Abstract
Bioleaching is a common and eco-friendly method for the metals mobilization from mines, contaminated soils and wastes and it can be used in metal extraction and bioremediation. Due to the fact that these processes are considered as metabolism-dependent processes, isolation and identifying of the strains with high resistance features in responsible bacteria are one of the most important steps to have. In present study, an Iranian new isolate of iron-oxidizing bacteria was characterized. The phylogenetic analysis and 16S rRNA gene sequence of strain ZT-94 indicated that this strain is related to Acidithiobacillus ferrooxidans. It is investigated that the ferrous iron oxidation was increased dramatically in the early hours of bacterial growth and the ferrous iron (Fe2+) was completely oxidized at 30 h. According to the results, strain ZT-94 had the high tolerance capability to U, Ba, Al and Se. Iron oxidation by strain ZT-94 was inhibited by the addition of 312.5 mg/L Se and 70 mg/L Te but concentrations above 5000 mg/L of Ba and 2000 mg/L Al had no inhibitory effect on bacterial growth. In addition, strain ZT-94 was able to grow in the pH range of 1–4, temperatures at 25–35 °C. Results showed no inhibitory effects on bacterial growth rate in presence of 0.1 w/v organic compounds like fructose and glucose but a delayed growth was detected in presence of yeast extract in comparison with the others. The results showed that strain ZT-94 had high environmental adaptability and can be introduced as a suitable and valuable candidate for research in the bioleaching and bioremediation processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, applying of the microbial processes in the mineral industry and metals extraction has been considerably increased (Olson et al. 2003). Microorganisms play an important role in the immobilization or mobilization of radionuclides and heavy metals (Lovley and Coates 1997; Valls and de Lorenzo 2002; Vijayaraghavan and Yun 2008; Volesky 1994). The bioleaching process has been mentioned as a new method, cost effective, less harmful and eco-friendly for metal extraction and mobilization from mine, sediment, sludge and soil (Chen and Chou 2016; Srichandan et al. 2019; Yang et al. 2016). The classical leaching bacteria now belong to the genus Acidithiobacillus. Acidophilic Acidithiobacilus ferrooxidans as a chemolithotrophic microorganism grows on ferrous or sulphur as its energy source. This bacteria obtains its required energy through the oxidation of ferrous or sulfide contents which leads to the production of ferric sulfate (Brierley 1982; Ruiz et al. 2012). Ferric sulfate as a strong oxidizing agent is able to mobilize metals in the environments containing toxic metals. This microorganism plays an important role in the bioleaching process, especially in the leaching of sulfide minerals. (Chen and Chou 2016; Rohwerder et al. 2003; Vera et al. 2013).
With the industrialization of mines and metal extraction, water safety has been challenged by organic compound and heavy metals and they have entered into the environment (Liu et al. 2020; Mohapatra et al. 2019; Xiao et al. 2020). Heavy metals unlike organic compounds are not degradable and remain in the environment causing serious challenge for remediation and bioleaching (Chen and Chou 2016; Mishra and Mohan 2017; Soares and Soares 2012; Srivastava et al. 2015; Tabak et al. 2005). It was recently demonstrated that the microbial population density and heavy metals resistance of bacteria is very important in bioleaching processes. The growth of microorganisms and their activities can be inhibited by the presence of some toxic metals. This problem can be solved by using the heavy metal resistance bacteria in these systems (Diels et al. 1999; Kanwar et al. 2017). Most bacteria that live in harsh environments have different strategies for surviving in hard conditions and microorganisms have been adapted to the presence of toxic heavy metals. This is due to the mechanism of resistance systems (Navarro et al. 2013). Also, some reports have shown that native microbes from harsh environments can tolerate a high concentration of heavy metals and play an important role in biotechnological processes (Irawati et al. 2016). For example, it is shown that Acidithiobacillus ferrooxidans can be used to remove heavy metals in acidic wastewater (Min et al. 2017). Also, it is indicated that Acidithiobacillus ferrooxidans is one of the efficient bioleaching bacteria modulating the bioleaching process (Gao et al. 2020).
Some organic compounds also can have an inhibitory effect on bacterial growth. Agar toxicity is a known issue in the growth of autotrophic bacteria which prevents the growth of colonies on ferrous-iron medium and inhibits their growth (Happold et al. 1954; Kelly 1971; Tuovinen and Kelly 1973). Therefore, the isolation of bacteria that have the ability to grow in solid medium is very important for further identify them. The purpose of this research was development of a fundamental guideline for isolation and cultivation of a strain of the fastidious iron (II)-oxidizing bacteria in a solid medium. This paper also introduces iron(II)-oxidizing isolated bacteria (Acidithiobacillus ferrooxidans strain ZT-94) resistant to high concentration of metals with high iron oxidation rate as a valuable leaching bacteria for application in bioleaching processes that is fitted with the principles of the green technology.
Material and methods
Isolation and purification of bacteria
Ore sample was collected from the Saghand uranium mine in Iran. Modified 9 K medium used for isolation experiments (Silverman and Lundgren 1959). The isolation medium contained (Silverman and Lundgren 1959): The basal salts (3 g (NH4)2SO4, 0.5 g MgSO4·7H2O, 0.5 g K2HPO4, 0.01 g Ca(NO3)2, 0.1 g KCl in 700 mL distilled H2O) was added to 300 mL of a 14.74% (w/v) FeSO4·7H2O solution and adjusted to pH 2 with 10 N H2SO4. The broth cultures were incubated aerobically for 14 days at 30 °C in a shaking incubator (150 rpm).
