Abstract
Objectives
Although surgical resection after induction therapy (IT) for locally advanced non-small cell lung cancer (NSCLC) is a possible treatment option, pneumonectomy may be avoided owing to high-surgical risks. However, reports exist that pneumonectomy after IT has acceptable safety and favorable outcomes. We reviewed pneumonectomies after IT in terms of surgical outcomes, perioperative management, and complications.
Methods
Between April 2004 and March 2015, 15 consecutive pneumonectomies were performed for locally advanced NSCLC after IT. Surgical outcomes, perioperative management, and complications were retrospectively reviewed.
Results
Thirteen patients were men, and 6 pneumonectomies were right-sided. One pneumonectomy was performed after induction chemotherapy and 14 followed induction chemoradiation. In all 15 cases the bronchial stumps were covered with autologous tissues. Pedunculated mediastinal fat pad and pedunculated intercostal muscles were used in 4 and 11 cases, respectively. Although postoperative complications were seen in 12 patients (80.0%), with major complications (Clavien–Dindo classification ≥ IIIa) in 5 patients (33.3%), there were no deaths within 30 days after pneumonectomy. Overall 3- and 5-year survivals were 80.0 and 57.1%, respectively.
Conclusions
Owing to high-surgical risks and complication rates, careful surgical technique and postoperative management are essential for successful pneumonectomy after IT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the leading causes of deaths worldwide. Induction therapy followed by surgery is thought to be a treatment option for locally advanced non-small cell lung cancer (LA-NSCLC) [1,2,3,4]. In particular, lobectomy after induction chemotherapy for LA-NSCLC (stage III with N2 disease) has been proven to be a feasible and useful treatment option in a randomized control trial [1]. On the contrary, pneumonectomy after induction therapy is not widely performed owing to the high-surgical risks and complication rates. Recently, however, there have been reports of the safety and usefulness of pneumonectomy following induction therapy for lung cancer [5,6,7,8,9]. In fact, little is known about the surgical risks, usefulness, and complications of pneumonectomy after induction therapy. In the present study, we retrospectively review our experiences with pneumonectomy after induction therapy and analyzed the surgical outcomes, perioperative management, and complications.
Patients and methods
Between April 2004 and March 2015, 2132 surgical resections for non-small cell lung cancer (NSCLC) and 155 surgical resections were performed after induction therapy for NSCLC at Kurashiki Central Hospital. Among these surgical resections, 15 pneumonectomies after induction therapy were performed for locally advanced lung cancer; these 15 cases were the subjects of the current study. Lung cancer staging was performed based on the 7th edition of the tumor node metastasis (TNM) classification of lung cancer [10]. We reviewed the 15 cases included in the present study and retrospectively analyzed surgical outcomes, perioperative management, and complications. The observation period was defined as the time between the date of operation and the last follow-up date or death. Follow-up was censored as of December 2016. This study was approved by the Kurashiki Central Hospital Review Board. The requirement of informed consent from each patient was waived owing to the retrospective nature of the study, with patient information obtained from the database.
Statistical analysis
All statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [11]. The data were evaluated with Student’s t test, and Fisher’s exact test was used for two-group analysis. Actuarial survival rates were calculated using the Kaplan–Meier method. All values were expressed as mean ± standard deviation. Statistical significance was defined as p < 0.05.
Results
Patient characteristics
Among 15 patients, the median age was 67 years (range 38–79 years). Thirteen patients were men and 2 were women. Fourteen patients had a smoking history (93.3%), and the median Brinkman index was 900 (range 0–2000). The following clinical stages of lung cancer were observed: stage IIA (n = 2), stage IIIA (n = 12), and stage IIIB (n = 1). The median tumor size was 47 mm (23–72 mm). The histology of lung cancer was as follows: squamous cell carcinoma (n = 9), adenosquamous carcinoma (n = 3), adenocarcinoma (n = 1), and others (n = 2). Regarding tumor response to induction therapy, there were 10 partial responses and 5 stable diseases (Table 1). The median observation period was 1074 days (range 38–4409 days).
