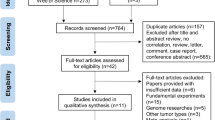
Abstract
Objective
The predictive importance of galectin-3 in non-small cell lung cancer (NSCLC) has not been elucidated. We examined whether galectin-3 could serve as a predictor for tumor recurrence in NSCLC.
Methods
In 42 consecutive patients with NSCLC who underwent radical resection, galectin-3 expression in tumor cells was examined by immunohistochemistry. Galectin-3 levels in serum were assessed before surgery and 1 month after surgery by enzyme-linked immunosorbent assays.
Results
Higher expression of galectin-3 in tumor cells was associated significantly with lymphatic invasion (p = 0.049) and tumor recurrence (p = 0.001). The Kaplan–Meier curves for relapse-free survival after radical resection showed that patients with high expression of galectin-3 had significantly shorter relapse-free survival than those with low expression of galectin-3 (p < 0.001). The serum level of galectin-3 was not reduced after radical resection, and there was no significant correlation between the serum level of galectin-3 and recurrence.
Conclusions
Galectin-3 expression in tumor cells could serve as a predictive factor for recurrence, but serum level of galectin-3 is not useful for predicting NSCLC recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide [1]. The prognosis of NSCLC has gradually improved through advances in therapeutic strategies, such as surgery, chemotherapy, radiotherapy, molecular targeted therapy, and immunotherapy [2,3,4,5,6]. However, 10–60% of patients with p-stage IA–IIIA NSCLC develop recurrent disease within 5 years after radical resection [6, 7]. Therefore, the identification of useful biomarkers to predict tumor recurrence is very important.
Galectins are a family of β-galactoside-binding lectins that can be classified into three groups: prototype, chimera, and tandem [8, 9]. Galectin-3 is the only member of the chimera group, and is expressed ubiquitously in healthy adult tissues, as well as in various tumor types [9,10,11]. Galectin-3 is localized mainly to the cytoplasm and nucleus [10, 11]. It is also expressed on the cell surface, and secreted via a non-classical pathway that is yet to be identified [9,10,11]. Galectin-3 has been reported to promote the aggressiveness of tumors, including increased proliferation of tumor cells, anti-apoptotic traits, angiogenesis, tumor cell motility, and metastatic activity [10,11,12,13].
In NSCLC, galectin-3 shows high expression in tumor cells, and has been reported to be associated with tumor progression and a poor prognosis [14,15,16]. Furthermore, scholars have reported that galectin-3 is secreted by tumor cells [17, 18], and the serum level of galectin-3 in patients with lung cancer is higher than that in healthy individuals [19]. Collectively, these data suggest that galectin-3 could be a predictive biomarker for NSCLC recurrence. However, the clinical importance of galectin-3 in post-operative recurrence in NSCLC patients has not been elucidated fully.
In the present study, we hypothesized that galectin-3 could serve as a predictive marker for post-operative recurrence of NSCLC. We wished to evaluate the predictive value of galectin-3 in NSCLC recurrence.
Materials and methods
Patient samples
Samples of tumor tissues and serum were obtained from 42 consecutive patients with p-stage IA–IIIA NSCLC who underwent radical surgical resection at Shiga University of Medical Science Hospital, between January 2014 and October 2015. Patients who received pre-operative anti-cancer therapy, such as chemotherapy or radiotherapy, were excluded. Patients with adenocarcinoma in situ/minimally invasive adenocarcinoma were also excluded. Serum samples were stored at − 80 °C until use. This study was approved by the Institutional Review Board of Shiga University of Medical Science (IRB #28–210). Written informed consent was obtained from all patients to participate in this study.
Enzyme-linked immunosorbent assay (ELISA)
The serum level of galectin-3 was measured before surgery and 1 month after surgery using a Human Galectin-3 ELISA kit (Abcam, Cambridge, UK), according to the manufacturer’s instructions. All ELISAs were repeated three times independently, and each was carried out using duplicate measurements.