For purification of bacteria, isolated bacteria were cultured on a solid culture medium (Yates and Holmes 1987). The solid culture media were incubated at 30 °C for 10 days. Single colonies appearing over this period were re-cultured onto solid plates, and stored at 4 °C. The solid medium components consisted of the following (Yates and Holmes 1987):
-
Solution A: A 35 mL of 10x modified 9 K salts (50 g/L (NH4)2SO4, 8.34 g/L MgSO4·7H2O, 0.84 g/L K2HPO4, 0.24 g/L Ca(NO3)2.4H2O, 1.66 g/L KCl) was added to 265 mL of H2O and its pH was adjusted to 2.5 with 10 N H2SO4.
-
Solution B:11 g/L FeSO4·7H2O was added to 75 mL of H2O and pH was adjusted to 2.5 with 10 N H2SO4.
-
Solution C: 2 g/L agarose was added to 125 mL of H2O.
-
Solutions A and C were sterilized at 121 °C for 15 min and mixed with solution B, which had been filter sterilized (Yates and Holmes 1987).
Bacterial culture medium
This isolate was grown in APH medium at 150 rpm and 30 °C. APH medium containing 2 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L K2HPO4, 0.01 g/L Ca(NO3), 0.1 g/L KCl, and 20 g/L FeSO4·7H2O with final pH adjusted to 2 with H2SO4 (Atlas 2005). Samples were taken to determine total soluble ferrous and ferric iron concentration and cell number at regular intervals. The number of bacterial cells was counted every 2 h and growth curve was plotted. The numbers of bacterial cells were estimated directly by a neubauer chamber cell counting (Tajer Mohammad Ghazvini et al. 2014).The pH and Eh were also measured with a pH meter (Metrohm 827).
Analytical methods
For ferric ion and total iron measurement, 5-sulfosalicylic acid (SSA) testing was used to determine of the bacterial activity in ferrous ion oxidation to ferric ion (Karamanev et al. 2002). The redox potential and the pH values were evaluated using a pH meter (Metrohm 827) and with a combined Pt-ring electrode (reference electrode Ag/AgCl, reference electrolyte 3 mol/L KCl) respectively (Zare Tavakoli et al. 2017).
Phylogenetic analysis of isolated Bacteria
The 16S rRNA gene sequence (1498 bases) analysis was carried out with Chromas Pro software version 1.5 (Technelysium Pty. Ltd., http://www.technelysium.com.au). For obtain the nearest phylogenetic neighbors from the databases, the 16S rRNA gene sequence was compared with the GenBank data using NCBI (http://blast.ncbi.nlm.nih.gov) and EzTaxon-e servers (http://eztaxon-e.ezbiocloud.net/) (Kim et al. 2012). Then 16S rRNA sequences were aligned using CLUSTAL W (Thompson et al. 1994). MEGA5 (Tamura et al. 2011) and the neighbour-joining algorithm were used for construction of phylogenetic tree (Saitou and Nei 1987; Tajer Mohammad Ghazvini et al. 2014; Tamura et al. 2004).
Microscopic examinations
This isolate was investigated by scanning electron microscope (SEM). In this study, lamellas were cut at size of 10 × 10 mm and immersed in 0.8% agar solution. After forming a thin layer of agar, the bacteria were expanded onto the lamellas and placed at 37 °C for 12 h. Then, they were immersed successively for 30 min in low to high concentrations of ethanol (10, 25, 50, 75, 96, 99.9%). Finally, ethanol evaporated for 1 h at 37 °C. A coating of gold was vacuum-deposited on the specimens by Ion-Coater (KIC-IA, COXEM), and then the specimens were examined by a scanning electron microscope (Piroeva et al. 2013).
Heavy metal susceptibility testing
For this test, 96 well microplates were used. All stages of the experiment were performed in sterile conditions and at least in 2 replicate (2 columns). For determination of minimum inhibitory concentration (MIC), APH medium containing Potassium tellurite (K2TeO3), Sodium selenite pentahydrate (Na2SeO3.5H2O), Aluminium sulfate hexadecahydrate (Al2(SO4)3.16H2O), Barium chloride dihydrate (BaCl2.2H2O), Sodium molybdate dihydrate (Na2MoO4.2H2O) and Uranyl nitrate hexahydrate (UO2(NO3)2.6H2O) (Merck) were made (Atlas 2005). Finally, the microplates were incubated at 30 °C and the results were monitored daily. The growth of microorganism was investigated apparently by the development of growth-induced turbidity (also by the production of red dye by bacterial iron oxidation). The lowest concentration of the metal salt inhibiting bacterial growth was considered as the MIC. The minimal bactericidal concentration (MBC) of metals was determined from dilutions that no signs of growth was detectable by sub culturing on the solid culture medium with no metal content (Yates and Holmes 1987). The plates were incubated at 30 °C for 10 days. That concentration in which its subculture showed no bacterial growth was indicated as MBC.
