Abstract
Background
Thoracic endometriosis syndrome gives rise to various clinical and radiological manifestations. We reviewed the records of patients operated for intrathoracic migration of abdominal viscera through a diaphragmatic hernia secondary to thoracic endometriosis.
Methods
We retrospectively reviewed the single-center prospective collected database of all patients operated for thoracic endometriosis during the twenty years. All cases in which an abdominal organ was found to be herniated into the thoracic cavity were retrieved. Clinical and pathological data are presented and analyzed.
Results
Twenty women of median age 36 (range 25–58) years were operated for endometriosis-related diaphragmatic hernia. The hernia was diagnosed concomitantly with endometriosis-related pneumothorax in 13 cases and during the exploration of catamenial thoracic pain in seven cases. There were 18 cases on the right side and two cases on the left side. The median diameter of the hernia was 8 cm (2.5–20 cm). In seventeen cases, the hernia was repaired by direct suture, and in three cases a heterologous prosthesis was positioned. At follow-up, two patients had an episode of recurrent pneumothorax.
Conclusions
Diaphragmatic hernia should be ruled out in the presence of endometriosis-related pneumothorax or catamenial thoracic pain. Surgery is indicated to make a pathological diagnosis, restore anatomy, and prevent recurrence in patients presenting with pneumothorax.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of endometriosis in the thorax may give rise to a large spectrum of clinical and radiological manifestations that constitute thoracic endometriosis syndrome (TES) [1]. Although thoracic dissemination is considered a rare event in the course of the disease, the thorax is the second most common site of extragonadal endometriosis [2]. In their historic publication collecting all published cases of TES in the English-language literature before 1995 [3], Joseph and Sahan described the syndrome as being characterized by four main clinical and/or radiological entities, namely: pneumothorax, hemothorax, hemoptysis, and lung nodules. These manifestations typically occur during the period of menses and are termed “catamenial”, though they may also be observed outside this particular period and to avoid misunderstanding the term “endometriosis related” can also be employed [4].
Endometriosis-related pneumothorax is the most common clinical presentation of TES, accounting for approximately 70% of cases, and has been widely studied in several prospective series exploring the clinical and pathological characteristics of spontaneous pneumothorax in women [5, 6]. On the other hand, the other manifestations of TES, because of their rarity, have mostly been published as case reports or small series, except for a 2017 single-center surgical study exploring clinical manifestations of TES other than pneumothorax which presented 21 cases of isolated catamenial thoracic pain, 5 cases of pleural effusion (including hemothorax), and 6 cases of endometriosis-related diaphragmatic hernia [7].
Although right diaphragmatic lesions in the form of nodules or holes have been a topic in TES since they were described in 1958 by Maurer et al. [8], the presence of large defects leading to intrathoracic migration of abdominal viscera is a recent observation in the medical literature and, as far as we could determine, the first case describing a diaphragmatic hernia secondary to endometriosis was published only in 2007 [9]; a 2021 literature review has since reported fourteen cases [10].
With a view to better understanding this particular clinical manifestation of TES, we reviewed all patients operated for TES in our department. The clinical and pathological data of patients presenting intrathoracic migration of abdominal viscera through an endometriosis-related diaphragmatic hernia were retrieved and analyzed.
Methods
The study included all patients operated on between December 2003 and March 2023 at the thoracic surgery department of the Hospital Hôtel-Dieu/Cochin, Paris, France. Since December 2003, all cases of proven or suspected thoracic endometriosis observed in our department have been prospectively collected in a database. Institutional review board approval to use data from the database was obtained from the French Thoracic Society Ethics Committee (CERC-SFCTCV-53 MaAl). All patients included in the database signed an informed consent.
Surgical reports of all operated cases of TES were reviewed and checked for intraoperative ascertainment of intrathoracic migration of abdominal viscera through a diaphragmatic hernia. Those surgical reports describing the presence of a few or numerous holes (perforations) in the tendinous portion of the diaphragm and communicating with the abdominal cavity, as shown by Forster et al. [11], but without the presence of intrathoracic migration of an abdominal organ, were excluded from the study. The exclusion was also applied in the absence of a pathological diagnosis of pelvic, abdominal, or thoracic endometriosis.
