Abstract
This investigation was carried out during 2012–2013 in order to bring out the forest vegetation of the Finike Forest Planning Unit. The study area is located in the Southwestern part of Turkey within the Mediterranean Basin. Field sampling was undertaken using the old Braun-Blanquet method, and 77 sampled relevés were sampled from the study area. The database consists of 214 vascular plant taxa, dominated by the Mediterranean phytogeographical region and Hemicryptophytes. The sampled relevés were classified using the Modified TWINSPAN, and distribution patterns of the plant communities were analysed using non-metric multidimensional scaling with the integration of the R-Project and JUICE program. Topographic factors were assessed for the interpretation of the differentiation among communities. Five associations, four (Lino corymbuloso-Genistetum acanthocladae, Rhamno nitidae-Quercetum cocciferae, Asparago acutifoli-Pinetum brutia, and Lamio striati-Cedretum libani) of which are new, were defined as belonging to the Mediterranean bioclimatic strata within five classes. The distribution of these five plant associations was highly affected by altitude.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest areas in the Mediterranean basin are estimated to be 73 million ha (Palahi et al. 2008). Mediterranean forests are highly diverse in terms of plant composition. Although these forests are generally characterized by evergreen Eu-Mediterranean vegetations such as sclerophyllous maquis, phrygana, garique, and pine forests (Pignatti 2003), there are also semi-humid and humid forests such as cold-tolerant deciduous oak and coniferous forests at high altitudes (Akman et al. 1978). Forest ecosystems have a complex structure and exhibit a constantly changing dynamic formation. As in all ecosystems that exhibit a dynamic structure, Mediterranean forest ecosystems are intertwined with many factors of natural or anthropogenic origin (Quézel and Barbero 1982). Forest ecosystems have always played an important role in the Mediterranean culture from past to present. Mediterranean forest ecosystems provide a wide range of important benefits and services to local people, contributing to their welfare (Gauquelin et al. 2018; Peris-Llopis et al. 2020). However, excessive and unplanned use of these opportunities has caused forest ecosystems to constantly decline and become fragile (Scarascia-Mugnozza et al. 2000). With the increasing pressure of human activities and climate change, Mediterranean forest ecosystems have faced with important natural and anthropogenic disturbances, such as forest fires (Peris-Llopis et al. 2020), over-grazing (Papanastasis et al. 2002), drought stress (Pumo et al. 2010), over-exploitation (Allen 2003), tourism activities (Pinna et al. 2019), urbanization (Rick et al. 2020), industrialization (Barbero et al. 1990), and deforestation (Karavani et al. 2018).
Turkey, located in the Mediterranean Basin, attracts attention with its natural plant resources (Terzioğlu et al. 2012) and is characterized by complex climatic and human factors of vegetation changes (Bakker et al. 2013). Due to its topography, microclimate diversity, and location at the junction of three different phytogeographical (Mediterranean, Euro-Siberian, and Irano-Turanian) regions, Turkey is one of the most important centers in the world in terms of plant biodiversity that hosts a great number of habitats (Terzioğlu et al. 2012). The presence of a wide variety of habitats makes Turkey a country rich in plant biodiversity. This also contributes to the high rate of endemism. Therefore, of the 374,000 (Christenhusz and Byng 2016) plant taxa registered in the world, 12,975 natural and cultivated plant taxa are distributed in Turkey, and the number of endemic plants has reached 4157 (with an endemism rate of 32%) (Güner et al. 2012; Özhatay et al. 2013, 2015, 2017, 2019). Forest ecosystems, which contain the majority of this plant richness, are very important for the continuity of biological diversity. In Turkey, the establishment of forests started 12,000 years ago and reached potential distribution 4000 years ago (Aytuğ and Görecelioğlu 1993). With the beginning of agriculture and animal husbandry in the Early Holocene period, negative human impact began to be felt in the forest areas in Anatolia (Van Zeist and Bottema 1988). With this human influence, the forest areas that covered 70% of Anatolia in the past have dropped to around 30% today (Aytuğ and Görecelioğlu 1993). According to forest inventory studies, Turkey’s forests cover an area of 22.6 million ha (OGM 2015). These forests are distributed to three phytogeographical regions and comprise coniferous (48%), broad-leaved (33%), and mixed coniferous-broadleaved (19%) forests (Terzioğlu et al. 2012).
Forest communities in Turkey’s Mediterranean-Type Ecosystem is creating a compelling environment for many researchers. Schwarz (1936), Czeczott (1939), Walter (1956), Zohary (1973), and Çetik (1976) carried out the first phytosociological studies related to Mediterranean ecosystems in Turkey. After that, comprehensive studies were undertaken by Kavgacı et al. (2021), Bonari et al. (2021), Uğurlu et al. (2012), Kavgacı et al. (2010), Akman et al. (1978), Akman et al. (1979b), and Akman et al. (1979a). These studies evaluate Mediterranean forest ecosystems of Turkey under numerous higher syntaxonomical alliances and orders within the classes of Quercetea ilicis, Quercetea pubescentis, Carpino-Fagetea sylvaticae (Syn. Querco-Fagetea), and Pinetea halepensis. Indeed, these phytosociological studies are expected to continue to be carried out with developing technologies and changing environmental conditions.
This research aims at the determination and evaluation of plant biological diversity in the Finike Forest Planning Unit (FPU) (Karaköse and Terzioğlu 2020). With this study, it was aimed to contribute to the forest vegetation of the Mediterranean Basin and Turkey’s Forest Vegetation Database.
Materials and methods
Study area
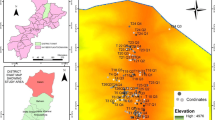
The Finike FPU is geomorphologically mountainous and steep and varies from the sea level to 2318 m a.s.l. (Fig. 1). The Finike FPU is mainly located in the Mediterranean (west side of the Antalya province) phytogeographic region in terms of phytogeographical regions (Davis 1965–1985). However, at higher altitudes, the Irano-Turanian phytogeographic region’s influence is felt. The area consists of a total area of 30,439.8 ha, 20,894.8 ha of which is forested and 9545 ha is non-forested. The climatic data for Finike region are based on records made between 1975 and 2005 at Finike (at 3 m altitude) and Elmalı (at 1095 m a.s.l.) meteorological stations (Karaköse and Terzioğlu 2020). The study area has a typical Mediterranean climate. This climate type is characterised by hot and dry summers and cold and rainy winters (Quézel and Barbero 1982; Pignatti 2003; Atalay et al. 2014). The annual average temperature and the average annual precipitation are 18.8 °C and 941.6 mm at the Finike station, and 12.8 °C and 475.7 mm at the Elmalı station. The records of the local meteorology stations showed that the rain regime was winter-spring-autumn-summer for both Finike and Elmalı. According to Emberger’s classification, Finike is rainy and sub-cool, and Elmalı semi-arid and upper-cold (Akman 2011).
The study area consists of Beydağları formation, Susuzdağ formation, Kasaba formation, slope debris, and alluvium-deposit cone (İslamoğlu and Taner 2002). Around Finike, bedrocks belonging to the Beydağları autochthonous hold an important place. These are mainly composed of bedrocks representing the Cenozoic and Mesozoic periods. Beydağları formation, which consists of the upper Cretaceous platform-type limestones (in some places it contains dolomite and dolomitic limestone) belonging to the Beydağları autochthonous, unconformably includes the Susuzdağ formation consisting of upper Lutetian-Priabonian nummulitic limestones and the unit ends with the Kasaba Formation consisting of Langhian aged conglomerate, sandstone, siltstone and claystone (İslamoğlu and Taner 2002).
Data collection and analysis
In order to identify the plant communties found in Finike FPU, 77 sampled relevés were gathered between 2012 and 2013. The plant sociology study was undertaken using old Braun-Blanquet (1932) approach. Relevé areas were 400 m2 for forested areas and 200 m2 for garique and riparian forests (Chytrý and Otýpková 2003). Flora of Turkey (Davis 1965–1985; Davis et al. 1988; Güner et al. 2000), Turkey Plant List (Vascular Plants) (Güner et al. 2012), and World Flora Online (WFO 2021) were used for the identification, nomenclature, and chorological types of the plant taxa. The life forms of plant taxa were determined according to Raunkiaer (1934)’s system. Gathered relevés were entered into TURBOVEG (version 2.12) program (Hennekens and Schaminée 2001). After that, the created dataset was exported to the JUICE (version 7.1) program (Tichý 2002) for dataset managing. For the hierarchical classification, the Modified TWINSPAN (Roleček et al. 2009), integrated into the JUICE program, was used. The diagnostic taxa for plant communities were determined using the presence/absence data (Chytrý et al. 2002). The lower threshold values for the diagnostic, constant, and dominant species were set to 30, 30, and 30, respectively.
The resulting classification was projected onto an ordination diagram using non-metric multidimensional scaling (NMDS) performed on a matrix of Simpson dissimilarities. Ordination was calculated using the R (version 3.6.1) program (R Development Core Team 2019) and its vegan (version 2.5–6) package (Oksanen et al. 2019). Syntaxonomical concepts and nomenclature of higher syntax were followed from Akman (1995), Mucina et al. (2016), Bonari et al. (2021), and Kavgacı et al. (2021). Descriptions of new syntax followed the rules of the International Code of Phytosociological Nomenclature (ICPN) (Theurillat et al. 2021).
Results
Classification and vegetation units
In this study, 77 sampled relevés were taken from the study area. Six sampled relevés were removed from the data set because they were outside (from the dune vegetation) the forest vegetation. Thus, the data set contains 71 sampled relevés and 214 plant species. Classification results are given in the Fig. 2. We calculated the diagnostic (in gray), constant, and dominant taxa for each cluster and presented them in the Online Supplement.
