Abstract
Objective
To evaluate the effects of administering tranexamic acid (TXA) after cardiopulmonary bypass, instead of after anesthesia induction, on postoperative seizures and blood transfusion requirements.
Methods
Adult patients who underwent valve surgery and/or coronary artery bypass grafting at West China Hospital between July 1, 2011 and December 31, 2016 were retrospectively analyzed. Patients either received TXA after bypass (n = 2062) or not (n = 4236). Logistic regression and propensity score matching analysis were performed to assess effects of TXA on postoperative seizures and blood product requirements in hospital.
Results
Among 6298 patients, seizures occurred in 2.4% (102/4236) in the no-TXA group and 2.7% (56/2062) in the TXA group (P = 0.46). The number of patients receiving any blood products was greater in the no-TXA group (57.3%, 2428/4236) than in the TXA group (53.1%, 1095/2062) (P < 0.01), and the volume of blood products was also greater in the no-TXA group (1.5 vs. 1.0 units, P < 0.01). TXA was not associated with increased incidence of postoperative seizures (adjusted OR 1.16, 95% CI 0.83–1.62) but was associated with lower incidence of a requirement for blood products (adjusted OR 0.82, 95% CI 0.73–0.92). Similar results were obtained after patients from the two groups were matched based on propensity scoring. TXA was associated with reduced requirements for fresh frozen plasma, platelets and cryoprecipitate, but not red blood cells.
Conclusions
Administering TXA after bypass may reduce requirements for blood products without increasing risk of seizures following cardiac surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac surgery under cardiopulmonary bypass (CPB) is often associated with a greater requirement for blood products than procedures without CPB [1], which may be due to excessive bleeding induced by the consumption of coagulation factors and activation of fibrinolysis during CPB [2, 3]. Tranexamic acid (TXA), a lysine analogue that inhibits fibrinolysis, has been increasingly used to reduce bleeding and requirements for allogeneic blood products in cardiac surgery [4,5,6]. However, some authors report a dose-dependent association between TXA and seizures [7, 8], which may lead to neurological deficits and perioperative mortality [9, 10].
TXA is recommended to be given as a bolus after induction of anesthesia, and then to be continuously administered intravenously to maintain an effective concentration in the plasma [11,12,13]. Since TXA inhibits fibrinolysis during CPB, microthrombi inevitably form, even when high-dose heparin is used [14]. This may explain why administering TXA after anesthesia induction increases risk of cerebral infarction [15]. To avoid the risk of thrombus formation, it may be safer to administer TXA after CPB instead of after anesthesia induction, because the vascular endothelium transitions from a procoagulation state during CPB to an anticoagulation state after CPB [16].
These considerations led us to administer TXA after CPB rather than after anesthesia induction at West China Hospital from July 1, 2011 onwards. The present study retrospectively examined patients’ records from this date until the end of 2016 to assess whether this alteration mitigated risk of postoperative seizures without compromising the drug’s ability to reduce blood product requirements.
Methods
Patients
This retrospective study considered patients at least ≥ 18 years old who underwent elective valve surgery and/or coronary artery bypass grafting (CABG) involving CPB in the Heart Center of West China Hospital between July 1, 2011 and December 31, 2016. Those who died within 24 h after surgery or for whom medical records were incomplete were excluded.
This study was approved by the Ethics Committee of West China Hospital (no. 256, 2017), which waived the requirement for informed consent since it was retrospective.
Data collection
Demographic characteristics, medical and medication history, duration of stay in the intensive care unit (ICU) and postoperative hospitalization stay were collected from the Hospital Information System. Previous medication included beta blockers, anticoagulant and antiplatelets. Laboratory results, blood cell counts, conventional coagulation examinations and blood biochemistry tests were collected from the Laboratory Information System. Intra-operative data, including type of surgery, duration of CPB, aortic clamp duration and use of TXA were collected from operating records. Data on blood product use were collected from the laboratory information system of the Department of Blood Transfusion.
Cardiac surgical procedure
Based on recommendations [4], aspirin was used until the day of surgery, and clopidogrel use was suspended for 5 days before surgery.