Effects of temperature, pH and organic compounds on the growth of the bacteria
The effect of different temperatures (20, 25, 30, 35, 40, 45 °C) and different pH (pH 1–6) on bacterial growth were separately evaluated in APH medium at 30 °C and 150 rpm monitoring the Eh changes as the representative factor of bacterial growth during the test. Furthermore, the possible changes of bacterial growth in presence of some organic compounds that are considered as the growth inhibitors for autotrophic microorganisms were studied. Organic compounds such as yeast extract, glucose and fructose monohydrate were added at a final concentration of 0.1 w/v to the medium and Eh changes in the media were monitored daily as the representative factor of bacterial growth. The initial pH of the medium was adjusted to 2 by sulfuric acid and the culture medium with no organic compounds was used as control in this study.
Results and discussion
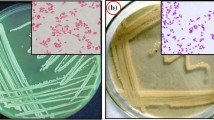
In general, most autotrophic microorganisms are sensitive to low-molecular-weight organic compounds that it make their isolation and purification on solid agar medium difficult (Johnson and Hallberg 2007; Ngom et al. 2015). But, this isolation procedure resulted in the purification of an isolate of Iron (II)-oxidizing bacteria at the solid culture medium containing agarose, which presented as colonies with an iron-oxidized zone, orange red color. Microscopic examination of the isolate showed gram negative and rod-shaped bacterial cells (Fig. 1). Genetic analysis demonstrated that new strain ZT-94 belongs to the class Acidithiobacillia, phylum Proteobacteria. The phylogenetic tree showed the relationship between Acidithiobacillus ferrooxidans strain ZT-94 and other related bacteria (Fig. 2). The 16S rRNA gene sequence was submitted in the National Center for Biotechnology Information GeneBank database (www.ncbi.nlm.nih.gov) with the accession number KU726246 for Acidithiobacillus ferrooxidans strain ZT-94.
Phylogenetic tree based on 16S rRNA gene sequences is showing the phylogenetic position of new isolated Acidithiobacillus ferrooxidans strain ZT-94. The resulting was evaluated by using bootstrap analysis based on 1000 replicates (Felsenstein 1985). Sulfobacillus acidophilus strain DSM 10332 was taken as an out-group. GenBank accession numbers are indicated after the name in parentheses. Bar 0.02 substitutions per nucleotide position
The growth curve of Acidithiobacillus ferrooxidans strain ZT-94 in APH medium was presented in Fig. 3. The lag phase was observed in the first 2 h. The maximum number of bacterial cells (1.77 × 107) was obtained after 48 h. According to the bacterial growth curve, the logarithmic growth phase continued for 48 h. Afterward, it entered the stationary phase.
In bioleaching process there are some responsible factors as the metal sulfide-oxidizing agent, iron (III) ions, which are made as result of oxidizing reactions in iron (II)-oxidizing bacteria. These compounds can soluble metals in the environment containing metals. Therefore bioleaching depends on the iron oxidation ability of the acidophilic microorganism such as Acidithiobacillus ferrooxidans (Valdés et al. 2008; Vera et al. 2013). In this study, the change in the ferrous and ferric concentration and redox potential of APH medium Acidithiobacillus ferrooxidans strain ZT-94 was showed in Fig. 4. Results showed that the increase of the ferric iron concentration complete the decrease of the ferrous iron concentration during the time. As previously reported, the main role of these microorganisms is regenerating the leaching agents (Fe3+) and to facilitate the reaction by creating an environment in which the leaching process occurs (Venugopal 2005; Zare Tavakoli et al. 2017). The oxidation and reduction potential (ORP) represents the Fe3+/ Fe2+ proportion, as can be seen in Fig. 4. It is indicated that bacterial oxidation of ferrous iron to ferric iron enhanced the redox potential of the culture medium from 361.7 to 621.8 mV. Generally, the redox potential (Eh) values increases with decreasing ferrous ion in the culture medium. The important point is the ratio of ferric to ferrous ions in the culture medium, which can change Eh. According to this relationship, with increasing ferric ions and decreasing ferrous ions in the medium, Eh increases (Zare Tavakoli et al. 2017). At the middle or end of the bioleaching process, the concentrations of Fe3+ and reached at a certain level which facilitated the production of jarosite precipitation and consequently the iron concentration decreased through the time (Fig. 4). As shown in Fig. 4, the ferrous iron oxidation was increased dramatically in the early hours of bacterial growth and the ferrous iron (Fe2+) was completely oxidized at 30 h. The measurement results of the Eh also confirmed the high oxidation of iron. The high ferrous iron oxidation rate will be very important when the process is used on an industrial scale which many authors have reported this phenomenon (Falagán and Johnson 2016; Nemati et al. 1998; Zhan et al. 2019). Acidithiobacillus ferrooxidans belongs to the group of inorganic autotrophic bacteria that it can be take its energy required from oxidation of (Fe2+) and reduced inorganic sulfur for CO2 and nitrogen fixation. Many researchers have shown that the growth and iron oxidation rate in these bacteria are usually slow and long and culture of bacteria takes some days according to the optimal conditions (Cabrera et al. 2005; LIU et al. 2006; Zhan et al. 2019; Zhang et al. 2018). Therefore, it is valuable to use strains with high oxidation rate and rapid growth for industrial processes.