In all included cases, we noted the intraoperative presence of associated lesions on the diaphragm, in the form of nodules, holes, or cysts, as well as the presence of pulmonary parenchymal lesions such as blebs or bullae. Details of gynecological history in terms of medical or surgical interventions and the presence of pathologically proven abdominal or pelvic endometriosis were collected. Previous proven or suspected thoracic endometriosis and previous surgical thoracic intervention were noted. Pathologic diagnosis of thoracic endometriosis was based on either the association of endometrial stroma and glands or endometrial stroma alone with positive staining for CD10 and estrogen/progesterone receptors. The size of the diaphragmatic defects and the type of herniated organ were collected. Follow-up was completed by a telephone call in Mars 2023. The data on six patients operated before August 2016 were published previously [7].
General rules of surgical technique in the case of endometriosis-related diaphragmatic hernia
In our department, surgical treatment of patients with proven or suspected TES is based on standard rules. In all cases, surgery is performed with the patient in the standard posterolateral position. The intervention begins with a video-assisted evaluation of the whole pleural cavity through a trocar for the camera visualization positioned in the anterior axillary line at the sixth intercostal space. The visceral and parietal pleura are fully explored, and all visible foci of presumed endometriosis (nodular or cystic brown lesions, isolated pleural thickening, or blackish area) are completely removed. The diaphragm is completely inspected, and all lesions are noted. When the diagnosis of diaphragmatic hernia and intrathoracic migration of an abdominal organ is established, posterior thoracotomy at the level of the eighth intercostal space is performed. The first step is the intra-abdominal repositioning of the herniated organ by section of the inflammatory adhesions which are always present between the diaphragm and the abdominal organ. The second step is the circumferential excision of the edges of the hernia and, if present, of all other visible diaphragmatic lesions such as nodules, cysts or holes. Before diaphragmatic reconstruction, the liver must be released by sectioning the coronal and triangular ligament on the peritoneal surface of the diaphragm. This allows tension-free repair by non-absorbable U-shaped separate stitches without the need (in almost all cases) for a prosthesis. In patients with a history of pneumothorax, a pleural symphysis is induced by talc insufflation. Postoperative pain is controlled via a paravertebral catheter and all patients are referred to the gynecology team for evaluation of a medical treatment. Holistic evaluation taking into account previous treatments and tolerability, the presence of residual disease, severity of symptoms, and possibly the desire for pregnancy is considered before prescribing individual treatment.
Results
During the twenty-year period of the study in the database collecting all cases of TES there were 114 cases of pathologically proven endometriosis-related pneumothorax (catamenial or not), 73 cases of catamenial pneumothorax with no pathological proof of thoracic endometriosis, 52 cases of catamenial thoracic pain (operated or not) and 10 cases of endometriosis-related pleural effusion, including hemothorax. Among the 187 patients with pneumothorax associated with TES, 13 had diaphragmatic hernia and among the 52 patients observed with catamenial thoracic pain, diaphragmatic the hernia was finally found in 7 patients. So, these 20 patients represent 8.03% of the 249 patients surgically managed for a manifestation of TES in our department in the time frame of the study. These 20 patients represent the study population.
Clinical and pathological data are listed in Table 1. The median age of patients was 36 years (range 27–58). Interestingly, in the resected specimen of the 58-year-old patient, endometrial stroma and glands with positive staining for progesterone receptors were found.
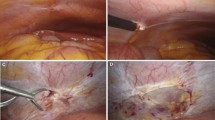
Among the 13 patients with diaphragmatic hernia found concurrently with endometriosis-related spontaneous pneumothorax, seven were seen at their first episode of pneumothorax. In seven cases it was the first episode of spontaneous pneumothorax (which was bilateral synchronous in one case, Fig. 1), whereas among the other 6 patients, a previous surgical intervention for pneumothorax was recorded, in five cases on the same side as the hernia, and in one case on the other one. Among the five patients previously operated on the same side as the hernia, the surgical intervention included the resection of nodules and holes in the diaphragm in three cases, whereas in two cases previous surgery had consisted of isolated resection of pulmonary blebs or bullae.