Cluster 1: Lamium garganicum subsp. striatum-Cedrus libani community.
Diagnostic species: Acer hyrcanum subsp. sphaerocaryum, Amelanchier parviflora var. parviflora, Anthemis rosea subsp. carnea, Arabis alpina subsp. brevifolia, Aubrieta deltoidea, Briza humilis, Cedrus libani, Conium maculatum, Dactylis glomerata subsp. hispanica, Doronicum orientale, Epilobium lanceolatum, Euphorbia falcata, Galium incanum subsp. elatius, Juniperus excelsa subsp. excelsa, Juniperus foetidissima, Juniperus oxycedrus subsp. oxycedrus, Lamium garganicum subsp. striatum var. striatum, Leontodon asperrimus, Lonicera nummulariifolia subsp. glandulifera, Medicago minima var. minima, Milium vernale subsp. vernale, Ostrya carpinifolia, Paeonia kesrouanensis, Poa alpina, Prunus x domestica, Rosa canina, Salvia tomentosa, Saponaria kotschyi, Satureja cuneifolia, Scaligeria napiformis, Scutellaria brevibracteata subsp. brevibracteata, Silene italica, Sorbus torminalis var. torminalis, Verbascum salviifolium, Vicia cracca subsp. atroviolacea.
Constant species: Cynosurus echinatus, Hyparrhenia hirta, Quercus coccifera, Stipa bromoides, Styrax officinalis, Vulpia ciliate.
Dominant species: Cedrus libani.
Cluster 2: Linum corymbulosum-Genista acanthoclada community.
Diagnostic species: Anthemis cretica subsp. cassia, Arum dioscoridis var. dioscoridis, Bupleurum gracile, Carduus argentatus, Centaurium pulchellum, Ceratonia siliqua, Crepis foetida subsp. foetida, Crucianella latifolia, Filago pyramidata, Galium murale, Genista acanthoclada, Lagoecia cuminoides, L. pentagonia, Linum corymbulosum, Olea europaea var. europaea, Phagnalon graecum, Phleum subulatum subsp. subulatum, Pistacia lentiscus, Polypogon maritimus subsp. maritimus, Quercus aucheri, Salvia viridis, Sarcopoterium spinosum, Torilis arvensis subsp. arvensis.
Constant species: Avena wiestii, Cynosurus echinatus, Daphne gnidioides, Hyparrhenia hirta, Micromeria myrtifolia, Phillyrea latifolia, Ptilostemon afer subsp. eburneus, Quercus coccifera, Sideritis montana subsp. curvidens, Smilax aspera, Teucrium chamaedrys subsp. chamaedrys.
Dominant species: Genista acanthoclada.
Cluster 3: Nerium oleander-Plantanus orientalis community.
Diagnostic species: Dioscorea communis, Dracunculus vulgaris, Ficus carica subsp. carica, Mentha longifolia subsp. typhoides, Nerium oleander, Papaver rhoeas, Platanus orientalis, Rubus sanctus, Rumex pulcher, Veronica anagallis-aquatica, Vitex agnus-castus.
Constant species: Catapodium rigidum, Clinopodium nepeta subsp. nepeta, Crucianella latifolia, Dryopteris pallida, Galium murale, Hedera helix, Helichrysum pamphylicum, Hordeum murinum subsp. murinum, Laurus nobilis, Onosma frutescens, Origanum onites, Oryzopsis miliacea subsp. thomasii, Phleum subulatum subsp. subulatum, Phlomis grandiflora var. grandiflora, Pistacia palaestina, Smilax aspera, Stachys annua subsp. annua var. annua, Styrax officinalis, Torilis arvensis subsp. arvensis, Urtica dioica.
Dominant species: Platanus orientalis.
Cluster 4: Rhamnus nitida-Quercus coccifera community.
Diagnostic species: Arbutus andrachne, Avena wiestii, Capparis sicula subsp. sicula, Ephedra foeminea, Euphorbia rigida, Genista acanthoclada, Hyparrhenia hirta, Melica minuta, Micromeria myrtifolia, Phillyrea latifolia, Pistacia palaestina, Quercus aucheri, Quercus coccifera, Rhamnus nitida.
Constant species: Catapodium rigidum, Ceratonia siliqua, Cistus creticus, Cynosurus echinatus, Daphne gnidioides, Lagoecia cuminoides, Olea europaea var. europaea, Origanum onites, Oryzopsis miliacea subsp. thomasii, Phlomis grandiflora var. grandiflora, Picnomon acarna, Smilax aspera, Stipa bromoides, Teucrium chamaedrys subsp. chamaedrys.
Dominant species: Quercus coccifera.
Cluster 5: Asparagus acutifolius-Pinus brutia community.
Diagnostic species: Allium flavum subsp. tauricum var. tauricum, Asparagus acutifolius, Asperula brevifolia, Cistus creticus, Cotinus coggygria, Crataegus monogyna subsp. monogyna, Crepis reuterana subsp. reuterana, Eryngium falcatum, Ferulago galbanifera, Jasminum fruticans, Lathyrus aphaca, Ononis pusilla, Phlomis grandiflora var. grandiflora, Pinus brutia, Ptilostemon afer subsp. eburneus, Quercus coccifera, Ruscus aculeatus, Styrax officinalis.
Constant species: Avena wiestii, Cynosurus echinatus, Dactylis glomerata subsp. hispanica, Daphne gnidioides, Euphorbia rigida, Micromeria myrtifolia, Milium vernale subsp. vernale, Phillyrea latifolia, Pistacia palaestina, Smilax aspera, Stipa bromoides, Teucrium chamaedrys subsp. chamaedrys, Torilis arvensis subsp. arvensis.
Dominant species: Pinus brutia.
Chorology, life form and ordination of the units
The plant communities identified within the scope of the study were evaluated according to altitude levels, not according to JUICE analysis, in order to avoid confusion due to the Mediterranean bioclimatic strata. The elevation of the Finike FPU ranges between 0 and 2328 m a.s.l. The planning unit is bordered by the Mediterranean Sea in the south and remains totally within the Mediterranean phytogeographical region (Eastern Mediterranean). However, while the effect of the Irano-Turanian region is felt after 1500 m a.s.l., the influence of Euro-Siberian is found in moist and shady areas. When all plant communities are examined, the dominance of taxa belonging to the Mediterranean region stands out (Mediterranean, 50 taxa; East Mediterranean, 44 taxa; East Mediterranean [Mountain], one taxon, and Omni-Mediterranean, one taxon). This phytogeographical region is followed by Euro-Siberian with 13 taxa and the Irano-Turanian phytogeographic regions with 10 taxa. The phytogeographic region of 95 taxa in the region is not exactly known. This situation clearly shows itself when we examine the plant collections. In fact, taxa belonging to the Mediterranean phytogeographical region are represented at the highest level in the Genista acanthacloda community, which is at the lowest altitude level in the study area. This representation decreases linearly depending on the altitude level (Table 1). Likewise, with the increase in altitude, Irano-Turanian phytogeographic elements are included in plant communities.
Table 1 The dispersion of taxa according to the Raunkiaer’s life forms.
When plant communities are examined as a whole (214 plant taxa) according to their life forms, hemicryptophytes are represented with 84 taxa, phanerophytes with 50 taxa, therophytes with 46 taxa, chamaephytes with 26 taxa, and cryptophytes with eight taxa. Therophytes stand out as dominant only in the G. acanthoclada community (Table 2). Similarly, because it is a chamaephytic plant community, the excession of the chamaephyte taxa is also seen in this G. acanthoclada community. In plant communities where the influence of the Mediterranean climate decreases with altitude, it shows itself with the increase of plant taxa in the hemicrytophyte character. Looking at Table 2, the representation of the species in the hemicryptophyte character has reached the highest level in the Cedrus libani community. As expected in the therophytes, it reaches the lowest level in the C. libani community. Phanerophytes are among the most important elements of the Mediterranean phytogeographical region and make themselves felt in almost every woody plant community. Thus, the phanerophytes reach the highest representation rate in the Quercus coccifera community, which is maquis vegetation.
Table 2 Chorological dispersion of the clusters in the Finike FPU.
The dendrogram (Fig. 2) divides the data set into two main clusters. The first on the left includes the first cluster and represents the vegetation of the C. libani community. The second cluster is further divided into four clusters including clusters 2, 3, 4, and 5 (G. acanthoclada, Platanus orientalis, Q. coccifera, and Pinus brutia communities). Topographic factors were useful in the interpretation of the two first gradients of NMDS (Fig. 3). The NMDS of the five clusters with a passive projection of topographic factors shows an evident gradient along axes 1 with community changes among the clusters. The NMDS diagram (Fig. 3) shows that the five clusters have minimum overlap (stress 0.13). Looking at the Fig. 3, it is seen that the changes of sampled relevés differs mainly the first axis, and the topographic factors have a view in the same way. Altitude and inclination are the topographical factors mostly affecting the vegetation composition and spatial distribution of the Finike forest vegetation (Fig. 3, Fig. 4). Of the topographical factors, altitude is correlated to the first axis. While inclination and aspect are correlated to the second axis.
On the left side of the NMDS diagram, vegetation types of xerophytic (except P. orientalis community) communities and on the right side, humid communities are grouped. C. libani community spreads at higher altitudes in the study area. This situation also shows itself in the NMDS diagram. G. acanthoclada and Q. coccifera communities spread in lower altitude belts in the study area. Temperature and light intensity stand out as the most important factors in the distribution of these sclerophyllous plant communities. P. orientalis community is a riparian community forming gallery-type communities on the edges of temporary running waters in the study area. This community consists of moisture-loving and shade-tolerating plants in relatively low inclination areas. P. brutia community, like P. orientalis community, distributes at the low inclination areas in the study area. P. brutia communities naturally spread with or upper altitudes of the Q. coccifera community, and lower altitudes of the C. libani community in the study area. Therefore, the P. brutia community interacts with both plant communities (Fig. 3). The temperature and light demand of P. brutia community is lower than that of the Q. coccifera and G. acanthoclada communities.