Induction therapy
One patient received induction chemotherapy, and the remaining 14 received induction therapy with concurrent chemoradiation. The chemotherapy regimen was two courses of carboplatin and docetaxel in one case and two courses of carboplatin and paclitaxel in the remaining 14 cases. The radiation dose varied from 30 to 40 Gy; 7 patients received 30 Gy and 7 patients received 40 Gy.
Surgical procedure
Six patients underwent right pneumonectomy and 9 underwent left pneumonectomy. In all cases, pneumonectomy was performed through anterior axillary thoracotomy. Complete tumor resection was achieved in all 15 patients. In all cases, it was not possible to achieve complete resection with the use of bronchoplasty and/or pulmonary angioplasty to avoid pneumonectomy. The bronchial stump was closed with hand-suturing in one patient and with Proximate® reloadable staplers TLH 30 (Ethicon, Tokyo, Japan) and reinforced with several 3-0 Ethibond® (Ethicon) stitches in the remaining 14 patients. Bronchial stump covering was performed in all 15 cases. In 11 cases, pedunculated intercostal muscle was used for bronchial stump covering, and pedunculated mediastinal fat pad was used in the remaining 4 cases. Combined resection was performed in 5 patients, with the superior vena cava (n = 1), with the vagal nerve (n = 1), with the pericardium (n = 2) and with the parietal pleura (n = 1).
Therapeutic effect and postoperative pathological stage
The therapeutic effect on pathology was as follows: 2 patients were Ef. 1, 7 patients were Ef. 2, and 6 patients were Ef. 3 (Table 2). Postoperative pathological stage consisted of 6 cases of pathological complete response, 1 case of stage IA, 2 cases of stage IB, 2 cases of stage IIA, 2 cases of stage IIB, and 2 cases of stage IIIA. Downstaging was achieved in 13 cases.
Postoperative management
Patients undergoing pneumonectomy after induction therapy received follow-up at the outpatient clinic after the hospital discharge once every 1 or 2 weeks until 3 months after the operation. Thereafter, they underwent follow-up once every 3 or 6 months.
Adjuvant therapy
Adjuvant therapy for patients with NSCLC with pathological complete response following pneumonectomy was generally not performed. In other cases, whether adjuvant therapy was administered was discussed at the cancer board, which included thoracic surgeons, thoracic oncologists, and radiotherapists. Adjuvant chemotherapy was administered in 6 patients, and adjuvant radiotherapy was not performed in any patients. The adjuvant chemotherapy regimen consisted of oral intake of UFT® (Taiho, Tokyo, Japan, n = 1) or the same regimen as the induction therapy (n = 5).
Postoperative complications and surgical mortality
Fifteen postoperative complications occurred in 12 patients (80.0%), and 6 postoperative major complications defined as not less than Grade IIIa in the Clavien–Dindo classification occurred in 5 patients (33.3%) [12, 13]. Postoperative complications consisted of tachyarrhythmia (n = 5), empyema without bronchopleural fistula (BPF) in late phase (n = 4), late BPF (n = 1), chylothorax (n = 1), and hypoxemia (n = 4). All postoperative major complications were surgically treated and resolved (Table 3). All grade II complications and chylothorax occurred within 30 days after pneumonectomy, while the remaining major complications occurred after that period.
Regarding the major complications which were surgically managed, since the chylothorax after pneumonectomy is considered difficult to be managed conservatively, surgical management for chylothorax was performed as a semi-emergency operation in a few days after the diagnosis. The BPF in late stage was surgically managed right after the diagnosis from chest X-ray as an emergency operation. The empyema without BPF was also surgically managed a few days after the diagnosis and the insertion of the chest drain tube as a semi-emergency operation.
The 30-day mortality was 0%, and the 90-day mortality was 6.7% (one case). In this case, the patient’s postoperative course in our hospital was uneventful and he was able to return home; however, he chose to be transferred to a different hospital for rehabilitation by his will. He unfortunately died from acute heart failure at that hospital on postoperative day (POD) 38.