In the bioleaching, living and active microorganisms is an important factor in the process. Hence, the microbial population density and their resistance to metals have an important role in this process. Metal sulfides bioleaching increases the concentration of metal in the bioleaching liquid. Different species of microorganisms involved in the bioleaching have been shown to have different sensitivities to heavy metals (Bosecker 1997). Reports have suggested that differences in the toxicity of heavy metals may be due to differences in the mechanism of metal interactions by microorganisms. The toxicity mechanism of many of these metals is the inactivation, degradation, or occupation of active sites in microbial enzymes (De et al. 1997). In addition, there are some evidences that metal toxicity can be related to the disturbance of the cellular radical balance and according to this the evolution of metal tolerance probably can make changes the capacity of the cellular defense against radicals (De Vos and Schat 1991). In this study, the resistance of Acidithiobacillus ferrooxidans to 6 toxic metals was investigated (Table 1). Our previous research had shown that uranium does not have an inhibitory effect on the activity of (Fe2+) oxidizing enzyme in the strain ZT-94 up to concentration 455 mg/L. According to the results, minimal bactericidal concentration of uranium for Acidithiobacillus ferrooxidans strain ZT-94 was 570 mg/L (Bahrami-Bavani et al. 2017). Studies show that the resistance to uranium is often very low in the leaching microorganisms. For instance, among the three strains of Acidithiobacillus ferrooxidans, the maximum resistance was 9 mM of uranium (Merroun and Selenska-Pobell 2001). Mostly the uranium- containing leaching solutions inhibit the microbial oxidation of (Fe2+) in the leaching microorganisms. For example, it was showed that oxidation of ferrous iron by Acidithiobacillus ferrooxidans was inhibited by 0.2–0.9 mM of the UO22+ ions. Also iron oxidation was stopped completely in the liquid medium by 1–1.5 mM of the UO22+ ions. This finding confirms the negative impact and inhibitory effect of uranium on the microbial oxidation rate of ferrous iron (Satyavathi et al. 2008; VanEngelen et al. 2011). However, obtaining uranyl resistant strains of Acidithiobacillus ferrooxidans is available through mutation or adaptation (Tuovinen and Kelly 1973).
Our other study has shown that strain ZT-94 can tolerate 60 mg/L molybdenum. Researchers have shown that molybdenum inhibits Acidithiobacillus oxidation systems through inactivation of the iron oxidase and cytochrome c oxidase enzymes. In this inhibitory mechanism, the iron (II) present in the bacterial growth medium reduces Mo+6 to the Mo+5 that attaches to the cells plasma membrane and decrease the cytochrome c oxidase activity which leads to iron (II) oxidation inhibin and the consequent stop in bacterial growth. Therefore, the resistance of Acidithiobacillus oxidation systems can results in a good resistance to external molybdenum in this bacteria (Kasra-Kermanshahi et al. 2017; Kasra-Kermanshahi et al. 2020; Yong et al. 1997). Also, Yong and et al. showed that among seventy-five strains of iron-oxidizing bacteria, only one strain grew in the presence of 1.25 mM sodium molybdate (Yong et al. 1997). Therefore, this strain is a molybdenum resistant isolate.
Tellurium is a metal-like element which exists in four oxidized forms such as telluride (Te2−), tellurium (Te0), tellurite (TeO32−) and tellurate (TeO42−). Due to the higher toxicity of tellurite in comparison with elemental tellurium and tellurate, it is not commonly found in living systems. This toxicity is related to its activity as a strong oxidant. Therefore, the effect of different tellurium concentrations on Acidithiobacillus ferrooxidans growth was investigated in this study. The results showed that tellurium had no inhibitory effect on bacterial growth in concentrations less than 50 mg/L, but the growth of bacteria stopped in 70 mg/L. Moreover, previous studies have shown that tellurium was toxic in concentrations of 50–100 mg /L (Tuovinen et al. 1971).
Selenium is a non-metal element that is often found in two forms of selenate (Se6+, SeO42−) and selenite (Se4+, SeO32−) in agricultural soils, wastewater and metal sulfide mines. Both of these oxyanions have been of great concern due to their toxicity and bioaccumulation potential (Frankenberger Jr and Arshad 2001; Ohlendorf et al. 1986; Presser and Ohlendorf 1987; Weres et al. 1989). The result of minimum inhibitory concentration (MIC) for selenium was 156.5 mg/L and 312.5 mg/L of selenium stopped the growth of bacteria (MBC). Prior studies have shown the inhibitory effect of this metal on the growth of iron oxidizing bacteria in 100 mg/L (Tuovinen et al. 1971).
Aluminum toxicity has been described by its competition with magnesium and iron, and its binding to the DNA, membranes and cell walls in microorganisms (Piña and Cervantes 1996). The results of bacterial resistance to different concentrations of this metal showed its growth ability in solutions with aluminum concentrations above 2000 mg/L. Also, Tuovinen and et al. showed that Thiobacillus ferrooxidans can tolerate more than 10 g/L of Aluminum (Tuovinen et al. 1971). In addition, it has been reported that barium as antagonists of calcium and potassium ions can also have toxic effects on several biochemical reactions in both eukaryotic and prokaryotic cells. This toxic effect on bacteria, fungi and algae has been confirmed by different studies (Baldi et al. 1996). Acidithiobacillus ferrooxidans resistance test to barium showed high resistance to concentrations below 5000 mg/L.
Increase of the heavy metal ions in the environment may results in deactivation or even destroying the leaching bacteria. It has been reported that heavy metal ions can inhibit the microbial ferrous oxidation and stop bioleaching process by blocking or destroying the active sites of microbial enzymes. Variation in heavy metal toxicity may be due to the different interaction mechanisms between the bacteria and variable heavy metals (De et al. 1997; Navarro et al. 2013; Tabak et al. 2005). Therefore, according to the results of this study, the high metal resistance capacity in Acidithiobacillus ferrooxidans strain ZT-94 can introduce this strain as an interesting and promising candidate to be applied in bioleaching processes.