Discussion
This study was undertaken in the Mediterranean bioclimate strata. In Finike FPU, the Thermo-Mediterranean, Eu-Mediterranean, Supra-Mediterranean, and Mediterranean montane belts are located between 0 and 2000 m a.s.l. (Akman and Ketenoǧlu 1986). Within the scope of the study, only the high mountain Mediterranean (anthropogenic steppe) vegetation belt was excluded due to the fact that it was a non-forested area. The change and interrelationship of a plant community along a certain environmental factor is one of the main topics on vegetation ecology (Pignatti et al. 1996). Generally, altitudinal and climatic gradients highly affect species structure and spatial distribution in the Mediterranean Basin (Ozenda 1975; Akman et al. 1978; Quézel and Barbero 1982; Fontaine et al. 2007). Actually, in the Finike FPU, as determined in the NMDS study, the change in climatic conditions due to altitude has enabled the formation of different vegetation belts. This alteration in vegetation types has also been observed in previous researches (Akman et al. 1978; Eminağaoğlu et al. 2007; Kavgacı et al. 2010; Aksoy and Çoban 2017; Karaköse 2019; Kavgacı et al. 2021). Five plant communities belonging to garique, maquis, riparian, and forest vegetation were identified in these vegetation belts. Life forms reflect the adaptation of plant species to the climate prevailing in any habitat, and this allows them to be micro or macro-climate indicators (Raunkiaer 1934; Cain 1950). Phanerophytes are concentrated in temperate regions, hemicryptophytes in temperate and colder regions, and therophytes in arid climatic regions dominated by intense human pressure, over-grazing, and fire regime, as in the Mediterranean Basin (Pignatti 2003). When the life forms spectra of the plant communities were identified in the study area, this situation clearly emerged. The density of theropytes and hemicryptophytes proves the adaptation of the plant communities to the habitats (Dimopoulos and Georgiadis 1992; Christodoulakis 1996). In fact, therophytes have reflected their commitment to the Mediterranean climate mostly by being intensely in the G. acanthoclada community. Hemicryptophytes, on the other hand, were densely distributed in the C. libani community, located in a transitional zone between the South Anatolian Mediterranean climate and the Central Anatolian steppe climate and adapting to a cold-continental climate (Čarni et al. 2014; Messinger et al. 2015). Phanerophytes are abundant in the sclerophyllous Q. coccifera community. This situation caused a decrease in herbaceous plant taxa due to the canopy formed by the phanerophytes. In the C. libani community, however, the reverse of this formation was experienced. The detection of phytogeographic regions is another indicator of the spatial-temporal distribution of plant taxa and their adaptation to environmental factors. In the study area, the distribution of the plant taxa belonging to the phytogeographical regions also changes with the altitude. The diversity of the plant taxa belonging to the Mediterranean phytogeographical region decreases with altitude. This decline fills in shady and moist areas with Euro-Siberian elements and Irano-Turanian elements towards the anthropogenic tree line. This situation explains the changes in life forms depending on environmental variables in the field (Čarni et al. 2018).
Mediterranean forest ecosystems are known for highly degraded and fragile vegetation structures (Tsiourlis et al. 2007). Maquis and garique, which are among the characteristic vegetation structures of the Mediterranean phytogeographical region, are the secondary and tertiary the vegetation types formed after the degradation of P. brutia forest (Şık and Gemici 1994; Atalay et al. 2014; Kavgacı et al. 2017). An endemic garique community was identified in the Thermo-Mediterranean vegetation belt. Garique occurs because of the destruction of the maquis vegetation (Atalay et al. 2014). This garique community is a xerophytic-heliophilic plant community and composed of G. acanthoclada and endemic Linum corymbulosum taxa. The Thermo-Mediterranean belt, where the community is described, is an area with very intense human activity. In the Mediterranean region, anthropogenic effects such as tourism, fire, agriculture, animal husbandry, and urbanization are quite evident in this vegetation layer (Tsiourlis et al., 2007). For that reason, classification of this vegetation type sometimes becomes difficult as the human factor constantly destroys this vegetation belt. Zohary and Orshan (1959) and Akman (1995) define this vegetation layer as xerophilic Pistacia lentiscus-Olea europaea-Ceratonia siliqua communities. In the study area, anthropogenic effects destroyed these xerophilic communities, and this allowed the garique community to take a dominant position in the field. In previous studies in Turkey, three associations related to the G. acanthoclada (Alysso carici-Genistetum acanthocladae, Genisto acanthocladae-Daphnetum gnidioides, and Genisto acanthocladae-Serratuletum cerinthifoliae) have been identified (Barbero and Quézel 1989; Vural et al. 1995; Kavgacı et al. 2021). In two of these studies, G. acanthoclada is included in the tables as a co-dominant species. Alysso carici-Genistetum acanthocladae association was identified within alliance Cistion orientale Oberd. 1954 as a garique association from Muğla-Köyceğiz (Vural et al. 1995). While creating the syntaxonomy of the G. acanthoclada, some confusion has arisen from the past to the present. Namely, in these studies, they evaluated G. acanthoclada in the order of Cisto-Micromerietalia julianae Oberd. 1954 and the class of Cisto-Micromerietea julianae Oberd. 1954 (Barbero and Quézel 1989; Vural et al. 1995). Likewise, G. acanthoclada, in some studies in the East Mediterranean (Asensi et al. 2007; Tsiourlis et al. 2007), was evaluated under the same order and class. In addition to this, Mucina et al. (2016), Cisto-Micromerietea julianae has become the synonym of Ononido-Rosmarinetea Br.-Bl. in A. Bolòs y Vayreda 1950. However, G. acanthoclada was accepted within the order of the Quercetalia ilicis Br.-Bl. ex Molinier 1934 and the class of Quercetea ilicis Br.-Bl. ex A. Bolòs et O. de Bolòs in A. Bolòs y Vayreda 1950 by Barbero et al. (1976) and Tsiourlis et al. (2009). In related studies, the alliances of Gonocytiso pterocladi-Pinion brutiae (Barbero et al. 1976), Quercion ilicis Br.-Bl. ex Molinier 1934 and Oleo-Ceratonion siliquae Br.-Bl. Ex Guinochet et Drouineau 1944, Hyperico empetrifolii-Micromerion graecae Barbero et Quézel 1989, and Origano syriaci-Hypericion thymifolii Mucina et Theurillat in Mucina et al. 2016 (Syn.: Helichryso sanguinei-Origanion syriaci Barbero et Quézel 1989) were preferred for this taxon. According to result of this study, we decided that this garique community in the Thermo-Mediterranean belt should be treated as a new association as Lino corymbuloso-Genistetum acanthocladae ass. nova (type relevé 1 in Online Supplement). When evaluated as a whole, the association of G. acanthoclada (based on the representation values of the taxa belonging to the alliance of Origano syriaci-Hypericion thymifolii Mucina et Theurillat in Mucina et al. 2016) was evaluated under the order of Hyperico empetrifolii-Genistetalia acanthocladae Mucina in Mucina et al. 2016 and the Ononido-Rosmarinetea class. Comprehensive study by Kavgacı et al. (2021) was also confirmed this situation.
The maquis community defined in the Finike FPU is an endemic plant community consisting of Quercus coccifera (Syn.: Quercus calliprinos Webb) (WFO 2021) and Rhamnus nitida taxa that spread between 200 and 800 m a.s.l. at Thermo- and Eu-Mediterranean vegetation belts. Q. coccifera (Kermes oak) is distributed across the Mediterranean (except Egypt) Basin (Tsiourlis et al. 2009; Jasprica et al. 2016). Q. coccifera is an evergreen sclerophyllous oak species spreading up to 1500 m a.s.l., dominated by the Mediterranean climate (Akman et al. 1978). In Turkey, Q. coccifera dominated scrub communities usually arise due to the destruction of P. brutia (Turkish pine) forests and form different associations with different taxa depending on climate, bedrock, soil, and topography (Şık and Gemici 1994; Tsiourlis et al. 2009; Atalay et al. 2014; Jasprica et al. 2016). Similar maquis communities formed by Q. coccifera have been described in previous studies (Akman et al. 1978; Serin and Eyce 1994; Kutbay et al. 1998; Karaer et al. 1999; Kavgacı et al. 2010; Özen 2010; Altay et al. 2012; Sağlam 2013). Looking at the previous studies, plant associations were mostly linked to the orders of Quercetalia ilicis and Quercetalia calliprini Zohary 1955 within the Quercetea ilicis class. In addition to these two orders, Pistacio lentisci-Rhamnetalia alaterni Rivas-Mart. 1975 has also gained representation in another study in Antalya province (Kavgacı et al. 2017). On the other hand, there are also plant associations that belong to Quercetea pubescentis Doing-Kraft ex Scamoni et Passarge 1959 class (Serin and Eyce 1994; Kavgacı et al. 2010; Sağlam 2013). According to the alliance level, while Quercion ilicis and Quercion calliprini Zohary 1955 were frequently used, alliances of Quercion frainetto Horvat 1954 (Altay et al. 2012) and Lonicero nummulariafoliae-Cedrion libani Quézel, Barbéro & Akman 1978 (Kavgacı et al. 2010) were also preferred. In the western Mediterranean, non-calcareous maquis is mostly dominated by Quercus ilex but in the Eastern Mediterranean (also including Turkey) by calcicole Quercus coccifera (Şık and Gemici 1994). For that reason, Quercion calliprini (East Mediterranean) is vicariant of Quercion ilicis (West Mediterranean) (Akman 1995). In a recent study by Kavgacı et al. (2021), the taxonomic mistake related to Quercus calliprinos has been corrected. The names of alliance and order, which were previously Quercion calliprini and Quercetalia calliprini, have been revised as Quercion cocciferae Zohary 1955 nom. Corr. (cf. Art. 44/3) and Quercetalia cocciferae Zohary 1955 nom. Corr. (cf. Art. 44/3) (Kavgacı et al. 2021). The order Quercetalia cocciferae is dominant at the Thermo−Supra Mediterranean vegetation belts in Palestine, Syria, Israel, Lebanon, and Turkey (Zohary and Orshan 1959; Akman 1995), and in parallel with this, characteristic taxa of Quercion cocciferae are better represented in the community (Online Supplement). Because of this, the maquis community determined within the scope of the study should be connected to Quercetalia cocciferae in the Quercetea ilicis class. In line with these data, it was found appropriate in this study to define a new association in these vegetation belts. We decided that the name of the association is Rhamno nitidae-Quercetum cocciferae ass. nova with type relevé 48 (Online Supplement).