Prognosis
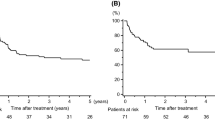
Lung cancer recurred in 6 patients (40%) during the follow-up period. There was no local recurrence; all recurrences were distant metastases such as brain metastases and contralateral lung metastases. During the follow-up period, there were 5 deaths among the 15 patients. Three patients died from progression of lung cancer, and the remaining 2 died from other causes: gallbladder cancer in one patient and acute heart failure in the other. Overall 3- and 5-year survival were 80.0% and 57.1%, respectively (Fig. 1). Moreover, 3- and 5-year relapse-free survival were 59.3 and 39.5%, respectively (Fig. 2).
There were no differences in overall survival between the patients with and without adjuvant therapy, or between the patients with right pneumonectomy and left pneumonectomy. There were also no differences in the therapeutic effect on pathology or the histology of the tumor.
Discussion
There were several important findings from this study. First, pneumonectomy after induction therapy was performed relatively safely in our institution. Second, although the group of patients was very select, all 15 underwent complete resection with pneumonectomy. Finally, as postoperative complications frequently occurred, careful postoperative management was essential for the success of pneumonectomy following induction therapy.
As a treatment option for locally advanced lung cancer, induction therapy followed by surgery is widely performed with satisfactory outcomes [1,2,3,4, 14,15,16,17]. However, following reports of high-mortality rates with pneumonectomy [1], the performance of pneumonectomy after induction therapy has often been avoided. In contrast, there have been reports of successful pneumonectomy after induction therapy, with favorable outcomes [5,6,7,8,9, 18, 19]. In the present study, although there was one death within 90 days, the remaining 14 patients left the hospital and returned to their daily activities. In the patient who died within 90 days, the postoperative course was uneventful, and he was able to return home. However, he elected to be transferred to another hospital, where no thoracic surgeon was available, for rehabilitation. After the hospital transfer, he unfortunately died from acute heart failure. We regret that the patient was not followed-up by thoracic surgeons, as pneumonectomy after induction therapy is very invasive. Considering these points, pneumonectomy after induction therapy was found to be acceptable with careful postoperative management.
In this series, 14 patients out of 15 underwent induction chemoradiation. It is controversial which of induction chemoradiation and induction chemotherapy is better for the treatment of locally advanced NSCLC. Our treatment option for locally advanced NSCLC is basically induction chemoradiation followed by surgical resection. The reason for our policy is that since induction chemoradiation would be superior for local control of NSCLC to induction chemotherapy [16, 20, 21], we consider that the complete resection rate of locally advanced NSCLC would increase by induction chemoradiation. Consequently, all patients underwent complete resection of lung cancer. Furthermore, there were no locoregional recurrences. Indeed, surgical resection after induction therapy could be more difficult to perform than initial resection owing to adhesions and fibrotic change of the hilum. However, for the purpose of achieving complete resection, induction therapy might be considered worth performing in some cases, as complete resection leads to better survival after surgery.
As mentioned above, the high-morbidity and -mortality of pneumonectomy after induction therapy is of great concern. In this regard, two features concerning pneumonectomy after induction therapy in this study are notable. First, when induction radiation therapy was performed, the dose varied from 30 to 40 Gy. In terms of safe operation, as radiotherapy with a dose of more than 45 Gy has been reported to increase postoperative complications such as BPF [22], the dose in our institution might be reasonable. In terms of therapeutic effect, as a major pathological response was achieved in 12 out of 15 patients and postoperative results revealed no local recurrences, the procedure could be considered effective. Second, when bronchial stumps were closed, autologous tissue covering was routinely performed after reinforcement with several stitches. The bronchial healing of the stumps might be frequently impaired owing to the effect of induction therapy and blood supply impairment related to radical lymph node dissection. As a result, complications such as BPF have been reported to occur occasionally. In our series, although postoperative empyema occurred in 4 patients, BPF only in the late phase occurred in one patient. Moreover, with early surgical treatment, BPF in that patient was successfully managed without the development of postoperative empyema. From this perspective, our management of bronchial stumps was thought to be useful.