Among the seven other patients with diaphragmatic hernia found at work-up of catamenial thoracic pain, one had a history of laser cauterization of implants of the abdominal aspect of the diaphragm during operation for abdominopelvic endometriosis, and another had a history of pleurodesis for a pneumothorax considered idiopatic 20 years previously.
The diagnosis was established by a diaphragmatic MRI in six cases (Fig. 2) and by a CT scan in the remaining one.
At the time of diagnosis of diaphragmatic hernia, a previous clinical or pathological diagnosis of abdominopelvic endometriosis, but without any clinical or radiological suspicion of thoracic endometriosis, was present in 10/20 patients. On the other hand, there were four patients with previously proven pathological thoracic endometriosis but without any previous clinical or pathological history of abdominopelvic endometriosis. In 4 patients, there was no previous clinical, radiological, or pathological diagnosis of either abdominopelvic or thoracic endometriosis. Finally, two patients had a history of abdominopelvic and thoracic endometriosis.
On questioning, all patients reported catamenial basal-thoracic pain with radiation to the scapula and neck. At the time of diagnosis, dyspnea was observed in six patients: two patients with complete pneumothorax, two patients with large herniation of abdominal organs, one case with hemopneumothorax, and one another case with concomitant bilateral pneumothorax, respectively. All patients but one with pneumothorax had a preoperative CT scan, and the suspicion of abdominal organ herniation could be established because of the presence of abnormal convexity on the burden of the diaphragm in 12/13 cases; in the remaining one the hernia was discovered intraoperatively.
On the right side the liver was always herniated into the thoracic cavity and in one case, characterized by a large diaphragmatic defect, a segment of the transverse colon was also found. The greater omentum and a part of the stomach fundus were noted in both cases with the hernia on the left side.
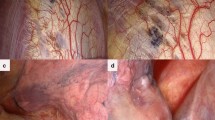
At surgery, the median size of the defect was 8 cm (range 2.5–22). In 10 patients, a single diaphragmatic defect was the sole perioperative finding, whereas in 10 cases there were two or more diaphragmatic defects. In eight cases there were associated diaphragmatic and/or pleural nodules (Fig. 3).
Reconstruction of the diaphragm was performed by a direct suture in 17 cases and by the interposition of a prosthetic mesh in three. Talc insufflation was done in the 13 patients presenting with pneumothorax. At pathologic examination, endometriosis was found in 18 patients on the edges of diaphragmatic resection; the remaining two pathological diagnoses of endometriosis had been obtained in previous operations for abdominopelvic endometriosis.
The median hospital stay was 6 days (range 5–12). There were two cases of postoperative air leaks. Long-term follow-up showed no recurrence of hernia, but pneumothorax recurrence in two patients; both underwent successful iterative talc insufflation.
Discussion
This study shows that in women of childbearing age or even older, as observed in one case and previously reported in the literature [12], TES may be responsible for a diaphragmatic hernia with an intrathoracic migration of abdominal organs. As for endometriosis-related pneumothorax, a high predominance on the right thoracic side was observed, which led us to speculate that pathophysiological mechanisms leading to endometriosis-related pneumothorax and/or hernia share some common pathways [13, 14]. A recent study on laparoscopic bilateral diaphragm inspection of women with abdominal-pelvic endometriosis has shown a prevalence of the diaphragmatic disease in 5% of the study population, a high predominance at 94% on the right side and its association with specific diaphragmatic pain (basal-thoracic with radiation into the homolateral scapula and shoulder) [15]. In endometriosis, the cyclic phases of proliferation and desquamation, associated with local chronic inflammation as well as immune evasion, lead to irreversible modification of normal anatomy [16]. Typically, thoracoscopic findings of patient operated for endometriosis-related pneumothorax are diaphragmatic nodules and small perforations, and it can be postulated that the diaphragmatic hernia is the consequence of severe disease secondary to the coalescence of these lesions. On the other hand, it cannot be excluded that in some cases the diaphragm weakened by endometriosis could undergo a spontaneous rupture favored by a trans diaphragmatic pressure gradient.