The other important parameter that has major effects on bioleaching processes is density of microbial population. Maximum efficiency of metal extraction by microbial reactions can be obtained when the bioleaching conditions correspond to the optimal growth conditions of the microorganisms. The best temperature for maximum oxidation of ferrous and sulfides by Acidithiobacillus ferrooxidans has been reported at 28 to 30 °C. The results of temperature test showed that temperatures 25–35 °C were suitable for the growth of strain ZT-94 (Table 2). Also, measuring of Eh changes as a growth indicator at different temperatures showed that Acidithiobacillus ferrooxidans strain cannot grow at 20 °C and 40 °C (Fig. 5). According to the growth rate in this temperature range, strain ZT-94 was detected as a suitable candidate for industrial processes.
The pH of the environment is a key factor in the bioleaching. It is important for the growth of the responsible bacteria for bioleaching processes and metal solubilization. Researchers have reported the pH 2–2.5 as the optimal pH for ferrous oxidation (Bosecker 1997; Tuovinen and Kelly 1974). On the other hand, the growth potential of Acidithiobacillus ferrooxidans has been reported in the range of pH 3.1–4.5. In addition, it has been reported that the pH required for dissolution of iron is less than pH 1.5. Therefore, it is necessary to adjust the pH in mentioned range to provide the optimum conditions for bioleaching (Waksman and Joffe 1922). Researchers have shown that the best chemical material to regulate pH is sulfuric acid which can simultaneously provide required sulfate for bacteria during the iron oxidation. In this study, the growth ability of strain ZT-94 was investigated at acidic pH range 1–4 regulated by sulfuric acid. According Table 3, Acidithiobacillus ferrooxidans strain ZT-94 had high ability to grow at the acidic pH and this pH range had no inhibitory effect on bacterial growth. In general, strains with the ability to grow in a wider range of pH are more favorable to use in industrial applications due to their ability to survive in harsh environments (Takeuchi and Suzuki 1994). Also, resistant bacteria to acidic pH are very valuable in the bioleaching processes.
Most of the important bioleaching bacteria are autotrophic microorganisms that their growth and iron bio-oxidation can be inhibited by organic compounds. According to this in industrial processes and heap leaching where there is a consortium of microorganisms and organic matter it can be an issue to overcome (Fang and Zhou 2006; Fournier et al. 1998; Gu and Wong 2004). Organic compounds can target enzymatic iron oxidation systems directly and react with extracellular iron (Bacelar-Nicolau and Johnson 1999; Brandl 2001; Tuttle and Dugan 1976). However, it is not possible to report a general rule for the negative impact of organic compounds on different bioleaching process as many studies have reported the positive effects of these compounds on process efficiency (Puhakka and Tuovinen 1987). In this regard, three organic compounds were selected to investigate their toxicity effect on strain ZT-94 growth. The results showed that the presence of fructose and glucose in the medium decreased the rate of iron oxidation and Eh values but the delay in bacterial growth and iron oxidation in the presence of yeast extract was clearly longer than the others. However, no complete inhibition was detected in iron oxidation and bacterial growth in the presence of these organic substances (Fig. 6). These delays are due to the time required for the bacterial adaptation to these media. Also, the Eh changes showed the inhibitory effect of yeast extract on bacterial growth. The toxicity of yeast extract was more than glucose and fructose for Acidithiobacillus ferrooxidans strain ZT-94 (Fig. 7). The ferrous oxidation rate is one of the essential factors affecting the economic efficiency of bioleaching. Hence, the factors that change the rate of iron oxidation are very important for this process (Mazuelos et al. 1999). This study confirmed that strain ZT-94 is able to tolerate these three organic substances.
Growth of the cells and ferrous ion oxidation to ferric ion by Acidithiobacillus ferrooxidans strain ZT-94 in APH medium containing organic compounds at 48 h. (1) Control sample without any organic compounds (2) APH medium with 0.1 w/v yeast extract (3) APH medium with 0.1 w/v glucose (4) APH medium with 0.1 w/v fructose
Conclusion
The bioleaching is a metal dissolution process from ores by of microorganisms. This cost effective and eco-friendly method also used to remove and mobilize the heavy metals from solid wastes. The growth and activities of microorganisms can be inhibited by the presence of toxic metals, organic compounds, high temperatures or harsh pH. Therefore, the microbial population resistance to the harsh conditions of leaching processes is very important in this process. The results of this study showed that Acidithiobacillus ferrooxidans strain ZT-94 isolated from the ore sample located in the harsh site is a suitable candidate for industrial bioleaching or bioremediation applications due to its rapid growth, high iron oxidation rate, tolerance to organic matter and high resistance to metals.