Immunohistochemistry
Sections of formalin-fixed paraffin-embedded tumor tissues (thickness, 4 µm) were stained with anti-human galectin-3 monoclonal antibody (1:100; Abcam) using the Envision-kit, according to the manufacturer’s instructions (DAKO, Glostrup, Denmark). Briefly, tissue sections were deparaffinized in xylene, and then rehydrated in ethanol and distilled water. Antigen retrieval was performed in Tris–EDTA buffer (pH 9.0) for 5 min using a microwave. Endogenous peroxidase activity was inactivated using 3% H2O2 for 10 min, and slides were blocked with 5% normal goat serum in PBS at room temperature for 1 h. Sections were incubated with anti-galectin-3 at room temperature for 1 h, before incubating with secondary anti-mouse antibody-coated polymer peroxidase complexes for 1 h. Then, slides were then treated with substrate/chromogen (DAKO) for 5 min, followed by counterstaining with hematoxylin. For negative controls, the primary antibody was omitted. Ten fields at 400× magnification were randomly selected for each slide. Stained slides were evaluated under guidance by an experienced a pathologist. Galectin-3 expression was described as being high if more than 10% of tumor cells were positively stained.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). The Fisher’s exact test or Mann–Whitney U test was applied to investigate significant differences between groups. Curves for relapse-free survival or overall survival were calculated using the Kaplan–Meier method and compared by the log-rank test. Statistical analysis was carried out using SPSS 22 Statistics V.22.0 software (IBM, Armonk, NY, USA). p < 0.05 was considered significant.
Results
Characteristics of the study cohort
Forty-two patients (31 men and 11 women) with NSCLC who underwent radical resection formed the study cohort (Table 1). The median age of patients was 70 years (range 49–83 years). Twenty-nine patients (69.0%) were habitual smokers. The pathological stages (TNM version 8) were IA (n = 24, 57.1%), IB (n = 9, 21.4%), IIA (n = 3, 7.2%) and IIIA (n = 6, 14.3%). Positive N1 or N2 lymph node metastases were identified in eight cases (19.0%). The major histological type was adenocarcinoma (n = 27, 64.2%), followed by squamous cell carcinoma (n = 13, 31.0%), pleomorphic carcinoma (n = 1, 2.4%), and adenosquamous carcinoma (n = 1, 2.4%). Nineteen patients (45.2%) had received post-operative adjuvant chemotherapy (uracil/tegafur or platinum-based chemotherapy). Among ten cases (23.8%) who had disease reappearance after radical resection, one case had local recurrence and nine cases had distant recurrences. The sites of distant recurrence were bone (five cases), lung (five cases), distant lymph nodes (two cases), liver (one case) and adrenal glands (one case). Five patients diagnosed with distant recurrence had two types of distant site.
Correlation between galectin-3 expression and clinicopathological characteristics
High expression of galectin-3 was observed in 14 cases (33.3%) (Fig. 1a; Table 2). Univariate analysis showed that high expression of galectin-3 in tumor cells was associated significantly with lymphatic invasion (p = 0.049) (Table 2). In patients diagnosed with recurrence, galectin-3 expression in tumor cells was associated significantly with distant recurrence (p = 0.046) (Table 3). However, age, sex, smoking habits, tumor size, pathological stage, lymph node metastasis, histological grade, pleural invasion, and invasion into microblood vessels were not correlated with galectin-3 expression (Table 2). Univariate analysis revealed significant associations between tumor recurrence and the following factors: low-grade histological tumor (p = 0.013), lymphatic invasion (p = 0.003), microblood vessel invasion (p = 0.010), and high galectin-3 expression (p = 0.001) (Table 4).