Among those patients in whom the hernia was diagnosed concomitantly with pneumothorax, there were four cases without any previous gynecological or thoracic pathologic proof of endometriosis, and in seven other cases, the hernia was diagnosed at the time of the first episode of pneumothorax. On the other hand, in six of the seven patients in whom the hernia was discovered at the time of exploration of catamenial chest pain, there was no previous proof of thoracic endometriosis. Such findings show that the hernia could form without any abdominal or thoracic symptoms other than catamenial thoracic pain.
The discovery of a diaphragmatic hernia must be considered a formal indication for surgery. In 80% of our patients, the reconstruction of the diaphragm was performed by direct suture and with this objective it is important to divide the coronal and triangular ligaments of the liver, otherwise in the presence of a large defect the positioning of a heterologous prosthesis is needed. In our experience, a PTFE mesh gives us the best results. An observational follow-up study conducted by Redwine suggested that full-thickness resection of the diaphragm and repair performed at laparotomy improved symptoms in 100% of patients and procured a complete treatment of symptoms in 88% of patients without postoperative medical therapy after a follow-up of 7 years [17].
In all cases of endometriosis-related pneumothorax, we induce pleural symphysis with talc, unless we observe two recurrences of a pneumothorax that was newly operated. It is our policy that the prescription of drugs after surgery is under the responsibility of the gynecology team and is decided based on a tailored discussion with the patient, including the considerations regarding private and social life. Inhibition of ovarian function for a period of six months after surgery in endometriosis-related pneumothorax is generally indicated to reduce the risk of recurrence. Gonadotropin-releasing hormone analogues or continuous oral progesterone are evaluated but may not be tolerated and/or strongly interfere with the quality of life of these young women [18,19,20].
Despite recent numerous articles on TES, reports on endometriosis-related diaphragmatic hernia are rare [21]. We can propose two explanations for the large number of patients operated on in our center, namely the fact that ours is a referral center for thoracic endometriosis, and, probably more importantly, our policy of prescribing specific imaging (ideally MRI) when caring for patients with catamenial thoracic pain [22, 23]. We believe that timely diagnosis of thoracic endometriosis, when the presentation is pain, leads to the rapid specific treatment of diaphragmatic endometriosis and management of complications (including hernia), thereby avoiding more important events, such as strangulation of a herniated hollow viscus.
In conclusion, diaphragmatic herniation of abdominal organs is a possible manifestation in TES and may be observed concomitantly with pneumothorax or discovered during the work-up of catamenial thoracic pain. Surgery is fundamental in making a pathological diagnosis, resection of all visible implants, restoring anatomy, and, in patients presenting with pneumothorax, preventing the recurrence of pneumothorax.
Data availability
Data are available on demand to the corresponding author.
References
Alifano M, Trisolini R, Cancellieri A, Regnard JF. Thoracic endometriosis: current knowledge. Ann Thorac Surg. 2006;81:761–9.
Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193–219.
Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996;100:164–70.
Alifano M, Jablonski C, Kadiri H, Falcoz P, Gompel A, Camilleri-Broet S, et al. Catamenial and noncatamenial, endometriosis-related or nonendometriosis-related pneumothorax referred for surgery. Am J Respir Crit Care Med. 2007;176:1048–53.
Legras A, Mansuet-Lupo A, Rousset-Jablonski C, Bobbio A, Magdeleinat P, Roche N, et al. Pneumothorax in women of child-bearing age: an update classification based on clinical and pathologic findings. Chest. 2014;145:354–60.
Fukuoka M, Kurihara M, Haga T, Ebana H, Kataoka H, Mizobuchi T, et al. Clinical characteristics of catamenial and non-catamenial thoracic endometriosis-related pneumothorax. Respirology. 2015;20:1272–6.