References
Atlas RM (2005) Handbook of media for environmental microbiology (2nd edn.). CRC Press. https://doi.org/10.1201/9781420037487
Bacelar-Nicolau P, Johnson DB (1999) Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures. Appl Environ Microbiol 65:585–590. https://doi.org/10.1128/AEM.65.2.585-590.1999
Bahrami-Bavani M, Kasra-Kermanshahi R, Tajer-Mohammad-Ghazvini P (2017) Study of the resistance of Acidithiobacillus ferrooxidans strain ZT-94 to uranium and its importance in bioremediation process. The 4th International Conference on Environmental Planning and Management. https://doi.org/10.1007/s10967-019-06819-9
Baldi F, Pepi M, Burrini D, Kniewald G, Scali D, Lanciotti E (1996) Dissolution of Barium from Barite in Sewage Sludges and Cultures of Desulfovibrio desulfuricans. Appl Environ Microbiol 62:2398–2404
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20:591–604. https://doi.org/10.1111/j.1574-6976.1997.tb00340.x
Brandl H (2001) Microbial leaching of metals. Biotechnology 10:191–224
Brierley CL (1982) Microbiological mining. Sci Am 247:44–53 https://www.jstor.org/stable/24966658
Cabrera G, Gomez J, Cantero D (2005) Kinetic study of ferrous sulphate oxidation of Acidithiobacillus ferrooxidans in the presence of heavy metal ions. Enzym Microb Technol 36:301–306. https://doi.org/10.1016/j.enzmictec.2004.09.008
Chen S-Y, Chou L-C (2016) Relationship between microbial community dynamics and process performance during thermophilic sludge bioleaching. Environ Sci Pollut Res 23:16006–16014. https://doi.org/10.1007/s11356-016-6716-z
De Vos C, Schat H (1991) Free radicals and heavy metal tolerance. In: Ecological responses to environmental stresses. Springer, pp. 22–31. https://doi.org/10.1007/978-94-009-0599-3_3
De GC, Oliver D, Pesic B (1997) Effect of heavy metals on the ferrous iron oxidizing ability of Thiobacillus ferrooxidans. Hydrometallurgy 44:53–63. https://doi.org/10.1016/S0304-386X(96)00030-8
Diels L, De Smet M, Hooyberghs L, Corbisier P (1999) Heavy metals bioremediation of soil. Mol Biotechnol 12:149–158. https://doi.org/10.1385/0-89603-437-2:283
Falagán C, Johnson DB (2016) Acidithiobacillus ferriphilus sp. nov., a facultatively anaerobic iron-and sulfur-metabolizing extreme acidophile. Int J Syst Evol Microbiol 66:206. https://doi.org/10.1099/ijs.0.049759-0
Fang D, Zhou L (2006) Effect of sludge dissolved organic matter on oxidation of ferrous iron and sulfur by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Water Air Soil Pollut 171:81–94. https://doi.org/10.1007/s11270-005-9014-9
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Fournier D, Lemieux R, Couillard D (1998) Essential interactions between Thiobacillus ferrooxidans and heterotrophic microorganisms during a wastewater sludge bioleaching process. Environ Pollut 101:303–309. https://doi.org/10.1016/S0269-7491(98)00035-9
Frankenberger WT Jr, Arshad M (2001) Bioremediation of selenium-contaminated sediments and water. Biofactors 14:241–254. https://doi.org/10.1002/biof.5520140130
Gao X-Y et al (2020) Novel strategy for improvement of the bioleaching efficiency of Acidithiobacillus ferrooxidans based on the AfeI/R quorum sensing system. Minerals 10:222. https://doi.org/10.3390/min10030222
Gu X, Wong JW (2004) Identification of inhibitory substances affecting bioleaching of heavy metals from anaerobically digested sewage sludge. Environ Sci Technol 38:2934–2939. https://doi.org/10.1021/es0347134
Happold F, Johnstone K, Rogers H, Youatt JB (1954) The isolation and characteristics of an organism oxidizing thiocyanate. Microbiology 10:261–266. https://doi.org/10.1099/00221287-10-2-261
Irawati W, Wijaya Y, Christian S, Djojo ES (2016) Characterization of heavy metals resistant yeast isolated from activated sludge in Rungkut, Surabaya, Indonesia as biosorbent of mercury, copper, and lead. In: AIP Conference Proceedings, vol 1. AIP Publishing LLC, p 020061. https://doi.org/10.1063/1.4953535
Johnson DB, Hallberg KB (2007) Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms. In: Biomining. Springer, pp. 237–261. https://doi.org/10.1007/978-3-540-34911-2_12
Kanwar P, Mishra T, Mukherjee G (2017) Microbial bioremediation of hazardous heavy metals. In: Bioremediation and Sustainable Technologies for Cleaner Environment. Springer, pp. 281–293. https://doi.org/10.1007/978-3-319-48439-6_21
Karamanev DG, Nikolov LN, Mamatarkova V (2002) Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner Eng 15:341–346. https://doi.org/10.1016/S0892-6875(02)00026-2
Kasra-Kermanshahi R, Bahrami-Bavani M, Tajer-Mohammad-Ghazvini P (2017) Evaluation of molybdenum resistance of Acidithiobacillus ferrooxidans strain ZT-94: a valuable ability in biotechnological applications. In 18th International Iranian Congress of Microbiology, School of Medicine, Tehran University of Medical Sciences, 29–31 August 2017
Kasra-Kermanshahi R, Tajer-Mohammad-Ghazvini P, Bahrami-Bavani M (2020) A biotechnological strategy for molybdenum extraction using Acidithiobacillus ferrooxidans. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-020-03468-7