Galectin-3 expression in human NSCLC tissues. Galectin-3 positivity in tumor tissues was evaluated by immunohistochemical staining. a Staining at 400× magnification in patients with squamous cell carcinoma. Scale bar, 100 µm. b, c Curves showing relapse-free survival for NSCLC patients with p-stage IA–IIIA (b) and p-stage I (c). Patients with high expression of galectin-3 had significantly shorter disease-free survival than those with low expression (p < 0.001, p = 0.001, log-rank test). d Curves showing overall survival for patients with NSCLC. There was no significant difference in overall survival for patients with high expression of galectin-3 compared those with low expression of galectin-3 (p = 0.213, log-rank test)
Galectin-3 expression in tumor cells, relapse-free survival and overall survival
In the 42 patients included in this analysis, median follow-up duration after surgery was 32.5 months (range 20–43 months). The median relapse-free survival after surgery was not reached in patients with low galectin-3 expression compared with 18.0 months (95% CI 5.3–30.7) in patients with high galectin-3 expression. The Kaplan–Meier curve for relapse-free survival after surgery showed that patients with high expression of galectin-3 (n = 14) had significantly shorter relapse-free survival than those with low expression of galectin-3 (n = 28) (p < 0.001) (Fig. 1b). Furthermore, in 33 patients with p-stage I, patients with high expression of galectin-3 (n = 9) had significantly longer relapse-free survival rate compared with patients with low expression of galectin-3 (n = 24) (p = 0.001) (Fig. 1c). However, there was no significant difference in the overall survival for patients with high expression of galectin-3 (n = 14) compared to those with low expression of galectin-3 (n = 28) (p = 0.213) (Fig. 1d).
Serum levels of galectin-3 in NSCLC patients
The mean serum level of galectin-3 before surgery in NSCLC patients was 16.37 ± 5.13 ng/ml. One month after surgery, however, serum levels of galectin-3 had not decreased significantly (18.13 ± 11.54 ng/ml, p = 0.262) (Fig. 2). Next, we then evaluated the correlation between the serum level of galectin-3 before surgery and clinicopathological characteristics (Table 5). There was no association between the serum level of galectin-3 and patients’ sex, smoking habits, tumor size, pathological stage, lymph node metastasis, histological grade, pleural invasion, lymphatic invasion, invasion of microblood vessels, or tumor recurrence. Furthermore, a significant correlation was not observed between serum level of galectin-3 and galectin-3 expression in tumor cells (Table 5).
Discussion
In this study, we evaluated the predictive value of galectin-3 expression in NSCLC recurrence. We demonstrated that expression of galectin-3 is a potential biomarker for predicting tumor recurrence in NSCLC after radical resection. However, the serum level of galectin-3 did not have a prognostic role in NSCLC, and a significant correlation was not observed between expression of galectin-3 in tumor cells and serum.
Reports have indicated that galectin-3 is overexpressed in several types of tumor including pancreatic and tongue cancers, and is correlated with tumor aggressiveness and a poor prognosis [20, 21]. Conversely, in breast, and clear cell renal cancers, the loss of galectin-3 expression has been reported to be associated with reduced overall survival [22, 23].
In NSCLC, the prognostic importance of galectin-3 expression is controversial [14, 24,25,26]. In one study of NSCLC, Kosacka et al. [24] reported that galectin-3 expression was not associated with poor overall survival. By contrast, Mathieu et al. [25] showed nuclear expression of galectin-3 to be related to shorter relapse-free survival compared with cytoplasmic expression of galectin-3. Those studies included patients with distant metastases or those treated by non-radical resection. The diversity of patients or treatment variation might have influenced those controversial outcomes. With regard to NSCLC patients who underwent curative resection, Szöke et al. [14] suggested that patients with high expression of galectin-3 achieved shorter overall survival than those with low expression of galectin-3 in stage II NSCLC, and Puglisi et al. [26] found that concomitant nuclear expression of nuclear galectin-3 and thyroid transcription factor-1 was associated independently with shorter overall survival. However, those two reports did not detail the prevalence of relapse-free survival.
Whether galectin-3 expression in tumor cells could serve as a predictive biomarker for recurrence has not been clarified. Therefore, in the present study, we evaluated the correlation between galectin-3 expression in tumor cells and relapse-free survival in consecutive patients with NSCLC who underwent radical resection. Immunohistochemical analysis showed that high expression of galectin-3 in tumor cells was correlated significantly with lymphatic invasion and tumor recurrence after radical resection. Furthermore, NSCLC patients with a high level of galectin-3 had significantly shorter relapse-free survival than those with a low level of galectin-3. In patients with tumor reappearance, galectin-3 expression was related significantly to distant recurrence. These findings suggested that galectin-3 expression in tumor cells might be involved in the progression and post-operative tumor recurrence in NSCLC patients.