Nerium oleander-Platanus orientalis community belonging to hygrophile vegetation type was detected in the Finike FPU. P. orientalis (Oriental plane) establishes gallery-type forests on narrow valley bases and along riverines in almost every region in Turkey (Yaltırık 1982). The Oriental plane, which is estimated to have a wider spread in the study area in the past, is currently spreading only along the Akçasu riverine due to water shortage and anthropogenic effects. This community is a community in which moisture-loving and shade-tolerating plants are formed in a low-inclination area within the Turkish pine forest. Studies with the Oriental plane are very limited in Turkey. The Salix alba-P. orientalis association (Kutbay and Kılınç 1995) has been identified in the Middle Black Sea, and there are a few studies in Antalya province and its surroundings in the Mediterranean region (Ayaşlıgil 1987; Çinbilgel and Gökçeoglu 2010). Nerio-Platanetum orientalis community was first identified in Albania (Kárpáti 1962). Because of the species in the association, the community identified in the study area was linked to the alliance of Platanion orientalis I. Kárpáti et V. Kárpáti 1961 and the order of Populetalia albae within Alno glutinosae-Populetea albae P. Fukarek et Fabijanić 1968 class.
P. brutia is widely distributed (about 5.6 million ha) and one of the most important forest tree species in Turkey (Terzioğlu et al. 2012). It generally has a vertical habitat width from the Thermo-Mediterranean to the Supra-Mediterranean vegetation belts in the Mediterranean and Aegean regions. In the Black Sea and Southern Marmara regions, it forms communities in the form of enclaves in the Meso-Mediterranean belt under the influence of the Mediterranean climate (Akman et al. 1978; Akman 1995). The community identified in the study area, on the other hand, spreads between the Thermo- and Supra-Mediterranean belts at an altitude of 200–1100 m a.s.l. on limestone and conglomerate bedrock on mainly the south and southeast facing aspects. There are numerous Turkish pine associations that have different co-dominant taxon described (Akman et al. 1978; Serin and Eyce 1994; Kutbay and Kılınç 1995; Özen and Kılınç 1995; Vural et al. 1995; Akman et al. 1998; Özel 1999; Varol and Tatlı 2001; Şanda 2006; Varol et al. 2006; Sağlam 2007; Kaya et al. 2010; Öner and Akbin 2010; Korkmaz et al. 2011; Dönmez and Şanda 2015; Kurt et al. 2015). However, some of these associations (Yurdakulol 1981; Ayaşlıgil 1987; Gemici 1988; Akman et al. 1998) were named and published invalidly according to ICPN rules (Theurillat et al. 2021), due to they did not indicate the nomenclatural type (Art.5a). In addition to these, 3 new sub-associations were defined by Kurt et al. (2015) depending on the Phlomido bourgaei-Pinetum brutiae association, described by Akman et al. (1998). As explained previously, the Phlomido bourgaei-Pinetum brutiae association was invalidly published (nom. inval. [Art. 5a]) and not validated by Kurt et al. (2015). Furthermore, none of the sub-associations described by Kurt et al. (2015) carry the epithet ‘typicum’ (inval. [Art. 4d]). Associations of P. brutia were attached to two different higher syntax plant sociology units due to the variation in habitat requirements in Turkey. Among these, the Turkish pine associations in the Thermo- and Eu-Mediterranean vegetation belts are assigned to the Quercetea ilicis class, while in the Supra-Mediterranean vegetation belt to Quercetea pubescentis (Akman et al. 1978; Akman 1995). When previous studies were examined, P. brutia associations were generally linked to the alliance of Quercion ilicis and the order of Quercetalia ilicis under the Quercetea ilicis class. The alliance of Oleo-Ceratonion siliquae Br.-Bl. ex Guinochet et Drouineau 1944 (Syn. Oleo-Ceratonion Br.-Bl. 1936) in Muğla province (Vural et al. 1995; Akman et al. 1998) and the alliance of Gonocytiso pterocladi-Pinion brutiae Barbéro, Chalabi, Nahal et Quézel ex Quézel et al., 1993 were used for P. brutia forests spread in Kahramanmaraş province (Varol et al. 2006). However, in areas close to the Mediterranean montane belt, the alliances of Geranio-Cedrion Quezel et al., 1977 and Abieto cilicicae-Cedrion libani Quézel et al., 1978 connected to the Querco-Cedretalia libani Barbero et al., 1974 order were also grouped under the Quercetea pubescentis class (Serin and Eyce 1994; Varol et al. 2006; Dönmez and Şanda 2015). Thus, for P. brutia forests in southern and southwestern Anatolia, the alliance of Quercion cocciferae (Syn.: Quercion calliprini) and the order of Pistacio lentisci-Rhamnetalia alaterni were also used (Özen and Kılınç 1995; Şanda 2006; Kurt et al. 2015; Kavgacı et al. 2017). Taxa belonging to the Quercetea pubescentis class are well represented in the P. brutia community due to the presence of the C. libani communities (unlike the Central and Eastern Taurus, there are, interestingly, no Pinus nigra, Abies cilicica, and Quercus cerris forests here) on the upper zone of the Turkish pine forests in the Western Mediterranean (Akman 1995; Kavgacı et al. 2021). On the other hand, although there are also some plant taxa belonging to the order of Pistacio lentisci-Rhamnetalia alaterni in the community, their representation remained low. In addition, taxa belonging to the Ononido-Rosmarinetea class are also included in the community due to the intense anthropogenic effect such as forestry activities, grazing, agriculture, etc. At the alliance level, it is under the influence of alliances of Quercion cocciferae, Arbuto andrachnes-Quercion cocciferae Barbero et Quézel 1979 and Oleo-Ceratonion siliquae. In fact, when the species composition is examined, taxa of these alliances are included such as Arbutus andrachne, Phillyrea latifolia, Torilis arvensis, Juniperus oxycedrus, Daphne gnidioides, Oryzopsis miliacea subsp. thomasii, Eryngium falcatum, and Onosma frutescens (Online Supplement). However, the presence and cover values of the taxa of the Quercion cocciferae alliance are quite high. Although Kurt et al. (2015) evaluated the P. brutia communities in this region within the Ceratonio-Pistacion lentisci Zohary et Orshan 1959 (Syn. Ceratonio-Rhamnion oleoidis Barbero et Quézel 1979) alliance, Akman (1995) classified the Turkish pine forests in the Western Mediterranean (Antalya and Muğla provinces) under the Quercion cocciferae alliance. Recently, Bonari et al. (2021) conducted a comprehensive study of the entire Mediterranean Basin (including Southern Europe, North Africa, Levant, Anatolia, Crimea and the Caucasus). Syntaxonomy of thermophilous pine forests (including P. brutia, Pinus pinaster, Pinus halepensis, and P. pinea) located between the Thermo-Mediterranean and Mediterranean montane vegetation belts was investigated in this study. According to this study, P. brutia communities in Turkey were connected to the alliances of Pinion brutia Feinbrun 1959 and Styraco officinalis-Pinion brutia under the Pinetalia halepensis Biondi, Blasi, Galdenzi, Pesaresi et Vagge in Biondi et al., 2014 order and Pinetea halepensis Bonari et Chytrý in Bonari et al., 2021 class. The Pinion brutiae alliance refers to Thermo- to Meso-MediterraneanP. brutia forests, while Styraco officinalis-Pinion brutiae alliance refers to Meso- to Supra-MediterraneanP. brutia forests. Since the sampled relevés of this community are mostly (nearly 66%) recorded Meso- to Supra-Mediterrenean belts (Online Supplement), Styraco officinalis-Pinion brutiae alliance is better represented in the Turkish pine association. Belonging to Styraco officinalis-Pinion brutiae, taxa such as Styrax officinalis, Asparagus acutifolius, Eryngium falcatum, Arbutus andrachne, Crepis reuterana, J. oxycedrus, Cistus creticus, Phillyrea latifolia, Quercus coccifera, and Crucianella latifolia have more prominent cover-abundance values (Online Supplement). As a result, we decided that this Turkish pine community would be a new association, namely Asparago acutifoli-Pinetum brutiae ass. Nova with type relevé 28 in Online Supplement. Therefore, this association should be included in the alliance of Styraco officinalis-Pinion brutiae (in order to coordinate with Kavgacı et al. (2021) and Bonari et al. (2021)) and the order of Pinetalia halepensis within the class of Pinetea halepensis.