Anesthesia induction and CPB were performed according to standard protocols at our hospital [17]. Briefly, anesthesia was induced with midazolam, sufentanil, and muscle relaxant; then maintained with continuous infusion of remifentanil and propofol, intermittent muscle relaxant, and/or inhalation of sevoflurane. Pump equipment included a roller pump, membrane oxygenator (Medtronic, Minneapolis, MN, USA), and tubing system. Heparin was used to maintain an activated clotting time > 480 s. During CPB, body temperature was maintained at 32–33 °C. Patients in both groups were primed with 1600–1800 mL solution (colloid and crystalloid in a 2:1 ratio with 3750 units of heparin) [18], and cardiac arrest was achieved by 4:1 cold blood cardioplegia. After bypass, heparin was neutralized by administering protamine in a 1:1 ratio with the initial heparin dose. After CPB, anesthesiologists administered TXA (15–50 mg kg−1) for 30 min when coagulation disorders and hemorrhaging in the surgical field and based on personal experience.
Outcomes
Outcomes were compared between patients who received TXA after bypass and those who did not receive TXA. The primary outcome was postoperative seizures, defined as new-onset transient dysfunction of the nerve system manifesting as abnormal involuntary movement of the limbs [19]. The diagnosis of postoperative seizures was examined by a neurologist who was blinded to whether a given patient received TXA or not.
The secondary outcome was perioperative requirements for blood products including packed red blood cells, fresh frozen plasma, platelets and cryoprecipitate. As per standard protocols at our hospital, red blood cells were given when hemoglobin concentration was lower than 8 g dL−1 during surgery and 9.5 g dL−1 in the intensive care unit (ICU). One unit (200 mL) of fresh frozen plasma was given when prothrombin time was longer than 15 s and mediastinal drainage was also in excess of 150 mL h−1 [20]. Platelets were transfused without strict adherence to predefined rules but rather at the attending physician’s discretion, typically when platelet count was below 50 × 109 L−1 during routine blood analysis performed as a result of obvious bleeding.
Other outcomes included length of ICU stay, length of postoperative hospitalization and surgical re-exploration for hemostasis.
Statistical analysis
Data were analyzed using the “MatchIt”, “sqldf” and “epiDisplay” packages in R 3.5.1 (https://www.r-project.org). Skewed data were reported as median and interquartile range. Differences in categorical variables between TXA and no-TXA patients were assessed for significance using chi-squared or Fisher’s exact tests; differences in continuous variables were assessed using Kruskal–Wallis tests. Multivariate logistic regression was used to determine factors associated with the risk of seizures and with consumption of blood products.
To balance patient characteristics between the TXA and no-TXA groups, patients from the two groups were matched based on propensity scores calculated for the following parameters: age, gender, history of smoking, history of hypertension, infective endocarditis, atrial fibrillation, cardiac catheterization, repeat surgery, respiratory dysfunction, stroke, diabetes mellitus, liver dysfunction and gastrointestinal bleeding, preoperative medication (beta blockers, anticoagulants, antiplatelet drugs), preoperative laboratory results (hemoglobin, white blood cell count, platelet count, international normalized ratio, fibrinogen), type of surgery, and CPB time. The matching ratio was 1:1. Patients were considered well matched if the two groups showed a mean difference of no more than 0.1 for the abovementioned variables.
Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). P < 0.05 was considered statistically significant, and all reported P values were two-tailed.
Results
Patient characteristics
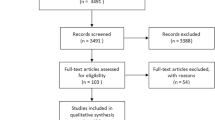
A total of 6333 adult patients were screened from the database, of whom 3 were excluded because they underwent aortic replacement surgery, 10 because CPB time was not recorded and 9 because outcomes data were incomplete. And 13 patients who died within 24 h after operation were also excluded, 4 received 15–20 mg kg−1 TXA after CPB. All of these four patients, together with other seven patients who did not receive TXA, died from severe circulatory failure. The other two patients died of cardiac rupture. The resulting 6298 were included in the final analysis (Fig. 1). These patients were divided into a TXA group (n = 2062) who received the drug after bypass at a median dose of 31 mg kg−1 (interquartile range 18–36), and a control group (n = 4236) who did not receive TXA at any time.
Patients screening. A total of 6333 adult patients were screened from the database. After 35 were excluded, 6298 were included in the final analysis, which were divided into a TXA group (n = 2062) and the no-TXA group (n = 4236) according to whether they received TXA. Propensity score matching was used to generate 2062 control-TXA pairs
Patients in the TXA group were more likely to have a higher preoperative level of fibrinogen, and their CPB lasted longer. Patients in the control group showed higher platelet counts and were more likely to undergo CABG or combined surgery and to have a history of infective endocarditis and use of β-blockers and anticoagulants.
Propensity score matching generated 2062 control-TXA pairs, which were well matched at baseline (Table 1).