Unlike results for relapse-free survival, for overall survival, Kaplan–Meier curves did not show a significant difference for galectin-3 expression. This observation might have been because: (i) only five patients died during follow-up; (ii) most patients who had disease recurrence received anti-cancer treatments.
The mechanism by which galectin-3 affects tumor progression and the prognosis is not known, but several mechanisms have been postulated. Some studies have shown that galectin-3 secreted from tumors binds to the receptors on the surface of tumor cells, such as integrins and receptor tyrosine kinases [11]. This linking prevents endocytosis of the receptors, promoting signal transduction and tumor progression [11]. In patients with bladder cancer, pancreatic carcinoma, or hepatocellular carcinoma, the circulating concentration of galectin-3 has been reported to be much higher than that in healthy individuals [27,28,29]. Also, a higher circulating concentration of galectin-3 has been shown to be an independent prognostic factor in colorectal and pancreatobiliary cancer [30, 31]. Iurisci and colleagues addressed the observation that the serum level of galectin-3 in patients with lung cancer was higher than those in normal controls [19]. However, the prognostic value of the serum level of galectin-3 in NSCLC patients has not been clarified.
From the findings in other types of cancers mentioned above, it was expected that the circulating level of galectin-3, as well as galectin-3 expression in tissue, would be a predictive marker for NSCLC recurrence. We used ELISAs to measure the serum concentration of galectin-3 in patients with NSCLC. However, our data showed: (i) no significant correlation between the serum level of galectin-3 and tumor recurrence or clinicopathological features in NSCLC; (ii) no association between the tissue expression and serum level of galectin-3.
Galectin-3 has intracellular and extracellular functions [10,11,12,13]. Galectin-3 has been reported to be secreted by tumor cells as well as normal epithelial cells, fibroblasts, and macrophages [10, 17, 18]. In HeLa cells and hepatocellular carcinoma cells, extracellular galectin-3 has been shown to influence cell motility via an autocrine process [32, 33]. Those studies reported a high level of galectin-3 (15–25 µg/ml) [32, 33]. However, Kuo et al. [16] showed that galectin-3 was not detected in the conditioned medium of NSCLC cell lines that express high galectin-3 in the cytoplasm, and also indicated that the effect of galectin-3 in NSCLC cells might be mainly through intracellular (not extracellular) function. Therefore, in the present study, the serum level of galectin-3 in NSCLC patients might represent galectin-3 secreted from normal (not tumor) cells. Importantly, the galectin-3 concentration did not change before and after surgery and, in fact, the median level of galectin-3 in serum was < 20 ng/ml in NSCLC patients. Based on our results for the serum level of galectin-3, although a high level of extracellular galectin-3 could enhance tumor cell motility, the predicted level in the tumor microenvironment of NSCLC patients could be much lower than that in vitro reported previously. In NSCLC patients, the serum level of galectin-3, as an extracellular function, did not seem to influence the progression or recurrence of tumor cells.
Our study had three main limitations. First, our study cohort was small and contained patients with early and locally advanced NSCLC, which limited our ability to perform multivariate analysis and predict tumor recurrence in patients with NSCLC. Therefore, extended immunohistochemical analyses in a much larger population of NSCLC patients should be performed to confirm the prognostic significance of galectin-3 in NSCLC recurrence. Second, the immunohistochemical analysis was done by researchers under the guidance of one pathologist. Third, we did not measure the serum level of galectin-3 from a control group of healthy individuals. Therefore, we could not evaluate the difference in the serum level of galectin-3 between NSCLC patients and healthy controls. One strength of our study was that patients were treated by radical surgery at the same institution and were followed up at regular intervals after surgery.
In conclusion, this is the first report to address the predictive importance of galectin-3 expression in the tissue and serum of NSCLC patients who underwent radical resection. In human NSCLC, galectin-3 expression in tumor cells could be a biomarker for predicting tumor recurrence after radical resection, but the serum level of galectin-3 is not helpful to predict tumor recurrence.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Lima JP, dos Santos LV, Sasse EC, Sasse AD. Optimal duration of first-line chemotherapy for advanced non-small cell lung cancer: a systematic review with meta-analysis. Eur J Cancer. 2009;45:601–7.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–33.