Bobbio A, Canny E, Mansuet-Lupo A, Lococo F, Legras A, Magdeleinat P, et al. Thoracic endometriosis syndrome other than pneumothorax: clinical and pathological findings. Ann Thorac Surg. 2017;104:1865–71.
Maurer ER, Schaal JA. Mendez FL Chronic recurring spontaneous pneumothorax due to endometriosis of the diaphragm. J Am Med Assoc. 1958;168:2013–4.
Bobbio A, Carbognani P, Ampollini L, Rusca M. Diaphragmatic laceration, partial liver herniation and catamenial pneumothorax. Asian Cardiovasc Thorac Ann. 2007;15:249–51.
Arakawa S, Matsudaira H, Noda Y, et al. Catamenial pneumothorax with partial liver herniation due to diaphragmatic laceration: a case report and literature review. J Cardiothorac Surg. 2021;16:23.
Forster C, Bénière C, Lattion J, et al. Evolutive diaphragmatic lesions causing recurrent catamenial pneumothorax. Thorax. 2022;77:105.
Yukumi S, Suzuki H, Morimoto M, Shigematsu H, Sugimoto R, Sakao N, Abe M, Watanabe A, Kitazawa S, Sano Y. A case of thoracic endometriosis-related pneumothorax in a menopausal woman. Gen Thorac Cardiovasc Surg. 2020;68(12):1584–6. https://doi.org/10.1007/s11748-020-01381-8.
Vercellini P, Abbiati A, Viganò P, et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum Reprod. 2007;22(9):2359–67.
Saito T, Saito Y, Fukumoto KJ, Matsui H, Nakano T, Taniguchi Y, Kaneda H, Konobu T, Tsuta K, Murakawa T. Clinical and pathological characteristics of spontaneous pneumothorax in women: a 25-year single-institutional experience. Gen Thorac Cardiovasc Surg. 2018;66(9):516–22. https://doi.org/10.1007/s11748-018-0952-8.
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;17:S1553–4650(23)00007–9.
Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–56.
Redwine DB. Diaphragmatic endometriosis: diagnosis, surgical management, and long-term results of treatment. Fertil Steril. 2002;77(2):288–96.
Campisi A, Ciarrocchi AP, Grani G, Sanna S, Congiu S, Mazzarra S, Argnani D, Salvi M, Stella F. The importance of diaphragmatic surgery, chemical pleurodesis and postoperative hormonal therapy in preventing recurrence in catamenial pneumothorax: a retrospective cohort study. Gen Thorac Cardiovasc Surg. 2022;70(9):818–24. https://doi.org/10.1007/s11748-022-01802-w.
Ciriaco P, Muriana P, Lembo R, Carretta A, Negri G. Treatment of thoracic endometriosis syndrome: a meta-analysis and review. Ann Thorac Surg. 2022;113(1):324–36.
Blair Marshall M, Ahmed Z, Kucharczuk JC, et al. Catamenial pneumothorax: optimal hormonal and surgical management. Eur J Cardiothorac Surg. 2005;27:662–6.
Yu PSY, Sihoe ADL. Beware the “raised right hemidiaphragm” in a female patient with previous pneumothorax surgery: liver herniation through a massive endometrosis-related diaphragmatic fenestration. J Thorac Dis. 2015;7:112–6.
Rousset P, Rousset-Jablonski C, Alifano M, et al. Thoracic endometriosis syndrome: CT and MRI features. Clin Radiol. 2014;69:323–30.
Picozzi G, Beccani D, Innocenti F, et al. MRI features of pleural endometriosis after catamenial haemothorax. Thorax. 2007;62:744.
Funding
No funding has been received for the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bobbio, A., Gherzi, L., Tormen, F. et al. A surgical series on endometriosis-related diaphragmatic hernia. Gen Thorac Cardiovasc Surg 72, 668–673 (2024). https://doi.org/10.1007/s11748-024-02016-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-024-02016-y