Abstract
Objective
We assessed the clinical effectiveness of coronary artery bypass grafting (CABG) in comparison with that of percutaneous coronary intervention (PCI) in octogenarians with triple-vessel disease (TVD) or left main coronary artery (LMCA) disease.
Methods
From the CREDO-Kyoto registry cohort-2, 527 patients, who were ≥ 80 years of age and underwent the first coronary revascularization for TVD or LMCA disease, were divided into the CABG group (N = 151) and the PCI group (N = 376).
Results
The median and interquartile range of patient’s age was 82 (81–84) in the CABG group and 83 (81–85) in the PCI group (P = 0.10). Patients > = 85 years of age accounted for 19% and 31% in the CABG and PCI groups, respectively (P = 0.01).
The cumulative 5-year incidence of all-cause death was similar between CABG and PCI groups (35.8% vs. 42.9%, log-rank P = 0.18), while CABG showed a lower rate of the composite of cardiac death/MI than PCI (21.7% vs. 33.9%, log-rank P = 0.005). After adjusting for confounders, the lower risk of CABG relative to PCI was significant for all-cause death (HR 0.61, 95% CI 0.43–0.86, P = 0.005), any coronary revascularization (HR 0.25, 95% CI 0.14–0.43, P < 0.001) and the composite of cardiac death/MI (HR 0.52, 95% CI 0.32–0.85, P = 0.009).
Conclusions
CABG compared with PCI was associated with a lower adjusted risk for all-cause death, any coronary revascularization, and a composite of cardiac death/MI in very elderly patients with TVD or LMCA disease. CABG seemed an acceptable option for selected octogenarians with severe coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reflecting the aging societies, there is an increasing number of older patients who undergo percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) [1]. Based on the randomized trials, CABG rather than PCI has been recommended as the first-line therapy in patients with complex coronary artery disease [2]. However, very old patients were often excluded from the randomized controlled trials comparing PCI with CABG. In the daily clinical practice, PCI is often selected in older patients with complex multiple-vessel disease, because they prefer less invasive procedure and shorter hospitalization. However, there is a scarcity of reports on the clinical outcomes comparing PCI versus CABG in elderly patients.
Subjects
we sought to compare the 5-year outcomes of PCI or CABG in patients > = 80 years of age with triple-vessel disease (TVD) and/or left main coronary artery (LMCA) disease in a large observational database of patients with first coronary revascularization in Japan.
Methods
Study population
As previously described in detail, the CREDO-Kyoto PCI/CABG Registry Cohort-2 is a physician-initiated non-company-sponsored multicenter registry enrolling consecutive patients who underwent first coronary revascularization among 26 centers in Japan from January 2005 to December 2007 [3].
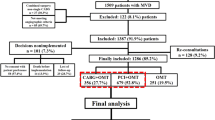
The relevant ethics committees in all 26 participating centers (Supplementary appendix A) approved the research protocol. Because of retrospective enrollment, written informed consents from the patients were waived; however, we excluded those patients who refused participation in the study when contacted for follow-up. This strategy is concordant with the guidelines of the Japanese Ministry of Health, Labor, and Welfare. Of 15,939 patients enrolled in the registry, we excluded 99 patients who refused to participate in the study, 609 patients with concomitant non-coronary surgery, 4892 patients with acute myocardial infarction presentation, 9023 patients < 80 years of age, and 789 patients with single- or double-vessel disease. As a result, the study population for the current analysis consisted of 527 patients ≥ 80 years of age with TVD or LMCA disease (CABG group: 151 patients/PCI group: 376 patients) (Fig. 1).
Endpoints
The primary outcome measure in the current study was all-cause death. The secondary outcome measures included a composite of cardiac death and MI, cardiac death, non-cardiac death, MI, stroke, hospitalization for heart failure, major bleeding after 30 day, and any coronary revascularization. Death was regarded as cardiac in origin unless obvious non-cardiac causes could be identified. MI was defined according to the definition in the Arterial Revascularization Therapy Study [4, 5]. In the first 7 days after the intervention, a definite diagnosis of MI was made if there was documentation of new abnormal Q waves and either a ratio of serum creatine kinase MB (CK-MB) isoenzyme to total cardiac enzyme that was greater than 0.1 or a CK-MB value that was five times the upper limit of normal. Beginning 8 days after the intervention (the length of the hospital stay after surgery), either abnormal Q waves or enzymatic changes were sufficient for a diagnosis of MI [5]. The endpoint of MI included peri-procedural MI. Stroke was defined as ischemic or hemorrhagic stroke either occurring during the index hospitalization or requiring hospitalization with symptoms lasting > 24 h. Hospitalization for heart failure was defined as hospitalization because of worsening heart failure requiring intravenous drug therapy. Bleeding was defined according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) classification. GUSTO moderate or severe bleeding was adjudicated as a major bleeding event [6]. Any coronary revascularization was defined as either PCI or CABG for any reasons. Scheduled staged coronary revascularization procedures performed within 3 months of the initial procedure were not regarded as follow-up events, but were included in the index procedure.