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229–35.
Hughes RC. The galectin family of mammalian carbohydrate-binding molecules. Biochem Soc Trans. 1997;25:1194–8.
Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17.
Ahmed H, AlSadek DM. Galectin-3 as a potential target to prevent cancer metastasis. Clin Med Insights Oncol. 2015;9:113–21.
Cardoso AC, Andrade LN, Bustos SO, Chammas R. Galectin-3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front Oncol. 2016;6:127.
Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–6.
Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–10.
Szöke T, Kayser K, Trojan I, Kayser G, Furak J, Tiszlavicz L, et al. The role of microvascularization and growth/adhesion-regulatory lectins in the prognosis of non-small cell lung cancer in stage II. Eur J Cardiothorac Surg. 2007;31:783–7.
Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY, Sun KH. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with β-catenin. Oncotarget. 2015;6:4936–52.
Kuo HY, Hsu HT, Chen YC, Chang YW, Liu FT, Wu CW. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology. 2016;26:155–65.
Chan YC, Lin HY, Tu Z, Kuo YH, Hsu SD, Lin CH. Dissecting the structure-activity relationship of galectin-ligand interactions. Int J Mol Sci. 2018; 19.
Dos Santos SN, Sheldon H, Pereira JX, Paluch C, Bridges EM, El-Cheikh MC, et al. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget. 2017;8:49484–501.
Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93.
Shimamura T, Sakamoto M, Ino Y, Shimada K, Kosuge T, Sato Y, et al. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–5.
Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6:4635–40.
Ilmer M, Mazurek N, Gilcrease MZ, Byrd JC, Woodward WA, Buchholz TA, et al. Low expression of galectin-3 is associated with poor survival in node-positive breast cancers and mesenchymal phenotype in breast cancer stem cells. Breast Cancer Res. 2016;18:97.
Merseburger AS, Kramer MW, Hennenlotter J, Serth J, Kruck S, Gracia A, et al. Loss of galectin-3 expression correlates with clear cell renal carcinoma progression and reduced survival. World J Urol. 2008;26:637–42.
Kosacka M, Piesiak P, Kowal A, Gołecki M, Jankowska R. Galectin-3 and cyclin D1 expression in non-small cell lung cancer. J Exp Clin Cancer Res. 2011;30:101.
Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, et al. Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2005;18:1264–71.
Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, et al. Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004;212:233–9.
Gendy HE, Madkour B, Abdelaty S, Essawy F, Khattab D, Hammam O, et al. Diagnostic and prognostic significance of serum and tissue galectin 3 expression in patients with carcinoma of the bladder. Curr Urol. 2014;7:185–90.
Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, He S, et al. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138:1035–43.
Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y, Shimosegawa T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098–111.
Shimura T, Shibata M, Goda K, Nakajima T, Chida S, Noda M, et al. Elevated serum galectin-3 is associated with poor prognosis in patients with colorectal carcinoma. Ann Cancer Res Ther. 2016;24:12–6.
Shimura T, Shibata M, Gonda K, Kofunato Y, Okada R, Ishigame T, et al. Significance of circulating galectin-3 in patients with pancreatobiliary cancer. Anticancer Res. 2017;37:4979–86.
Gao X, Balan V, Tai G, Raz A. Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway. Oncotarget. 2014;5:2077–84.
Serizawa N, Tian J, Fukada H, Baghy K, Scott F, Chen X, et al. Galectin 3 regulates HCC cell invasion by RhoA and MLCK activation. Lab Invest. 2015;95:1145–56.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI, Grant numbers 16K19976 and 18K16415). The authors thank Mitsuaki Ishida for immunohistochemical analysis support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declared that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kataoka, Y., Igarashi, T., Ohshio, Y. et al. Predictive importance of galectin-3 for recurrence of non-small cell lung cancer. Gen Thorac Cardiovasc Surg 67, 704–711 (2019). https://doi.org/10.1007/s11748-019-01074-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-019-01074-x