Postoperative seizures
Among all patients, postoperative seizures occurred in 158 (2.5%); seizure incidence was similar in the TXA group (2.7%) and no-TXA group (2.4%; P = 0.463) (Table 2). Logistic regression identified the following independent risk factors of seizures: male, older age, use of beta blockers, and longer CPB duration (Table 3). After adjustment for these factors, logistic regression suggested that TXA after CPB was unrelated to seizures (OR 1.16, 95% CI 0.83–1.62; P = 0.385; Table 4).
In the matched subset of 4124 patients, 102 (2.5%) developed seizures, for which incidence was similar in the TXA group (2.7%) as in the no-TXA group (2.2%, P = 0.316; Table 2). TXA after CPB was not associated with increased risk of postoperative seizures (OR 1.22, 95% CI 0.82–1.82; Table 4).
Restricted cubic spline (RCS) graph was performed to clarify if TXA dose-dependently affect the risk of convulsive seizures. The horizontal axis was referred to the dose of TXA (mg kg−1), while vertical axis represented risk index of seizures (log [riskTXA/riskno-TXA]). When the risk index was equal to zero, the corresponding dose of TXA was 31 mg kg−1 (Fig. 2), which indicated higher dose of TXA (more than 31 mg kg−1) may increase the risk of convulsive seizures. So, patients were divided into three groups including low-dose TXA (L-TXA for short, < 31 mg kg−1), high-dose TXA (H-TXA for short, ≥ 31 mg kg−1), and no-TXA group. Based on the chi-squared test, the incidence of seizures both in L-TXA group and H-TXA group had no significant difference compared with that of no-TXA group (P = 0.153 before matching; P = 0.119 after matching) (Table 5). These results suggested that the dose of TXA raging from 15 to 50 mg kg−1 had no significant influence on the postoperative seizures in this study, though there was an increasing trend of the incidence of seizures in patients received the high dose of TXA.
Blood product requirements
Among all 6298 patients, 55.9% (3523) received any type of blood products, of whom 40.4% required packed red blood cells; 30.2%, fresh frozen plasma; 14.7%, platelets; and 6.4%, cryoprecipitate. Uni- and multivariate logistic analysis identified the following factors associated with increased risk of requiring any blood product: older age, previous infective endocarditis, history of cardiac catheterization, repeat surgery, higher international normalized ratio, prolonged CPB duration, CABG or combined surgery. These analyses also identified higher hemoglobin level and anticoagulant use as associated with lower risk of requiring any blood product (Table 6). After adjustment for all these factors, TXA after CPB was associated with a reduced risk of requirement of any blood product (OR 0.82, 95% CI 0.73–0.92, P < 0.001) and more specifically of fresh frozen protein (OR 0.74, 95% CI 0.65–0.83, P < 0.001), platelets (OR 0.49, 95% CI 0.41–0.59, P < 0.001) or cryoprecipitate (OR 0.72, 95% CI 0.57–0.91, P = 0.005; Table 6). However, TXA after CPB did not affect the risk of requirement of red blood cells (OR 1.12, 95% CI 0.99–1.26, P = 0.067).
Among the subset of 4124 matched patients, 54.7% (2256) received any type of blood products, of whom 40.5% required packed red blood cells; 29.4%, fresh frozen plasma; 12.5%, platelets; and 6.3%, cryoprecipitate. The proportion of patients requiring any type of blood product was significantly smaller in the TXA group (53.1%) than in the no-TXA group (56.3%; OR 0.88, 95% CI 0.78–0.99, P = 0.039; Tables 2 and 7). Median blood product consumption was also significantly smaller in the TXA group [1.0 (0, 3.5) vs. 1.5 (0, 4.0) units, P = 0.014; Table 2). Similar trends were found regarding requirements for fresh frozen plasma, platelets and cryoprecipitate. The proportion of patients requiring red blood cells, however, was similar between the TXA and no-TXA groups (Table 2).
Other outcomes
Incidence of surgical re-exploration for hemostasis and length of ICU stay were similar between the TXA and no-TXA groups [21], but length of postoperative hospitalization was longer in the TXA group, even after propensity score matching (Table 2).
Discussion
TXA is routinely administered after anesthesia and during CPB at doses of 24–100 mg kg−1 to reduce requirement for blood products, but it is associated with a two to sevenfold increase in incidence of postoperative seizures [8, 13, 22]. In our previous study, we found that using TXA after CPB might reduce the risk of postoperative death during hospitalization but was not associated with ischemic or bleeding-related events [21]. The present study provides evidence that administering TXA after CPB at 31 mg kg−1 does not increase the risk of postoperative seizures following cardiac surgery, while still reducing the requirement for fresh frozen plasma, platelets and cryoprecipitate, although not necessarily the requirement for packed red blood cells.
