:101108. https://doi.org/10.1016/j.rsma.2020.101108
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
León-Alvarez D, Núñez M (2011) Géneros de algas marinas tropicales de México II: algas pardas. Ed. Las prensas de Ciencias, Facultad de Ciencias, UNAM, México
León-Alvarez D, Candelaria C, Hernández P, León H (2007) Géneros de algas marinas tropicales de México I. Algas verdes. Ed. Las prensas de Ciencias, Facultad de Ciencias, UNAM, México
León Álvarez D, López Gómez NA, Ponce Márquez ME et al (2019) Géneros de algas marinas tropicales de México. Algas rojas. Ed. Las prensas de Ciencias, Facultad de Ciencias, UNAM, México
Levenets IR, Ovsyannikova II, Lebedev EB (2010) Epibiotic macroalgae on the scallop Mizuhopecten yessoensis in Peter the Great Bay, Sea of Japan. Russ J Mar Biol 36:340–349
Li D (2020) Diversity Through Hill Numbers. In: https://github.com/daijiang/hillR. Accessed 22 Feb 2020
Littler DS, Littler MM (2000) Caribbean reef plants. Offshore Graphics. Inc., Washington, DC
Littler DS, Littler MM, Hanisak MD (2008) Submersed plants of the Indian River Lagoon : a floristic inventory and field guide. OffShore Graphics Inc, Washington, DC
Loza A (2011) Ecosistemas errantes: Epibiontes como indicadores biogeográficos de tortugas marinas de Canarias. Universidad de las Palmas, Gran Canaria
Machado GBO, Sanches FHC, Fortuna MD, Costa TM (2013) Epibiosis in decapod crustaceans by stalked barnacle Octolasmis lowei (Cirripedia: Poecilasmatidae). Zoologia 30:307–311. https://doi.org/10.1590/S1984-46702013000300007
Magurran AE, Henderson PA (2011) Commonness and rarity. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. xford University Press, Oxford, pp 97–104
Martinelli M, Betti F, Di Camillo C et al (2011) Patterns of epibiont colonisation on the spider crab Inachus communissimus (Decapoda, Inachidae) from the Northern Adriatic Sea (Mediterranean Sea). Ital J Zool 78:517–523. https://doi.org/10.1080/11250003.2011.560905
Mateo-Cid L, Sánchez-Rodríguez I, Rodríguez-Montecinos E (2014) Algas epífitas de Sargassum sinicola Setchell y Gardner (Fucales, Phaeophyceae), en las islas Magdalena y Margarita en Baja California Sur, México. Rev Investig Mar 34:31–44
McLay CL (2020) Camouflage by the masking crab, Notomithrax ursus (Herbst, 1788) (Decapoda: Brachyura: Majidae): is it a decorator or a dressmaker? J Crustac Biol 40:673–683. https://doi.org/10.1093/jcbiol/ruaa076
Moreno CE, Barragán F, Pineda E, Pavón NP (2011) Reanálisis de la diversidad alfa: alternativas para interpretar y comparar información sobre comunidades ecológicas. Rev Mex Biodivers 82:1249–1261
Moreno CE, Calderón-Patrón JM, Martín-Regalado N et al (2018) Measuring species diversity in the tropics: a review of methodological approaches and framework for future studies. Biotropica 50:929–941. https://doi.org/10.1111/btp.12607
Nava-Olvera R, Mateo-Cid LE, Mendoza-González ÁC, García-López DY (2017) Macroalgas, microalgas y cianobacterias epífitas del pasto marino Thalassia testudinum (Tracheophyta: Alismatales) en Veracruz y Quintana Roo, Atlántico mexicano. Rev Biol Mar y Oceanogr 52:429–439
Ortega MM, Godínez JL, Gorduño G (2001) Catálogo de algas bénticas de las costas mexicanas del Golfo de México y Mar Caribe. Universidad Nacional Autónoma de México
Parapar J, Fernández L, González-Gurriarán E, Muíño R (1997) Epibiosis and masking material in the spider crab Maja squinado (Decapoda: Majidae) in the Ría de Arousa (Galicia, NW Spain). Cah Biol Mar 38:221–234
Phang S-M, Yeong H-Y, Ganzon-Fortes ET et al (2016) Marine algae of the South China Sea bordered by Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. Raffles Bull Zool 34:13–59
Quiroz-González N, Aguilar-Estrada LG, Ruiz-Boijseauneau I, Rodriguez D (2020) Biodiversidad de algas epizoicas en el Pacifico tropical mexicano. Acta botánica Mex 127:e1645. https://doi.org/10.21829/abm127.2020.1645
Reyes de Armas LM (2016) Macroalgas epizoicas en Lobatus gigas en el Parque Nacional Jardines de la Reina, Camaguey. Cuba Hombre, Cienc y Tecnol 20:67–74