Kelly DP (1971) Autotrophy: concepts of lithotrophic bacteria and their organic metabolism. Annu Rev Microbiol 25:177–210. https://doi.org/10.1146/annurev.mi.25.100171.001141
Kim OS et al (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. https://doi.org/10.1099/ijs.0.038075-0
Liu J-S, Zhang Y-H, Li B-M, Xie X-H (2006) The study kinetic for growth of Acidithiobacillus ferrooxidans. J Microbiol 26:9–13
Liu K et al. (2020) Simultaneous disinfection of E. faecalis and degradation of carbamazepine by sulfate radicals: an experimental and modelling study. Environ Pollut 114558. https://doi.org/10.1016/j.envpol.2020.114558
Lovley DR, Coates JD (1997) Bioremediation of metal contamination. Curr Opin Biotechnol 8:285–289
Mazuelos A, Iglesias N, Carranza F (1999) Inhibition of bioleaching processes by organics from solvent extraction. Process Biochem 35:425–431. https://doi.org/10.1016/S0032-9592(99)00065-5
Merroun ML, Selenska-Pobell S (2001) Interactions of three eco-types of Acidithiobacillus ferrooxidans with U (VI). Biometals 14:171–179. https://doi.org/10.1023/A:1016658209397
Min G, Li M-M, Jian Z, Liu X-X, Zhu J-Y, Hu Y-H, Qiu G-Z (2017) Acidithiobacillus ferrooxidans enhanced heavy metals immobilization efficiency in acidic aqueous system through bio-mediated coprecipitation. Trans Nonferrous Metals Soc China 27:1156–1164. https://doi.org/10.1016/S1003-6326(17)60135-3
Mishra M, Mohan D (2017) Bioremediation of contaminated soils: an overview. In: adaptive soil management: from theory to practices. Springer, pp 323-337. https://doi.org/10.1007/978-981-10-3638-5_16
Mohapatra RK, Srichandan H, Mishra S, Parhi PK (2019) Native soil bacteria: potential agent for bioremediation. Soil microenvironment for bioremediation and polymer production 17-34. https://doi.org/10.1002/9781119592129
Navarro CA, von Bernath D, Jerez CA (2013) Heavy metal resistance strategies of acidophilic bacteria and their acquisition: importance for biomining and bioremediation. Biol Res 46:363–371. https://doi.org/10.4067/S0716-97602013000400008
Nemati M, Harrison S, Hansford G, Webb C (1998) Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: a review on the kinetic aspects. Biochem Eng J 1:171–190. https://doi.org/10.1016/S1369-703X(98)00006-0
Ngom B, Liang Y, Liu Y, Yin H, Liu X (2015) Use of an acidophilic yeast strain to enable the growth of leaching bacteria on solid media. Arch Microbiol 197:339–346. https://doi.org/10.1007/s00203-014-1051-6
Ohlendorf HM, Hoffman DJ, Saiki MK, Aldrich TW (1986) Embryonic mortality and abnormalities of aquatic birds: apparent impacts of selenium from irrigation drainwater. Sci Total Environ 52:49–63. https://doi.org/10.1016/0048-9697(86)90104-X
Olson G, Brierley J, Brierley C (2003) Bioleaching review part B. Appl Microbiol Biotechnol 63:249–257. https://doi.org/10.1007/s00253-003-1404-6
Piña RG, Cervantes C (1996) Microbial interactions with aluminium. Biometals 9:311–316. https://doi.org/10.1007/BF00817932
Piroeva I, Atanassova-Vladimirova S, Dimowa L, Sbirkova H, Radoslavov G, Hristov P, Shivachev BL (2013) A simple and rapid scanning electron microscope preparative technique for observation of biological samples: application on bacteria and DNA samples. Bulg Chem Commun 45:510–515
Presser TS, Ohlendorf HM (1987) Biogeochemical cycling of selenium in the San Joaquin Valley, California, USA. Environ Manag 11:805–821. https://doi.org/10.1007/BF01867247
Puhakka J, Tuovinen OH (1987) Effect of organic compounds on the microbiological leaching of a complex sulphide ore material. MIRCEN J Appl Microbiol Biotechnol 3:429–436. https://doi.org/10.1007/BF00935701
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A. Appl Microbiol Biotechnol 63:239–248. https://doi.org/10.1007/s00253-003-1448-7
Ruiz L, Castro M, Barriga A, Jerez C, Guiliani N (2012) The extremophile Acidithiobacillus ferrooxidans possesses ac-di-GMP signalling pathway that could play a significant role during bioleaching of minerals. Lett Appl Microbiol 54:133–139. https://doi.org/10.1111/j.1472-765X.2011.03180.x
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Satyavathi C, Craig RA, Kai-Uwe U, Daniel EG, Bradley MT (2008) Indirect UO2 oxidation by Mn(II)-oxidizing spores of Bacillus sp. strain SG-1 and the effect of U and Mn concentrations. Environ Sci Technol 42:8709–8714
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol 77:642–647
Soares EV, Soares HM (2012) Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: a review. Environ Sci Pollut Res 19:1066–1083. https://doi.org/10.1007/s11356-011-0671-5
Srichandan H, Mohapatra RK, Parhi PK, Mishra S (2019) Bioleaching: a bioremediation process to treat hazardous wastes. Soil microenvironment for bioremediation and polymer production 115-129. https://doi.org/10.1002/9781119592129
Srivastava S, Agrawal S, Mondal M (2015) A review on progress of heavy metal removal using adsorbents of microbial and plant origin. Environ Sci Pollut Res 22:15386–15415. https://doi.org/10.1007/s11356-015-5278-9