In general, postoperative seizures can be induced by focal and generalized lesions [7, 23]. Seizures after cardiac surgery involving arrhythmic movements are believed to result from generalized lesions, including drug toxicity such as TXA, and diffuse injury such as post-pump encephalopathy and multiple emboli [24]. Seizures occur in 0.1–11.0% of patients after cardiac surgery [8, 10, 13, 23, 25,26,27,28], and this wide range may reflect differences in the definition of seizures [23, 29], study populations [30], types of cardiac surgery [7, 9, 31] and administration of TXA [8, 10]. The incidence of postoperative seizures in patients who did not receive any TXA ranged to 0.1–1.2% [8, 9] while that was 2.4% in present study, one of the important reasons was the limitations of the retrospective study. The diagnosis of seizures was highly dependent to clinic observation and many patients suffered related symptoms in ICU which were easily missed or misdiagnosed. Therefore, the incidence of seizures after cardiac surgery might be difficult to calculate authentically. To reduce subjectivity in the observation of seizures in the present study, they were examined according to the definition strictly by a neurologist blinded to whether a patient received TXA or not. The incidence of postoperative seizures in our large cohort was 2.5%. Similar to other reports [25, 32], the independent risk factors for seizures in our study were identified to be male gender, older age, and longer CPB time. Interestingly, we also found that the rate of seizures in patients with CABG (21/518, 4.1%) was higher than that in those who underwent valve surgery or combined surgery (137/5780, 2.4%, P = 0.019). But, surgery type was not associated with seizures after adjusted by gender, age, atrial fibrillation, history of cardiac catheterization, respiratory dysfunction, beta blockers, surgery type and CPB duration. These findings suggest caution in administering TXA after cardiac surgery to older patients, male patients, and patients who undergo prolonged CPB.
Different with previous conclusions that TXA after anesthesia induction dose-dependently increased the seizures risk [7, 8], present study indicated that TXA at the dose of 15–50 mg kg−1 after CPB did not increase seizures. Given these results, we believe that RCT studies are required to distinguish whether the effect of TXA due to its low dosage or the different time window of its administration.
The molecular pathway(s) linking TXA with postoperative seizures is unclear. One link appears to be drug toxicity, i.e., inhibition by TXA of γ-aminobutyric acid (GABA) binding to GABAA receptors in the central nervous system [33, 34]. Another link may be the formation of microthrombi in the cerebral circulation. The results of the present study suggest that simply changing the TXA administration window can affect risk of postoperative seizures. It may be that TXA increases the risk of microthrombus formation when it is administered before CPB, but not when administered after CPB.
Although TXA is used globally to reduce requirements for blood transfusion, evidence about its efficacy remains controversial. Two randomized controlled trials showed that TXA could reduce exposure to any blood product transfusion as well as the amount of total blood products consumed [5, 8]. On the other hand, one study reported no such reduction [35], while a retrospective study suggested that TXA increased the incidence of fresh frozen plasma transfusion by 19.4% and of platelet transfusion by 68.5% [15]. Our results with TXA administered after CPB are similar to those of the randomized controlled trials of TXA administered before and during CPB [5, 8]: the drug reduced the requirement among our patients for any blood product and specifically for fresh frozen plasma, platelets and cryoprecipitate. These results suggest that TXA exerts similar hemostatic effects regardless of whether it is administered after CPB or after anesthesia induction.
We were slightly surprised to find that TXA in our study did not reduce consumption of red blood cells. One explanation may be that since TXA was administered after CPB in our study, anesthesiologists had the opportunity to decide whether or not to use the drug. They may have tended to use TXA in patients with high risk of bleeding, and this assessment may be quite subjective, as reflected in the significant heterogeneity in TXA use among anesthesiologists [36]. In any event, administering TXA after CPB may help reduce its unnecessary use by giving anesthesiologists the opportunity to observe the complexity of the operation and the amount of bleeding after CPB.