C. libani (Taurus cedar) forests occur around the Mediterranean Basin (including Turkey, Syria, and Lebanon), covering an area, along the Taurus mountain between Muğla and Kahramanmaraş provinces, of approximately 483,000 ha in Turkey (Sevim 1952; Akman et al. 1978; Terzioğlu et al. 2012). Taurus cedar makes its optimum development in the Supra−Mediterranean and Mediterranean montane vegetation belts on the south and southeast-facing slopes between 1100 and 1800 m a.s.l. in these areas (Akman 1995). While Taurus cedar usually coexists with Pinus nigra or Abies cilicica taxa in the middle and eastern Taurus, it manifests itself in the Supra-Mediterranean belt just after the P. brutia taxon in the western Taurus and forms pure or mixed forest communities up to the anthropogenic steppe zone (Akman et al. 1978; Akman 1995; Kavgacı et al. 2021). C. libani also spreads in the Meso-Mediterranean belt as an enclave in the Blacksea (Tokat-Erbaa) region (Sevim 1952; Quézel et al. 1980). The study area in this state remains in the western part (Ancient Lycia) of the actual spread of C. libani. The first study on cedar forests was carried out in Elmalı (Antalya province) (Çetik 1976) in Turkey. After that, phytosociological researches related to C. libani were undertaken by different investigators using two alliances (Abieto cilicicae-Cedrion libani and Lonicero nummulariifoliae-Cedrion libani Quézel et al., 1978) (Akman et al. 1979a; Akman 1995; Kavgacı et al. 2010). While the Abieto cilicicae-Cedrion libani alliance is dominant in the Middle and Eastern Taurus, this alliance loses its effect in the Western Taurus and leaves its place to the Lonicero nummulariifoliae-Cedrion libani alliance (Quézel and Pamukcuoǧlu 1973; Akman 1995; Kavgacı et al. 2010; Kavgacı et al. 2021). In studies carried out in the Middle and Eastern Taurus (Ocakverdi and Çetik 1987; Serin and Eyce 1994; Duman 1995; Varol and Tatlı 2001; Şanda 2006), C. libani forests were included in the Abieto cilicicae-Cedrion libani alliance. The Lonicero nummulariifoliae-Cedrion libani (except (Sağlam 2013, 2014)) alliance was used in studies conducted in western Taurus (Akman et al. 1979a; Sağlam 2007; Kavgacı et al. 2010; Kavgacı and Čarni 2012; Kavgacı et al. 2021). In addition, the Carpino betuli-Acerion hyrcani Quezel et al. 1978 alliance was also choosen for C. libani forests spread in Tokat-Erbaa (Quézel et al. 1980). Nevertheless, this definition, for the Tokat-Erbaa, is invalid according to ICPN rules, due to the original diagnosis of the association contains no type relevé (nom. inval. Art. 5a) (Theurillat et al. 2021). Quézel et al. (1992) later typified this association, but at this stage, the table number was given incorrectly. Cedar enclaves spreading in Tokat−Erbaa are not currently included in a valid syntaxonomic unit. The Lonicero nummulariifoliae-Cedrion libani alliance is well represented by Lonicera nummulariifolia subsp. glandulifera, Acer hyrcanum subsp. sphaerocaryum, Astragalus pinetorum. The Querco-Cedretalia libani order is also characterized by Briza humilis, Vicia cracca subsp. atroviolacea, Milium vernale subsp. vernale, Juniperus excelsa, Galium incanum subsp. elatius, Paeonia kesrouanensis, and Juniperus foetidissima (Online Supplement). Therefore, this community, observed between 1100 and 1900 m a.s.l., is connected to the Querco-Cedretalia libani order within the class of Quercetea pubescentis (Kavgacı et al. 2021). Since this community spreads between the Supra−Mediterranean and the anthropogenic steppe, it also feels the influence of different phytosociological classes. Actually, plant taxa belonging to the classes of Quercetea ilicis at lower altitudes and Astragalo microcephali-Brometea tomentelli Quézel 1973 at the range of 1700–1900 m a.s.l. have been included in the floristic structure of the community. C. libani community described in the Finike FPU should be accepted as a new association, namely Lamio striati-Cedretum libani with type relevé 20 in Online Supplement and thus, has been evaluated within the scope of the alliance of Lonicero nummulariifoliae-Cedrion libani, due to the predominance of the taxa belonging to this alliance.
In accordance with these assessments, the syntaxonomic scheme of plant associations and syntaxonomical interpretation of these associations given below:
Garique vegetation:
Ononido-Rosmarinetea Br.-Bl. in A. Bolòs y Vayreda 1950.
Hyperico empetrifolii-Genistetalia acanthocladae Mucina in Mucina et al. 2016.
Origano syriaci-Hypericion thymifolii Mucina et Theurillat in Mucina et al. 2016.
Lino corymbuloso-Genistetum acanthocladae Karaköse et Terzioğlu ass. nova
Maquis vegetation:
Quercetea ilicis Br.-Bl. ex A. Bolòs et O. de Bolòs in A. Bolòs y Vayreda 1950.
Quercetalia cocciferae Zohary 1955.
Quercion cocciferae Zohary 1955.
Rhamno nitidae-Quercetum cocciferae Karaköse et Terzioğlu ass. nova
Riparian vegetation:
Alno glutinosae-Populetea albae P. Fukarek et Fabijanić 1968.
Populetalia albae Br.-Bl. ex Tchou 1949.
Platanion orientalis I. Kárpáti et V. Kárpáti 1961.
Nerio oleandri-Platanetum orientalis Karpati 1962.
Forest vegetation:
Pinetea halepensis Bonari et Chytrý in Bonari et al. 2021.
Pinetalia halepensis Biondi, Blasi, Galdenzi, Pesaresi et Vagge in Biondi et al. 2014.
Styraco officinalis-Pinion brutiae Bonari et al. 2021.
Asparago acutifoli-Pinetum brutia Karaköse et Terzioğlu ass. nova
Quercetea pubescentis Doing-Kraft ex Scamoni et Passarge 1959.
Querco pseudocerridis-Cedretalia libani Barbero, Loisel & Quézel. 1974.
Lonicero nummulariifoliae-Cedrion libani Quézel, Barbéro & Akman 1978.
Lamio striati-Cedretum libani Karaköse et Terzioğlu ass. nova
1. Lamio striati-Cedretum libani: This new Taurus Cedar association consists of 22 sampled relevés and 92 vascular plant taxa. It has been identified in the regions of Üçkuzuluk, Ekinalan, Erendağı, Boldağ, and Sirken Mount in the Finike FPU. The association is spreaded in the N-facing hillsides between the altitudes of 1100 and 1900 m a.s.l. and in 14°–34° inclination. This association occurs in Supra- to Montane-Mediterranean belts on the limestone parent rock. The coverage of the tree layer (98.1%) is quite high. Total coverages of the shrub and herb layers vary between 0% and 30%, and 5% and 25% respectively. The nomenclature type of the association Lamio striati-Cedretum libani ass. nova holotypus hoc. Loco: M. Karaköse (07.07.2012). Plot size: 400 m2, Altitude: 1544 m a.s.l., Inclination: 29o, Aspect: N, 0233061 N, 4032155 E, cover of tree layer: 100%, cover of shrub layer: 5%, cover of herb layer: 20% (Relevé 20 in Online Supplement).
Tree layer: C. libani 4, Acer hyrcanum subsp. sphaerocaryum 1, Ostrya carpinifolia 1, Prunus x domestica + .
Shrub layer: Lonicera nummulariifolia subsp. glandulifera 1.
Herb layer: Lamium garganicum subsp. striatum 1, Epilobium lanceolatum 1, M. vernale subsp. vernale 1, Vulpia ciliata subsp. ciliata 1, Dactylis glomerata subsp. hispanica 1, Stipa bromoides 1, Silene italica +, Picnomon acarna +, Cichorium intybus +, Leontodon asperrimus +, Lapsana communis subsp. pisidica +, Diplotaxis tenuifolia +, Euphorbia rigida +, V. cracca subsp. atroviolacea +, Galium tricornutum +, Hordeum bulbosum +, Arabis alpina subsp. brevifolia +, Scaligeria napiformis +, Conium maculatum + .
2. Asparago acutifoli-Pinetum brutia: This new Turkish pine forest association contains 29 sampled relevés and 117 plant taxa. It has been identified in the regions of Alacadağ, Asarönü, Adala, Karanlıkdere, Gökbük, Gökçeyaka, Akyokuş, Kapıçayı, Boldağ, Şamlıyurt, Kızılcık, and Sekçam in the Finike FPU. The association is distributed in the east-facing slopes between the altitudes of 100 and 1100 m a.s.l. and in 5°–29° inclination. This Turkish pine association occurs in Thermo- to Supra-Mediterranean belts on mainly limestone and conglomerate parent rocks. The association spreads in the transition zone to the Taurus Cedar at altitudes above 1000 m a.s.l. The coverage of the tree layer (84.5%) is relatively high. Due to this relativity, shrub coverage becomes rather high (54.3%). The coverage of the herbaceous species is 10.1%. The nomenclature type of the association Asparago acutifoli-Pinetum brutia ass. nova holotypus hoc. Loco: M. Karaköse (11.06.2012). Plot size: 400 m2, Altitude: 484 m a.s.l., Inclination: 20o, Aspect: E, 0238585 N, 4029783 E, cover of tree layer: 90%, cover of shrub layer: 75%, cover of herb layer: 15% (Relevé 28 in Online Supplement).
Tree layer: P. brutia 4, S. officinalis 1.
Shrub layer: Quercus coccifera 3, Phillyrea latifolia 1, Crataegus monogyna subsp. monogyna +, Cotinus coggygria +, Daphne gnidioides + .
Herb layer: Asparagus acutifolius 1, Micromeria myrtifolia 1, C. creticus 1, Smilax aspera 1, Ruscus aculeatus 1, O. miliacea subsp. thomasii 1, M. vernale subsp. vernale 1, D. glomerata subsp. hispanica 1, Stipa bromoides 1, Origanum onites +, Clinopodium nepeta subsp. nepeta +, E. rigida +, Eryngium falcatum +, Scaligeria napiformis +, Ptilostemon afer subsp. eburneus +, Crepis reuterana subsp. reuterana +, Dryopteris pallida subsp. pallida +, B. humilis +, Alcea biennis +, Phlomis grandiflora var. grandiflora + .