Tabak HH, Lens P, van Hullebusch ED, Dejonghe W (2005) Developments in bioremediation of soils and sediments polluted with metals and radionuclides–1. Microbial processes and mechanisms affecting bioremediation of metal contamination and influencing metal toxicity and transport. Rev Environ Sci Biotechnol 4:115–156. https://doi.org/10.1007/s11157-005-2169-4
Tajer Mohammad Ghazvini P, Kasra Kermanshahi R, Nozad Golikand A, Sadeghizadeh M (2014) Isolation and Characterization of a Novel Magnetotactic Bacterium from Iran: Iron Uptake and Producing Magnetic Nanoparticles in Alphaproteobacterium MTB-KTN90 Jundishapur. J Microbiol 7:e19343. https://doi.org/10.5812/jjm.19343
Takeuchi TL, Suzuki I (1994) Effect of pH on sulfite oxidation by Thiobacillus thiooxidans cells with sulfurous acid or sulfur dioxide as a possible substrate. J Bacteriol 176:913–916
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101:11030–11035 https://www.jstor.org/stable/3372847
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tuovinen OH, Kelly DP (1973) Studies on the growth of Thiobacillus ferrooxidans. Arch Microbiol 88:285–298. https://doi.org/10.1007/BF00409941
Tuovinen OH, Kelly DP (1974) Studies on the growth of Thiobacillus ferrooxidans. II. Toxicity of uranium to growing cultures and tolerance conferred by mutation, other metal cations and EDTA. Arch Mikrobiol 95:153–164. https://doi.org/10.1007/BF02451757
Tuovinen OH, Niemelä S, Gyllenberg H (1971) Tolerance of Thiobacillus ferrooxidans to some metals. Antonie Van Leeuwenhoek 37:489–496. https://doi.org/10.1007/BF02218519
Tuttle JH, Dugan PR (1976) Inhibition of growth, iron, and sulfur oxidation in Thiobacillus ferrooxidans by simple organic compounds. Can J Microbiol 22:719–730. https://doi.org/10.1139/m76-105
Valdés J et al (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9:597–597. https://doi.org/10.1186/1471-2164-9-597
Valls M, de Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26:327–338. https://doi.org/10.1111/j.1574-6976.2002.tb00618.x
VanEngelen MR, Szilagyi RK, Gerlach R, Lee BD, Apel WA, Peyton BM (2011) Uranium exerts acute toxicity by binding to pyrroloquinoline quinone cofactor. Environ Sci Technol 45:937–942. https://doi.org/10.1021/es101754x
Venugopal R (2005) Mineral processing technology Mpt-2005. McGraw-Hill Education (India) Pvt Limited. https://books.google.com/books?id=zujA_cdC-msC
Vera M, Schippers A, Sand W (2013) Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl Microbiol Biotechnol 97:7529–7541
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Volesky B (1994) Advances in biosorption of metals: selection of biomass types. FEMS Microbiol Rev 14:291–302
Waksman SA, Joffe J (1922) Microörganisms concerned in the oxidation of sulfur in the soil: II. Thiobacillus Thiooxidans, a new sulfur-oxidizing organism isolated from the soil 1. J Bacteriol 7:239. https://doi.org/10.1128/jb.7.2.239-256.1922
Weres O, Jaouni A-R, Tsao L (1989) The distribution, speciation and geochemical cycling of selenium in a sedimentary environment, Kesterson Reservoir, California, USA. Appl Geochem 4:543–563. https://doi.org/10.1016/0883-2927(89)90066-8
Xiao R, He L, Luo Z, Spinney R, Wei Z, Dionysiou DD, Zhao F (2020) An experimental and theoretical study on the degradation of clonidine by hydroxyl and sulfate radicals. Sci Total Environ 710:136333. https://doi.org/10.1016/j.scitotenv.2019.136333
Yang Z, Zhang Z, Chai L, Wang Y, Liu Y, Xiao R (2016) Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90. J Hazard Mater 301:145–152. https://doi.org/10.1016/j.jhazmat.2015.08.047
Yates JR, Holmes DS (1987) Two families of repeated DNA sequences in Thiobacillus ferrooxidans. J Bacteriol 169:1861–1870
Yong NK, Oshima M, Blake RC II, Sugio T (1997) Isolation and some properties of an iron-oxidizing bacterium Thiobacillus ferrooxidans resistant to molybdenum ion. Biosci Biotechnol Biochem 61:1523–1526. https://doi.org/10.1271/bbb.61.1523
Zare Tavakoli H, Abdollahy M, Ahmadi S, Khodadadi Darban A (2017) Kinetics of uranium bioleaching in stirred and column reactors. Miner Eng 111:36–46. https://doi.org/10.1016/j.mineng.2017.06.003
Zhan Y, Yang M, Zhang S, Zhao D, Duan J, Wang W, Yan L (2019) Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans. World J Microbiol Biotechnol 35:60. https://doi.org/10.1007/s11274-019-2632-y
Zhang S, Yan L, Xing W, Chen P, Zhang Y, Wang W (2018) Acidithiobacillus ferrooxidans and its potential application. Extremophiles 22:563–579. https://doi.org/10.1007/s00792-018-1024-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavakoli, H.Z., Bahrami-Bavani, M., Miyanmahaleh, Y. et al. Identification and characterization of a metal-resistant Acidithiobacillus ferrooxidans as important potential application for bioleaching. Biologia 76, 1327–1337 (2021). https://doi.org/10.1007/s11756-021-00687-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00687-z