Román-Valencia C, Lehmann P, Rubio E (2017) Distribución y constancia de los peces del Río San Miguel y el zanjón Bagazal en el Arto río Cauca. Colombia Actual Biológicas 21(71):163–172
Ros RM, Suárez AM (1980) Epibiosis en el cangrejo moro Menippe mercenaria (Say, 1818). Rev Investig Mar 1:5–17
Santelices B (1977) Ecología de las algas marinas bentónicas. Universidad Católica de Chile, Santiago de Chile
Schroeder PJ, Jenkins DG (2018) How robust are popular beta diversity indices to sampling error. Ecosphere 9(2):e02100. https://doi.org/10.1002/ecs2.2100
Sentíes A, Espinoza-Avalos J, Zurita JC (1999) Epizoic algae of nesting sea turtles Caretta caretta (L.) and Chelonia mydas (L.) from the Mexican Caribbean. Bull Mar Sci 64:185–188
Serio D, Catra M, Collodoro D, Nisi A (2011) Ceramium cormacii sp. nov.(Ceramiaceae, Rhodophyta), a new Mediterranean species epizoic on loggerhead sea turtles (Caretta caretta). Bot Mar 54:545–550
Soares LP, Fujii MT (2012) Epiphytic macroalgae from Boa Viagem Beach, Recife, Pernambuco state, Brazil. Check List 8:662–665. https://doi.org/10.15560/8.4.662
Sokal R, Rohlf F (2012) Biometry: the principles and practice of statistics in biological research, 4th edn. W. H. Freeman and Co., New York
Steneck RS, Dethier MN (1994) A functional group approach to the structure of algal-dominated communities. Oikos 69:476–498. https://doi.org/10.2307/3545860
Stevens M, Ruxton GD (2019) The key role of behaviour in animal camouflage. Biol Rev 94:116–134
Suárez AM, Martínez-Daranas B (2020) Similitud de la ficoflora marina en zonas del Atlántico Occidental Tropical y Subtropical. Caldasia; 42(1): 85–95. https://doi.org/10.15446/caldasia.v42n1.73372
Suárez AM, Martínez-Daranas B, Alfonso Y (2015) Macralgas marinas de Cuba. Editorial UH, La Habana, Cuba
Széchy MTM, de Sá ADF, Menezes M, Faria A (2008) Variação sazonal do epifitismo por macroalgas em uma população de Sargassum vulgare C. Agardh (Phaeophyceae, Fucales) da baía da Ilha Grande, Rio de Janeiro. Oecologia Bras 12:299.314. https://doi.org/10.4257/oeco.2008.1202.11
Victorero L, Robert K, Robinson LF et al (2018) Species replacement dominates megabenthos beta diversity in a remote seamount setting. Sci Rep 8:4152. https://doi.org/10.1038/s41598-018-22296-8
Villegas MJ, Stotz W, Laudien J (2006) First record of an epibiosis between the sand crab Emerita analoga (Stimpson, 1857) (Decapoda: Hippidae) and the mussel Semimytilus algosus (Gould, 1850) (Bivalvia, Mytilidae) in southern Peru. Helgol Mar Res 60:25–31. https://doi.org/10.1007/s10152-005-0012-5
Wahl M (2008) Ecological lever and interface ecology: epibiosis modulates the interactions between host and environment. Biofouling 24:427–438
Wahl M (2015) Epibiosis : ecology , effects and defences. In: Marine hard bottom communities. pp 61–72
Wahl M, Hay ME, Enderlein P (1997) Effects of epibiosis on consumer-prey interactions. Hydrobiologia 355:49–59
WoRMS (2020) World Register of Marine Species. http://www.marinespecies.org. Accessed 21 Aug 2020
Wynne MJ (1986) A checklist of benthic marine algae of the tropical and subtropical Western Atlantic: fourth revision. Nov Hedwigia, Beihefte 145:2239–2281. https://doi.org/10.1139/b86-298
Acknowledgements
We thank the Student Scientific Group of Marine Ecology EcoMar "Dra. María Elena Ibarra Martín" of the Universidad de Oriente for the facilities provided in the field work, and the reviewers for their comments and suggestions that improved the manuscript. Thanks to Suzanne Fredericq for language reviewing and correcting.
Author information
Authors and Affiliations
Contributions
Asiel Cabrera: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data Curation, Writing – Original Draft and Writing – Review & Editing. Abdiel Jover: Methodology, Software, Formal analysis, Investigation, Data Curation, Writing – Review & Editing, Resources, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerrero, A.C., Capote, A.J. Composition and diversity of epizoic macroalgae growing on crustaceans on the southeastern coast of Cuba. Biologia 77, 1–10 (2022). https://doi.org/10.1007/s11756-021-00916-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00916-5