3. Rhamno nitidae-Quercetum cocciferae: This new sclerophyll maqui association contains 13 sampled relevés and 70 vascular plant taxa. It has been identified in the regions of mainly Gülmez and Asarönü but also Alacadağ and Boldağ in the Finike FPU. The association is distributed in the east-facing slopes between the altitudes of 100 and 700 m a.s.l. and in 14°–29° inclination. This association occurs in Thermo- to Eu-Mediterranean belts on the limestone parent rock. The coverage of the shrub layer is between 90 and 100%. The coverage of the tree and herbaceous layers is 5% and 11.8%, respectively. The nomenclature type of the association Rhamno nitidae-Quercetum cocciferae ass. nova holotypus hoc. Loco: M. Karaköse (08.06.2013). Plot size: 400 m2, Altitude: 426 m a.s.l., Inclination: 16o, Aspect: N, 0238630 N, 4027586 E, cover of tree layer: 0%, cover of shrub layer: 95%, cover of herb layer: 15% (Relevé 48 in Online Supplement).
Tree layer: Olea europaea var. europaea 1, C. siliqua 1, Quercus aucheri 1.
Shrub layer: Quercus coccifera 4, Phillyrea latifolia 2, Pistacia palaestina 1, Genista acanthoclada 1, Daphne gnidioides 1, Rhamnus nitida + .
Herb layer: C. creticus 1, O. onites 1, Micromeria myrtifolia 1, S. aspera 1, Catapodium rigidum 1, Trifolium campestre +, E. rigida +, Onosma frutescens +, Teucrium chamaedrys subsp. chamaedrys +, Phlomis grandiflora var. grandiflora +, Avena wiestii +, V. ciliata subsp. ciliata +, Crepis reuterana subsp. reuterana +, Cynosurus echinatus +, Melica minuta +, Stipa bromoides +, Hyparrhenia hirta +, O. miliacea subsp. thomasii + .
4. Lino corymbuloso-Genistetum acanthocladae: This new heliophilic garique association includes 5 sampled relevés and 49 vascular plant taxa. It spreads from the port area (centre of Finike district) to the Demre district. The association is distributed in the south-facing slopes from sea level to 100 m a.s.l. and in 10°–25° inclination. This garique association occurs in the Thermo-Mediterranean belt on the limestone parent rock. The coverage of the tree and herbaceous layers is rather low, with 22% and 7%, respectively. On the contrary, to these coverages, the coverage of the shrub (74%) is quite high. The nomenclature type of the association Lino corymbuloso-Genistetum acanthocladae ass. nova holotypus hoc. Loco: M. Karaköse (06.05.2012). Plot size: 200 m2, Altitude: 7 m a.s.l., Inclination: 20o, Aspect: SW, 0242928 N, 4018901 E, cover of tree layer: 20%, cover of shrub layer: 70%, cover of herb layer: 10% (Relevé 1 in Online Supplement).
Tree layer: O. europaea var. europaea 1, C. siliqua 1, Quercus aucheri 1.
Shrub layer: Genista acanthoclada 4, P. lentiscus 3,
Herb layer: Sarcopoterium spinosum 1, Anthemis cretica subsp. cassia 1, Crepis foetida subsp. foetida 1, T. chamaedrys subsp. chamaedrys 1, Avena wiestii 1, Linum corymbulosum +, Lagoecia cuminoides +, Bupleurum gracile +, Centaurium pulchellum +, Kickxia commutata subsp. graeca +, T. arvensis subsp. arvensis +, Sideritis curvidens +, Salvia viridis +, Filago pyramidata +, Galium setaceum +, Arum dioscoridis var. dioscoridis +, Polypogon maritimus subsp. maritimus + .
Conclusion
As a result, five plant associations (four of which are new), which belong to the forest and pre-forest vegetations, were described with this study in the Finike Forest Planning Unit. This study provides, on a small scale, a significant contribution to the knowledge of Turkish Mediterranean forests. In addition to this, as can be understood from this syntaxonomical study, there is some confusion regarding Mediterranean forest ecosystems that needs to be resolved. Despite the comprehensive studies of Kavgacı et al. (2021) and Bonari et al. (2021), many taxa still overlap with each other in the orders of Quercetalia ilicis, Quercetalia cocciferae, Pinetalia halepensis, and Pistacio lentisci-Rhamnetalia alaterni. In order to resolve these misunderstandings, a comprehensive syntaxonomy should be established by evaluating the entire Mediterranean ecosystems on a large scale.
References
Akman Y, Barbero M, Quézel P (1978) Contribution à l'étude de la végétation forestière d'Anatolie méditerranéenne. Phytocoenologia 5:1–79. https://doi.org/10.1127/phyto/5/1978/1
Akman Y, Barbero M, Quézel P (1979a) Contribution à l'étude de la végétation forestière d'Anatolie méditerranéenne. Phytocoenologia 5:189–276. https://doi.org/10.1127/phyto/5/1979/189
Akman Y, Barbero M, Quézel P (1979b) Contribution à l'étude de la végétation forestière d'Anatolie méditerranéenne. Phytocoenologia 5:277–346. https://doi.org/10.1127/phyto/5/1979/277
Akman Y, Ketenoǧlu O (1986) The climate and vegetation of Turkey. Proc R Soc Edinb B 89:123–134
Akman Y (1995) Türkiye Orman Vejetasyonu. Ankara Üniversitesi Fen Fakültesi, Ankara
Akman Y, Kurt L, Demiryürek E, Quézel P, Kurt F, Evren H, Küçüködük M (1998) Les groupements à Pinus brutia sur roches ultrabasiques et calcaires, dans la région de Marmaris et Bodrum (Muğla), à l’étage thermo-méditerranéen du sud-ouest Anatolien (Turquie). Ecol Mediterr 24:63–71. https://doi.org/10.3406/ecmed.1998.1848
Akman Y (2011) İklim ve Biyoiklim: Biyoiklim metodları ve Türkiye iklimleri. Palme Yayınları, Ankara
Aksoy N, Çoban S (2017) Forest communities and ecological differentiation of the Mt. Elmacık (Düzce, Turkey). J Environ Biol 38:923–929. https://doi.org/10.22438/jeb/38/5(SI)/GM-08
Allen HD (2003) Response of past and present Mediterranean ecosystems to environmental change. Prog Phys Geogr 27:359–377. https://doi.org/10.1191/0309133303pp387ra
Altay V, Serin M, Yarcı C, Severoğlu Z (2012) Gölcük (Kocaeli/Türkiye) bitki örtüsünün fitoekolojik ve fitososyolojik yönden araştırılması. Ekoloji 21:74–89. https://doi.org/10.5053/ekoloji.2012.849
Asensi A, Díez-Garretas B, Quézel P (2007) Plant communities of Juniperus turbinata Guss. Subsp. turbinata in the Mediterranean region. A biogeographical, bioclimatical and syntaxonomical survey. Phytocoenologia 37:599–623. https://doi.org/10.1127/0340-269X/2007/0037-0599
Atalay I, Efe R, Öztürk M (2014) Effects of topography and climate on the ecology of Taurus Mountains in the Mediterranean region of Turkey. Procedia Soc Behav Sci 120:142–156. https://doi.org/10.1016/j.sbspro.2014.02.091
Ayaşlıgil Y (1987) Der Köprülü Kanyon Nationalpark, seine Vegetation und ihre Beeinflussung durch den Menschen Landschaftsökologie. Landschaftsökologie Weihenstephan, Freising
Aytuğ B, Görecelioğlu E (1993) Anadolu bitki örtüsünün geç kuaterner'deki gelişimi. İstanbul Univ Orman Fak Derg 43:27–46
Bakker J, Paulissen E, Kaniewski D, Poblome J, Laet VD, Verstraeten G, Waelkens M (2013) Climate, people, fire and vegetation: new insights into vegetation dynamics in the eastern Mediterranean since the 1st century AD. Clim Past 9:57–87. https://doi.org/10.5194/cp-9-57-2013
Barbero M, Chalabi MN, Nahal I, Quézel P (1976) Les formations à conifères méditerranéens en Syrie littorale. Ecol Mediterr 2:87–99
Barbero M, Quézel P (1989) Contribution á l’étude phytosociologique des matorrals de Méditerranée orientale. Lazaroa 11:37–60
Barbero M, Bonin G, Loisel R, Quézel P (1990) Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean basin. Vegetatio 87:151–173. https://doi.org/10.1007/BF00042952
Bonari G, Fernández GF, Çoban S, Monteiro-Henriques T, Bergmeier E, Didukh YP, Xystrakis F, Angiolini C, Chytrý K, Acosta AT, Agrillo E, Costa JC, Danihelka J, Hennekens SM, Kavgacı A, Knollova I, Neto CS, Sağlam C, Škvorc Ž et al (2021) Classification of the Mediterranean lowland to submontane pine forest vegetation. Appl Veg Sci 24:e12544. https://doi.org/10.1111/avsc.12544
Braun-Blanquet J (1932) Plant sociology. The study of plant communities. McGraw-Hill, New York
Cain SA (1950) Life-forms and phytoclimate. Bot Rev 16:1–32
Čarni A, Matevski V, Šilc U, Ćušterevska R (2014) Early spring ephemeral therophytic non-nitrophilous grasslands as a habitat of various species of Romulea in the southern Balkans. Acta Bot Croat 73:107–129. https://doi.org/10.2478/botcro-2013-0017