Data acquisition and follow-up
The detailed definitions of baseline clinical characteristics were described previously3. Experienced clinical research coordinators from the independent clinical research organization (Research Institute for Production Development, Kyoto, Japan; Supplementary appendix B) collected baseline clinical, angiographic, and procedural characteristics from hospital charts or hospital databases according to the prespecified definitions. Collection of follow-up information was mainly conducted through review of inpatient and outpatient hospital charts by the clinical research coordinators, and additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by sending mail with questions regarding vital status, subsequent hospitalizations, and status of antiplatelet therapy. Death, MI, and stroke were adjudicated by the clinical event committee (Supplementary appendix C). Median follow-up duration for the surviving patients was 1861 days (interquartile range 1639–2168). Complete 1-, 3-, and 5-year clinical follow-up information was obtained for 98.1%, 95.3%, and 73.8% of the patients, respectively.
Statistical analysis
Categorical variables were presented as number and percentage and were compared with the Chi-square test. Continuous variables were expressed as mean with standard deviation or median with interquartile range. Continuous variables were compared using the Student’s t test or Wilcoxon rank-sum test based on their distributions. Cumulative incidence was estimated by the Kaplan–Meier method, and differences were assessed with the log-rank test. The effects of CABG relative to PCI for the individual end points were expressed as hazard ratios (HRs) and their 95% confidence intervals (CIs). We constructed multivariable Cox proportional hazard models adjusting for 22 clinically relevant factors listed in Table 1. In the model, we included age as a continuous variable. Continuous variables other than age were dichotomized by the clinically meaningful reference values or median values. Proportional hazard assumptions for the risk-adjusting variables were assessed on the plots of log (time) versus log [− log (survival)] stratified by the variable, and the assumptions were verified to be acceptable for all the variables. As prespecified subgroup analyses, clinical outcomes were compared between the CABG and PCI groups stratified by renal function, diabetic status, anemia, and the SYNTAX (Synergy between percutaneous coronary intervention with taxus and cardiac Surgery) score. The interaction p-values between CABG vs PCI and subgroup factors were obtained. We also compared the clinical outcomes between CABG and PCI stratified by age; 80–85 years and ≥ 86 years of age.
All statistical analyses were conducted using JMP 14.0 software (SAS Institute Inc., Cary, North Carolina). All reported P values were 2 tailed, and P values < 0.05 were considered statistically significant.
Results
Baseline clinical and procedural characteristics
Baseline characteristics were similar, except for several important aspects (Table 1). The PCI group had significantly more patients ≥ 85 years of age, and previous stroke, while the CABG group more often had patients with previous MI, and thrombocytopenia. Regarding angiographic and procedural findings, the CABG group more often had complex coronary artery disease as reflected by the higher SYNTAX score, the greater number of target lesions or anastomoses, and more chronic total occlusions target (Table 1 and Fig. 2). The proportion of administration of antiplatelet agents and evidence-based medications for secondary prevention, such as angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, beta-blockers and statins, were low in both groups (Table 1).
Clinical outcomes between the CABG and PCI groups in the entire cohort
The 30-day mortality rate was low and not different between the CABG and PCI groups (2.0% and 2.7%, P = 0.65). The rate of stroke at 30 days was also low (0.7% and 0.3%, P = 0.53) (Table 2). Cumulative 5-year incidence of all-cause death was not significantly different between the two groups (35.8% and 42.9%, P = 0.17) (Fig. 3). However, after adjusting for confounders, the lower mortality risk of CABG relative to PCI was highly significant (HR 0.58, 95% CI 0.41–0.82, P = 0.002) (Table 2). The lower adjusted risks of CABG relative to PCI were also significant for cardiac death, any coronary revascularization and a composite of cardiac death or MI. There were no significant differences between CABG and PCI in the risks for other outcomes such as MI, stroke, and heart failure hospitalization (Table 2).