Our study is limited by its reliance on retrospective data, which led to significant differences between the TXA and no-TXA groups at baseline. Therefore, propensity score matching was performed to reduce biases from confounding factors. Furthermore, it is very difficult to distinguish the seizures due to TXA or other reasons, although the same CPB procedure for each patient between the two groups was applied. And because the seizures were only diagnosed retrospectively by the investigators based on postoperative medical records, the authentic incidence of postoperative seizures may be difficult to calculate. Our results are also limited by the fact that they came from a single cardiology center, and that administration of TXA was subjective, because it was based on anesthesiologists’ assessment of bleeding in the surgical field. This subjectivity is shared by TXA studies because there is no international consensus on its use. We could not directly compare outcomes when TXA was administered after anesthesia induction or after CPB because of the paucity of patients at our hospital who were given the drug after anesthesia induction. Future RCT studies should examine this parallel comparison to verify and extend our results.
Conclusions
Our results suggest that TXA after CPB reduces blood product requirements without increasing risk of postoperative seizures. Simply changing the TXA administration window may avoid unnecessary use of TXA and allow the use of lower doses. Further multi-center, randomized, double-blind, controlled studies are warranted to improve patient management through TXA administration during cardiac surgery.
References
Paparella D, Guida P, Scrascia G, Fanelli V, Contini M, Zaccaria S, et al. On-pump versus off-pump coronary artery bypass surgery in patients with preoperative anemia. J Thorac Cardiovasc Surg. 2015;149(4):1018–26.
Casati V, Gerli C, Franco A, Della Valle P, Benussi S, Alfieri O, et al. Activation of coagulation and fibrinolysis during coronary surgery: on-pump versus off-pump techniques. Anesthesiology. 2001;95(5):1103–9.
Dixon B, Santamaria J, Campbell D. Coagulation activation and organ dysfunction following cardiac surgery. Chest. 2005;128(1):229–36.
Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations (Phila). 2012;7(4):229–41.
Horrow JC, Van Riper DF, Strong MD, Brodsky I, Parmet JL. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation. 1991;84(5):2063–70.
Nuttall GA, Oliver WC, Ereth MH, Santrach PJ, Bryant SC, Orszulak TA, et al. Comparison of blood-conservation strategies in cardiac surgery patients at high risk for bleeding. Anesthesiology. 2000;92(3):674–82.
Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–3.
Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136–48.
Goldstone AB, Bronster DJ, Anyanwu AC, Goldstein MA, Filsoufi F, Adams DH, et al. Predictors and outcomes of seizures after cardiac surgery: a multivariable analysis of 2,578 patients. Ann Thorac Surg. 2011;91(2):514–8.
Sharma V, Katznelson R, Jerath A, Garrido-Olivares L, Carroll J, Rao V, et al. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia. 2014;69(2):124–30.
Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358(22):2319–31.
Gertler R, Gruber M, Grassin-Delyle S, Urien S, Martin K, Tassani-Prell P, et al. Pharmacokinetics of tranexamic acid in neonates and infants undergoing cardiac surgery. Br J Clin Pharmacol. 2017;83(8):1745–57.
Koster A, Borgermann J, Zittermann A, Lueth JU, Gillis-Januszewski T, Schirmer U. Moderate dosage of tranexamic acid during cardiac surgery with cardiopulmonary bypass and convulsive seizures: incidence and clinical outcome. Br J Anaesth. 2013;110(1):34–40.
Edmunds LH. Cardiopulmonary bypass after 50 years. N Engl J Med. 2004;351(16):1603–6.
Zhou ZF, Zhang FJ, Huo YF, Yu YX, Yu LN, Sun K, et al. Intraoperative tranexamic acid is associated with postoperative stroke in patients undergoing cardiac surgery. PLoS ONE. 2017;12(5):e0177011.
Boyle EMJ, Verrier ED, Spiess BD. Endothelial cell injury in cardiovascular surgery: the procoagulant response. Ann Thorac Surg. 1996;62(5):1549–57.
Qiu Y, Lin J, Yang Y, Zhou J, Gong LN, Qin Z, et al. Lack of efficacy of ulinastatin therapy during cardiopulmonary bypass surgery. Chin Med J (Engl). 2015;128(23):3138–42.
Lin J, Tan Z, Yao H, Hu X, Zhang D, Zhao Y, et al. Retrograde Inferior Vena caval Perfusion for Total Aortic arch Replacement Surgery (RIVP-TARS): study protocol for a multicenter, randomized controlled trial. Trials. 2019;20(1):232.
Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–2.
Guo Y, Tang J, Du L, Liu J, Liu RC, Liu X, et al. Protamine dosage based on two titrations reduces blood loss after valve replacement surgery: a prospective, double-blinded, randomized study. Can J Cardiol. 2012;28(5):547–52.