Čarni A, Matevski V, Kostadinovski M, Ćušterevska R (2018) Scrub communities along a climatic gradient in the southern Balkans: maquis, pseudomaquis and shibljak. Plant Biosyst 152:1165–1171. https://doi.org/10.1080/11263504.2018.1435567
Christenhusz M, Byng J (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261:201–217. https://doi.org/10.11646/phytotaxa.261.3.1
Christodoulakis D (1996) The flora of Ikaria (Greece, E. Aegean Islands). Phyton 36:63–92
Chytrý M, Tichý L, Holt J, Botta-Dukát Z (2002) Determination of diagnostic species with statistical fidelity measures. J Veg Sci 13:79–90. https://doi.org/10.1111/j.1654-1103.2002.tb02025.x
Chytrý M, Otýpková Z (2003) Plot sizes used for phytosociological sampling of European vegetation. J Veg Sci 14:563–570. https://doi.org/10.1111/j.1654-1103.2003.tb02183.x
Czeczott H (1939) A contribution to the knowledge of the Flora and Vegetation of Turkey. Feddes Repert 107:1–135
Çetik R (1976) The phytosociological and ecological studies of the Cedrus woodland vegetation of Çığlıkara and Bucak at Elmalı. Commun Fac Sci Univ Ank Ser C Biol 2:1–37
Çinbilgel İ, Gökçeoglu M (2010) The vegetation of Altınbeşik cavern National Park (İbradı-Akseki/Antalya-Turkey). A synecological study. Span J rural dev 1:1−18 https://doi.org/10.5261/2010.GEN2.01
Davis P (1965−1985) Flora of Turkey and the East Aegean Islands. Edinburgh University press, Edinburgh
Davis P, Tan K, Mill RR (1988) Flora of Turkey and the East Aegean Islands and supplement I. Edinburgh University Press, Edinburgh
Dimopoulos P, Georgiadis T (1992) Floristic and phytogeographical analysis of mount Killini (NE Peloponnisos, Greece). Phyton 32:283–305
Dönmez Ş, Şanda MA (2015) Vegetation of Ulubey canyon (Usak, Turkey). Appl Ecol Environ Res 13:627–637. https://doi.org/10.15666/aeer/1303_627637
Duman H (1995) Engizek Dağı (Kahramanmaraş) Vejetasyonu. Turk J Bot 19:179–212
Eminağaoğlu Ö, Anşin R, Kutbay HG (2007) Forest vegetation of Karagöl-Sahara National Park Artvin-Turkey. Turk J Bot 31:421–449
Fontaine M, Aerts R, Özkan K, Mert A, Gülsoy S, Süel H, Waelkens M, Muys B (2007) Elevation and exposition rather than soil types determine communities and site suitability in Mediterranean mountain forests of southern Anatolia, Turkey. For Ecol Manage 247:18–25. https://doi.org/10.1016/j.foreco.2007.04.021
Gauquelin T, Michon G, Joffre R, Duponnois R, Génin D, Fady B, Dagher-Kharrat MB, Derridj A, Slimani S, Badri W (2018) Mediterranean forests, land use and climate change: a social-ecological perspective. Reg Environ Change 18:623–636. https://doi.org/10.1007/s10113-016-0994-3
Gemici Y (1988) Akdağ (Afyon-Denizli) ve çevresinin vejetasyonu. Doğa Turk J Bot 11:270–305
Güner A, Özhatay N, Ekim T, Başer KHC (2000) Flora of Turkey and the East Aegean Islands and supplement II. Edinburgh University Press, Edinburgh
Güner A, Aslan S, Ekim T, Vural M, Babaç MT (2012) Türkiye Bitkileri Listesi (Damarlı Bitkiler). ANG Vakfı, İstanbul
Hennekens SM, Schaminée JH (2001) TURBOVEG, a comprehensive data base management system for vegetation data. J Veg Sci 12:589–591. https://doi.org/10.2307/3237010
İslamoğlu Y, Taner G (2002) Kasaba Miyosen havzasında Uçarsu ve Kasaba formasyonlarının mollusk faunası ve stratigrafisi (Batı Toroslar, GB Türkiye). The Journal of MTA 125:31–57
Jasprica N, Škvorc Ž, Dolina K, Ruščić M, Kovačić S, Franjić J (2016) Composition and ecology of the Quercus coccifera L. communities along the eastern Adriatic coast (NE Mediterranean). Plant Biosyst 150:1140–1155. https://doi.org/10.1080/11263504.2014.1001461
Karaer F, Kılınç M, Kutbay HG (1999) The woody vegetation of the Kelkit Valley. Turk J Bot 23:319–344
Karaköse M (2019) Numerical classification and ordination of Esenli (Giresun) forest vegetation. Biologia 74:1441–1453. https://doi.org/10.2478/s11756-019-00321-z
Karaköse M, Terzioğlu S (2020) Finike (Antalya) Orman Planlama Biriminin vasküler bitki florası. KSU Doğa Bilim Derg 23:1144–1162. https://doi.org/10.18016/ksutarimdoga.vi.681247
Karavani A, Boer MM, Baudena M, Colinas C, Díaz-Sierra R, Pemán J, De Luis M, Enríquez-de-Salamanca Á, Resco de Dios V (2018) Fire-induced deforestation in drought-prone Mediterranean forests: drivers and unknowns from leaves to communities. Ecol Monogr 88:141–169. https://doi.org/10.1002/ecm.1285
Kárpáti I (1962) Überblick der zönologischen und ökologischen Verhältnisse der Auenwälder des Westbalkans. Mitt Ostalp-Din Pflanzensoz Arbeit 2:101–106
Kavgacı A, Başaran S, Başaran M (2010) Cedar forest communities in Western Antalya (Taurus Mountains, Turkey). Plant Biosyst 144:271–287. https://doi.org/10.1080/11263501003690720
Kavgacı A, Čarni A (2012) Diversity and gradients in cedar forests on Taurus mountain range (Turkey). J Environ Biol 33:977–984
Kavgacı A, Šilc U, Başaran S, Marinsek A, Başaran M, Kosir P, Balpınar N, Arslan M, Ergüler Ö, Čarni A (2017) Classification of plant communities along postfire succession in Pinus brutia (Turkish red pine) stands in Antalya (Turkey). Turk J Bot 41:299–307. https://doi.org/10.3906/bot-1609-34
Kavgacı A, Balpınar N, Öner H, Arslan M, Bonari G, Chytrý M, Čarni A (2021) Classification of forest and shrubland vegetation in Mediterranean Turkey. Appl Veg Sci 24:e12589. https://doi.org/10.1111/avsc.12589
Kaya ÖF, Cansaran A, Yıldırım C (2010) A syntaxonomical investigation of forest and pseudomaquis on transitional area in the Central Black Sea region (Amasya, Turkey). Acta Bot Gallica 157:469–482. https://doi.org/10.1080/12538078.2010.10516223
Korkmaz H, Engin A, Kutbay HG, Yalçın E (2011) A syntaxonomical study on the scrub, forest, and stepe vegetation of the Kızılırmak valley. Turk J Bot 35:121–165. https://doi.org/10.3906/bot-0908-152
Kurt L, Ketenoğlu O, Akman Y, Özdeniz E, Şekerciler F, Bölükbaşı A, Özbey BG (2015) Syntaxonomic analysis of the preforest and forest vegetation in the thermo-and eumediterranean zone around Antalya gulf, Turkey. Turk J Bot 39:487–498. https://doi.org/10.3906/bot-1402-63
Kutbay HG, Kılınç M (1995) Bafra Nebyan Dağı (Samsun) ve çevresinin vejetasyonu üzerinde fitososyolojik ve ekolojik bir araştırma. Turk J Bot 19:41–63
Kutbay HG, Karaer F, Kılınç M (1998) Orta Karadeniz Bölgesinde bulunan Quercus L. ormanlarının fitososyolojik yapısı. OMÜ Fen Derg 9:1–17
Messinger J, Güney A, Zimmermann R, Ganser B, Bachmann M, Remmele S, Aas G (2015)Cedrus libani: a promising tree species for central European forestry facing climate change? Eur J For Res 134:1005–1017. https://doi.org/10.1007/s10342-015-0905-z
Mucina L, Bültmann H, Dierßen K, Theurillat J-P, Raus T, Čarni A, Šumberová K, Willner W, Dengler J, García RG, Chytrý M, Hájek M, Di Pietro R, Iakushenko D, Pallas J, Daniëls FJA, Bergmeier E, Santos Guerra A, Ermakov N et al (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19:3–264. https://doi.org/10.1111/avsc.12257
Ocakverdi H, Çetik A (1987) Seydişehir Maden bölgesi (Konya) ve çevresinin vejetasyonu. Doğa Turk J Bot 11:100–148
OGM (2015) Türkiye Orman Varlığı. Orman Genel Müdürlüğü, Orman Genel Müdürlüğü Dış ilişkiler, Eğitim ve Araştırma Dairesi Başkanlığı, Ankara
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara R, Simpson G, Solymos P (2019) Vegan: community ecology package. R package (version 25–6) https://cranr-projectorg/web/packages/vegan/veganpdf Accessed 16 July 2019
Ozenda P (1975) Sur les étages de végétation dans les montagnes du bassin méditerranéen. Documents de Cartographie Ecologique 16:1–32
Öner H, Akbin G (2010) Kapıdağ Yarımadası’nın fitossoyolojik ve fitoekolojik yönden incelenmesi. Ege Ormancılık Ens, Müdürlüğü, İzmir
Özel N (1999) Kazdağları orman vejetasyonu üzerine fitososyolojik ve fitoekolojik araştırmalar. Ege Ormancılık Araştırma Enstitü Müdürlüğü, İzmir
Özen F, Kılınç M (1995) Alaçam-Gerze ve Boyabat-Durağan arasında kalan bölgenin vejetasyonu: II-orman ve bozuk orman vejetasyonları. Turk J Bot 19:87–105