Clinical outcomes between the CABG and PCI groups in patients with 80–85 and ≥ 86 years of age
In patients with 80–85 years of age, the 30-day mortality rate was 1.6% in the CABG group and 2.8% in the PCI group, while in patients ≥ 86 years of age, it was 4.6% in the CABG group and 2.4% in the PCI group (Table 3 and 4).
Cumulative 5-year incidence of all-cause death was not significantly different between the two groups in both patients with 80–85 and ≥ 86 years of age (Fig. 4). However, the lower adjusted long-term mortality risk of CABG relative to PCI was significant in patients with 80–85 years of age (HR 0.63, 95% CI 0.42–0.94, P = 0.02), while the difference did not reach statistical significance in patients ≥ 85 years of age (HR 0.66, 95% CI 0.32–1.26, P = 0.22) (Table 3 and 4).
Subgroup analysis
In the subgroup analyses, there were no interactions between the subgroup factors such as renal function, diabetic status, anemia, and the SYNTAX score, and the effect of CABG relative to PCI on all-cause death (Table 5).
Discussion
The main finding of the present study was that CABG as compared with PCI was associated with a significantly lower long-term adjusted risk for all-cause death, any coronary revascularization, and a composite of cardiac death/MI in elderly patients older than 80 years of age with TVD or LMCA disease.
We already reported that CABG, as compared with PCI, was associated with better long-term outcomes in patients with TVD or LMCA disease [7, 8]. Other randomized studies endorsed the result of our studies, showing better clinical outcomes in patients undergoing CABG even in the DES era [9,10,11,12]. Nevertheless, an increasing number of patients even with severe coronary artery disease have received PCI rather than CABG probably because of less invasion and shorter hospital stay. The selection of the most appropriate treatment method for elderly patients with complex coronary disease is very challenging, because of paucity of data in patients with this age group. We should make meticulous consideration of the risks and benefits for each treatment method including medical therapy only, PCI and CABG.
Basically, all the studies evaluating clinical outcomes after CABG and PCI in very elderly patients such as ones older than 70 or 80 years of age with complex coronary artery disease are based on observational data [13,14,15,16,17,18]. There are several reasons for conflicting results in these studies; the study population varies from patients with left main disease only to patients with left main disease and multi-vessel disease. The age of study population is also different from 70 to 80 years of age. As the period of cohort data is very wide, ranging from 2002 to 2013, used stents varies from paclitaxel-eluting and sirolimus-eluting stent to everolimus-eluting stent and usage of medications for secondary prevention might be different in each period. However, what is commonly observed in those reports is that there is no significant difference in all-cause mortality in most studies and that the studies demonstrated satisfactory acute and long-term outcomes after CABG [14, 18].
One study reported by Nicolini et al., which was very similar to ours, showed that PCI was an independent predictor of increased all-cause mortality at long-term follow-up [13]. Although there might be no clear reason for the difference of clinical outcomes in these studies, the age distribution of the study population is likely to affect clinical outcomes after CABG and PCI. The age 86–90 accounted for only 5.9% in the CABG group whereas it accounted for 18% in the PCI group. Among patients older than 90 years of age, 4% of the study patients underwent PCI, while no patient received CABG [13].
The present study has several limitations. First and most importantly, unmeasured confounders might influence clinical outcomes even after multivariate adjustment. In particular, age was a strong confounder in the current analysis and strict comparison is challenging, because the group of very old patients such as ones over 85 years old dominant in the PCI group might have negatively influenced clinical outcomes. Moreover, the selection of the revascularization strategy is still controversial even in aged patients and the information on frailty, which is important in the actual decision-making, is lacking in the current analysis. Second, the rate of collected SYNTAX score in the CABG group is lower than that in the PCI group and the difference is relatively large. We did not include SYNTAX score in the multivariate statistical adjustment and instead included other adjusters such as a target of proximal lesions in left anterior descending artery or a target of chronic total occlusion as we consistently did in other analyses from our registry [7, 8]. Therefore, the difference might not affect the result of this study. Third, the practice patterns including used stents and secondary intensive medications might be different from those in the current clinical practice. First-generation drug-eluting stents such as sirolimus-eluting stent are not currently used. Considering the low rate of statins use, secondary preventive medications were very insufficient in both groups. Furthermore, because patient demographics, practice patterns including the selection of revascularization procedure and medical therapy, and clinical outcomes in patients undergoing PCI and CABG in Japan may be different from those outside Japan, generalizing these results to populations outside Japan should be done with caution.