Liu J, Chen CW, Huang D, Luo D, Du L. Effects of tranexamic acid after cardiopulmonary bypass on the outcomes of patients undergoing cardiac surgery. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49(4):660–4.
Sander M, Spies CD, Martiny V, Rosenthal C, Wernecke KD, von Heymann C. Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis. Crit Care. 2010;14(4):R148.
Keyl C, Uhl R, Beyersdorf F, Stampf S, Lehane C, Wiesenack C, et al. High-dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur J Cardiothorac Surg. 2011;39(5):e114–21.
Hunter GR, Young GB. Seizures after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(2):299–305.
Manji RA, Grocott HP, Leake J, Ariano RE, Manji JS, Menkis AH, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth. 2012;59(1):6–13.
Martin K, Knorr J, Breuer T, Gertler R, Macguill M, Lange R, et al. Seizures after open heart surgery: comparison of epsilon-aminocaproic acid and tranexamic acid. J Cardiothorac Vasc Anesth. 2011;25(1):20–5.
Martin K, Wiesner G, Breuer T, Lange R, Tassani P. The risks of aprotinin and tranexamic acid in cardiac surgery: a one-year follow-up of 1188 consecutive patients. Anesth Analg. 2008;107(6):1783–90.
Berman M, Cardone D, Sharples L, Vuylsteke A, Klein A, Gerrard C, et al. Safety and efficacy of aprotinin and tranexamic acid in pulmonary endarterectomy surgery with hypothermia: review of 200 patients. Ann Thorac Surg. 2010;90(5):1432–6.
Gofton TE, Chu MW, Norton L, Fox SA, Chase L, Murkin JM, et al. A prospective observational study of seizures after cardiac surgery using continuous EEG monitoring. Neurocrit Care. 2014;21(2):220–7.
Montes FR, Pardo DF, Carreno M, Arciniegas C, Dennis RJ, Umana JP. Risk factors associated with postoperative seizures in patients undergoing cardiac surgery who received tranexamic acid: a case-control study. Ann Card Anaesth. 2012;15(1):6–12.
Manji RA, Grocott HP, Manji JS, Menkis AH, Jacobsohn E. Recurrent seizures following cardiac surgery: risk factors and outcomes in a historical cohort study. J Cardiothorac Vasc Anesth. 2015;29(5):1206–11.
Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335(25):1857–63.
Furtmuller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W, et al. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid(A) receptor antagonistic effect. J Pharmacol Exp Ther. 2002;301(1):168–73.
Kratzer S, Irl H, Mattusch C, Burge M, Kurz J, Kochs E, et al. Tranexamic acid impairs gamma-aminobutyric acid receptor type A-mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639–49.
Casati V, Bellotti F, Gerli C, Franco A, Oppizzi M, Cossolini M, et al. Tranexamic acid administration after cardiac surgery: a prospective, randomized, double-blind, placebo-controlled study. Anesthesiology. 2001;94(1):8–14.
Spence J, Long S, Tidy A, Raymer K, Devereaux PJ, Lamy A, et al. Tranexamic acid administration during on-pump cardiac surgery: a survey of current practices among Canadian anesthetists working in academic centers. Anesth Analg. 2017.
Acknowledgements
The authors would like to thank Professor Jerry Yu, M.D., Ph.D. in the Department of Pulmonary Medicine at the University of Louisville School of Medicine for advice and revision of the manuscript, and Dr. Linyu Tian in the Department of Neurology, West China Hospital, Sichuan University for the diagnosis of postoperative seizures.
Funding
This work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital of Sichuan University (20HXJS004 to LD).
Author information
Authors and Affiliations
Contributions
Conceptualization: LD, JL. Data curation: CC, JL. Formal analysis: CC, JL. Funding acquisition: LD. Investigation: CC, JL. Methodology: CC, JL, LD. Project administration: CC, JL. Supervision: CC, JL, LD. Writing—original draft: CC, JL. Writing—review and editing: LD, CC.
Corresponding author
Ethics declarations
Conflict of interest
Jing Liu has no conflict of interest. Changwei Chen has no conflict of interest. Lei Du has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Liu, J. & Du, L. Tranexamic acid after cardiopulmonary bypass does not increase risk of postoperative seizures: a retrospective study. Gen Thorac Cardiovasc Surg 70, 337–346 (2022). https://doi.org/10.1007/s11748-021-01709-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-021-01709-y