Özen F (2010) Yeniköy (Bursa) higrofil, orman ve maki vejetasyonunun sinekolojik ve sintaksonomik analizi. Ekoloji 19:50–64
Özhatay N, Kültür Ş, Gürdal B (2013) Check-list of additional taxa to the supplement Flora of Turkey VI. J Pharm Istanbul Univ 43:33–83
Özhatay N, Kültür Ş, Gürdal B (2015) Check-list of additional taxa to the supplement Flora of Turkey VII. J Pharm Istanbul Univ 45:61–86
Özhatay N, Kültür Ş, Gürdal B (2017)Check-list of additional taxa to the supplement flora of Turkey VIII. J Pharm Istanbul Univ 47:31–47. https://doi.org/10.5152/IstanbulJPharm.2017.006
Özhatay N, Kültür Ş, Gürdal B (2019)Check-list of additional taxa to the supplement flora of Turkey IX. J Pharm Istanbul Univ 49:105–120. https://doi.org/10.26650/IstanbulJPharm.2019.19037
Palahi M, Mavsar R, Gracia C, Birot Y (2008) Mediterranean forests under focus. Int For Rev 10:676–688
Papanastasis VP, Kyriakakis S, Kazakis G (2002) Plant diversity in relation to overgrazing and burning in mountain Mediterranean ecosystems. J Mediterr Ecol 3:53–64
Peris-Llopis M, González-Olabarria JR, Mola-Yudego B (2020) Size dependency of variables influencing fire occurrence in Mediterranean forests of eastern Spain. Eur J For Res 139:525–537. https://doi.org/10.1007/s10342-020-01265-9
Pignatti S, Ellenberg H, Pietrosanti S (1996) Ecograms for phytosociological tables based on ellenberg. Ann di Bot 54:5–14. https://doi.org/10.4462/annbotrm-9021
Pignatti S (2003) The mediterranean ecosystem. Flora Mediterr 16:29–40
Pinna M, Bacchetta G, Cogoni D, Fenu G (2019) Is vegetation an indicator for evaluating the impact of tourism on the conservation status of Mediterranean coastal dunes? Sci Total Environ 674:255–263. https://doi.org/10.1016/j.scitotenv.2019.04.120
Pumo D, Viola F, Noto L (2010) Climate changes' effects on vegetation water stress in Mediterranean areas. Ecohydrology 3:166–176. https://doi.org/10.1002/eco.117
Quézel P, Pamukcuoǧlu A (1973) Contribution à l'étude phytosociologique et bioclimatique de quelques groupements forestiers du Taurus. Feddes Repert 84:185–229
Quézel P, Barbero M, Akman Y (1980) Contribution à l'étude de la végétation forestière d'Anatolie septentrionale. Phytocoenologia 8:365–519. https://doi.org/10.1127/phyto/8/1980/365
Quézel P, Barbero M (1982) Definition and characterization of Mediterranean-type ecosystems. Ecol Mediterr 8:15–29
Quézel P, Barbero M, Akman Y (1992) Typification de syntaxa décrits en région méditerranéenne orientale. Ecol Mediterr 18:81–87. https://doi.org/10.3406/ecmed.1992.1708
R Development Core Team (2019) R: A language and environment for statistical computing (Version 3.6.1). R Foundation for Statistical Computing. http://www.R-project.org Accessed 16 July 2019
Raunkiaer C (1934) The life forms of plants and statictical plant geography. Clarendon Press, Oxford
Rick T, Ontiveros MÁC, Jerardino A, Mariotti A, Méndez C, Williams AN (2020)Human-environmental interactions in Mediterranean climate regions from the Pleistocene to the Anthropocene. Anthropocene 31:100253. https://doi.org/10.1016/j.ancene.2020.100253
Roleček J, Tichý L, Zelený D, Chytrý M (2009) Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J Veg Sci 20:596–602. https://doi.org/10.1111/j.1654-1103.2009.01062.x
Sağlam C (2007) Davras Dağı (Isparta) ve çevresinin orman ve çalı vejetasyonu. SDÜ Fen Bil Enst Der 11:140–157
Sağlam C (2013) A phytosociological study of the forest, shrub, and steppe vegetation of Kızıldağ and environs (Isparta, Turkey). Turk J Bot 37:316–335. https://doi.org/10.3906/bot-1205-46
Sağlam C (2014) Phytosociological features of Çiçek mountain and environs (Isparta, Turkey). Ekoloji 23:19–37. https://doi.org/10.5053/ekoloji.2014.923
Scarascia-Mugnozza G, Oswald H, Piussi P, Radoglou K (2000) Forests of the Mediterranean region: gaps in knowledge and research needs. For Ecol Manage 132:97–109. https://doi.org/10.1016/S0378-1127(00)00383-2
Schwarz O (1936) Die vegetations siqliederung West Anatoliens. Bot Jahrb Syst 67:297–436
Serin M, Eyce B (1994) Hadim (Konya) Aladağ (Orta Toroslar) ve çevresinin vejetasyonu. Turk J Bot 18:201–227
Sevim M (1952) Lübnan sedirinin (Cedrus libani Barr.) Türkiyedeki Tabii yayılışı ve ekolojik şartları. İstanbul Univ Orman Fak Derg 2:19–46
Şanda MA (2006) Geyik Dağı (Antalya) ve çevresinin orman ve subalpin vejetasyonu. Selçuk Univ Fen Fak Fen Derg 2:99–116
Şık L, Gemici Y (1994) Batı Anadolu’da Maki ve Frigana vejetasyonunda kayaca bağlı değişimler üzerine gözlemler. Turk J Bot 18:73–80
Terzioğlu S, Bilgili E, Karaköse M (2012) Türkiye Ormanları (Forests of Turkey). Orman Genel Müdürlüğü, Orman Genel Müdürlüğü Dış ilişkiler, Eğitim ve Araştırma Dairesi Başkanlığı, Ankara
Theurillat J-P, Willner W, Fernández GF, Bültmann H, Čarni A, Gigante D, Mucina L, Weber H (2021) International code of phytosociological nomenclature. 4th edition. Appl Veg Sci 24:e12491. https://doi.org/10.1111/avsc.12491
Tichý L (2002) JUICE, software for vegetation classification. J Veg Sci 13:451–453. https://doi.org/10.1111/j.1654-1103.2002.tb02069.x
Tsiourlis G, Konstantinidis P, Xofis P (2007) Taxonomy and ecology of phryganic communities with Sarcopoterium spinosum (L.) Spach of the Aegean (Greece). Isr J Plant Sci 55:15–34
Tsiourlis G, Konstantinidis P, Xofis P (2009) Syntaxonomy and synecology of Quercus coccifera Mediterranean shrublands in Greece. J Plant Biol 52:433–447. https://doi.org/10.1007/s12374-009-9056-4
Uğurlu E, Roleček J, Bergmeier E (2012) Oak woodland vegetation of Turkey–a first overview based on multivariate statistics. Appl Veg Sci 15:590–608. https://doi.org/10.1111/j.1654-109X.2012.01192.x
Van Zeist W, Bottema S (1988) Late Quaternary vegetational and climatic history of Southwest Asia. Proc Indian National Sci Acad 54:461–480
Varol Ö, Tatlı A (2001) The vegetation of Çimen mountain (Kahramanmaraş). Turk J Bot 25:335–358
Varol Ö, Ketenoglu O, Bingöl Ü, Geven F, Güney K (2006) A phytosociological study on the coniferous forests of Başkonuş Mts, anti-Taurus, Turkey. Acta Bot Hung 48:195–211. https://doi.org/10.1556/abot.48.2006.1-2.18
Vural M, Duman H, Güner A, Dönmez A, Şağban H (1995) The vegetation of Köyceğiz-Dalyan (Muğla) specially protected area. Turk J Bot 19:431–476
Walter H (1956) Vegetationsgliederung Anatoliens. Flora Allg Bot Ztg 143:295–326
WFO (2021) World Flora Online. Published on the Internet; http://www.worldfloraonline.org Accessed 26 Aug 2021
Yaltırık F (1982)Platanus L. in: Davis P (ed) Flora of Turkey and the East Aegean Islands. Edinburgh University press Edinburgh, p 655−656
Yurdakulol E (1981) A phytosociological and ecological research on the vegetation of the Pos forests (Adana, Distr. Karsanti) on the anti-Taurus Mountains. Commun Fac Sci Univ Ank Ser C Biol 24:1–50
Zohary M, Orshan G (1959) The maquis of Ceratonia siliqua in Israel. Vegetatio:285–297
Zohary M (1973) Geobotanical foundations of the Middle East. Gustav Fischer Verlag, Stutgart
Acknowledgments
This study is a part of the PhD dissertation of the corresponding author. The corresponding author wants to express his special thank to Prof. Dr. Emin Uğurlu, Prof. Dr. Jürgen Dengler, Drs. Stephan Hennekens, and Dr. Jan Roleček for the Education Workshop (SIGNAL) of Climate Change Researches, TURBOVEG and JUICE Softwares. In addition, the corresponding author would like to thank to Prof. Dr. Ladislav Mucina, Prof. Dr. Erwin Bergmeier, and Prof. Dr. Ali Kavgacı for the supplying some literature. The authors would like to thank to staff of Antalya (especially Directorate of Finike Forest) Regional Directorate for their logistic support and Prof. Dr. Ali İhsan KADIOĞULLARI for the preparation the map of study area.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karaköse, M., Terzioğlu, S. Numerical classification and ordination of Finike (Antalya) Forest vegetation. Biologia 76, 3631–3645 (2021). https://doi.org/10.1007/s11756-021-00910-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00910-x