Conclusion
CABG compared with PCI was associated with a lower adjusted risk for all-cause death, any coronary revascularization, and a composite of cardiac death/MI in octogenarians with TVD or LMCA disease. CABG seemed an acceptable strategy for selected octogenarians with severe coronary artery disease.
References
Steel N, Ford JA, Newton JN, Davis ACJ, Vos T, et al. Changes in health in the countries of the UK and 150 English Local Authority areas 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1647–61.
Head SJ, Milojevic M, Daemen J, Ahn J, Boersma E, Christiansen EH, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet (London, England). 2018;391:939–48.
Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, et al. Long-term safety and efficacy of sirolimus-eluting stents versus bare-metal stents in real world clinical practice in Japan. Cardiovasc Interv Ther. 2011;26:234–45.
Serruys PW, Ong ATL, Van HLA, Sousa JE, Jatene A, Bonnier JJRM, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol Elsevier Masson SAS. 2005;46:575–81.
Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schönberger JP, et al. Arterial Revascularization Therapies Study. Group Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–24.
GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–82.
Shiomi H, Morimoto T, Hayano M, Furukawa Y, Nakagawa Y, Tazaki J, et al. Comparison of long-term outcome after percutaneous coronary intervention versus coronary artery bypass grafting in patients with unprotected left main coronary artery disease (from the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2012;110:924–32.
Shiomi H, Morimoto T, Furukawa Y, Nakagawa Y, Tazaki J, Sakata R, et al. Comparison of five-year outcome of percutaneous coronary intervention with coronary artery bypass grafting in triple-vessel coronary artery disease (from the Coronary Revascularization Demonstrating Outcome Study in Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2015;116:59–65.
Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 2014;35:2821–30.
Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38.
Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84.
Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76.
Nicolini F, Contini GA, Fortuna D, Pacini D, Gabbieri D, Vignali L, et al. Coronary artery surgery versus percutaneous coronary intervention in octogenarians: long-term results. Ann Thorac Surg. 2015;99:567–74.
Hannan EL, Zhong Y, Berger PB, Walford G, Curtis JP, Wu C, et al. Comparison of intermediate-term outcomes of coronary artery bypass grafting versus drug-eluting stents for patients ≥75 years of age. Am J Cardiol. 2014;113:803–8.
Conrotto F, Scacciatella P, D’Ascenzo F, Chieffo A, Latib A, Park SJ, et al. Long-term outcomes of Percutaneous coronary interventions or coronary artery bypass grafting for left main coronary artery disease in octogenarians (from a drug-eluting stent for left main artery registry substudy). Am J Cardiol. 2014;113:2007–12.
Alam M, Virani SS, Shahzad SA, Siddiqui S, Siddiqui KH, Mumtaz SA, et al. Comparison by meta-analysis of percutaneous coronary intervention versus coronary artery bypass grafting in patients with a mean age of ≥70 years. Am J Cardiol. 2013;112:615–22.
Gunn J, Kuttila K, Vasques F, Virtanen R, Lahti A, Airaksinen J, et al. Comparison of results of coronary artery bypass grafting versus percutaneous coronary intervention in octogenarians. Am J Cardiol. 2012;110:1125–9.
Chang M, Lee CW, Ahn JM, Cavalcante R, Sotomi Y, Onuma Y, et al. Outcomes of coronary artery bypass graft surgery versus drug-eluting stents in older adults. J Am Geriatr Soc. 2017;65:625–30.
Acknowledgements
We appreciate the collaboration of the co-investigators in the CREDO-Kyoto PCI/CABG Registry Cohort-2.
Funding
This study was supported by the Pharmaceuticals and Medical Devices Agency, Tokyo, Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose concerning the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hara, H., Watanabe, H., Esaki, J. et al. Five-year outcomes after coronary artery bypass grafting and percutaneous coronary intervention in octogenarians with complex coronary artery disease. Gen Thorac Cardiovasc Surg 70, 419–429 (2022). https://doi.org/10.1007/s11748-021-01711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-021-01711-4