Regarding postoperative complications, 15 complications (not less than grade II in the Clavien–Dindo classification) occurred in 12 patients. Five of these were tachyarrhythmia, which is sometimes observed after surgery for lung cancer, especially pneumonectomy. This complication was successfully managed with medical therapy in all 5 patients. In terms of major complications (not less than Grade IIIa), 6 major complications occurred in 5 patients; these consisted of 4 empyemas without BPF more than 3 months after pneumonectomy, 1 BPF in late phase, and 1 chylothorax. Four empyemas without BPF were successfully treated with surgical debridement through video-assisted thoracoscopic surgery (VATS). For the successful management of the empyema without BPF, early detection was essential. In this series, all empyemas were detected at the outpatient clinic. Moreover, as empyemas could occasionally recur, careful postoperative observation following VATS was also important. With regard to this point, our policy regarding postoperative management for pneumonectomy after induction therapy seemed valuable. The main cause of empyema after induction therapy followed by pneumonectomy is unknown, but it might be related to suppression of immunity after induction therapy.
The present study has several limitations. This study was a nonrandomized retrospective study performed at a single institution, and the observation period was relatively short. Moreover, a relatively small number of patients who underwent pneumonectomy after induction therapy were evaluated. This was probably because as far as possible, we aimed to avoid pneumonectomy with the use of bronchoplasty and pulmonary arterioplasty; during this period, 19 pneumonectomies were avoided through the performance of 10 bronchoplasties, 6 pulmonary arterioplasties, and 3 double plasties. However, ultimately, achievement of complete resection is most important.
Finally, although lung cancers at several stages were included in this study, the favorable mid-term survival, 80.0% at 3 years and 57.1% at 5 years, was encouraging.
Conclusion
Pneumonectomy after induction therapy for lung cancer was relatively safely performed, and the surgical outcomes were favorable. On the contrary, postoperative complications often occurred after pneumonectomy, and careful postoperative management was essential for success. In some selected cases, pneumonectomy after induction therapy for lung cancer was worth performing.
References
Albain KS, Swann RS, Rusch VW, Turrisi IIIAT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86.
Venuta F, Anile M, Diso D, Ibrahim M, De Giacomo T, Rolla M, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg. 2007;31:714–8.
Koshy M, Fedewa SA, Malik R, Ferguson MK, Vigneswaran MT, Feldman L, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non–small-cell lung cancer. J Thorac Oncol. 2013;8:915–22.
Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386:1049–56.
Weder W, Collaud S, Eberhardt WEE, Hillinger S, Welter S, Stahel R, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg. 2010;139:1424–30.
Doddoli C, Barlesi F, Trousse D, Robitail S, Yena S, Astoul O, et al. One hundred consecutive pneumonectomies after induction therapy for non-small cell lung cancer: an uncertain balance between risks and benefits. J Thorac Cardiovasc Surg. 2005;130:416–25.
Broderick SR, Patel AP, Crabtree TD, Bell JM, Morgansztern D, Robinson CG, et al. Pneumonectomy for clinical stage IIIA non-small cell lung cancer: the effect of neoadjuvant therapy. Ann Thorac Surg. 2016;101:451–8.
d’Amato TA, Ashrafi AS, Schuchert MJ, Alshehab DSA, Seely AJ, Shamji FM, et al. Risk of pneumonectomy after induction therapy for locally advanced non-small cell lung cancer. Ann Thorac Surg. 2009;88:1079–85.
Kim AW, Faber LP, Warren WH, Basu S, Wightman SC, Weber JA, et al. Pneumonectomy after chemoradiation therapy for non-small cell lung cancer: does “side” really matter? Ann Thorac Surg. 2009;88:937–44.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Clavien PA, Barkun J, de Oliverla ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Kim AW, Liptay MJ, Bonomi P, Warren WH, Basu S, Farlow EC, et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg. 2011;92:233–43.
Speicher PJ, Englum BR, Ganapathi AM, Onaitis MW, D’Amico TA, Berry MF. Outcomes after treatment of 17 378 patients with locally advanced (T3N0–2) non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47:636–41.
Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer. 2012;118:6126–35.
Friedel G, Budach W, Dippon J, Spengler W, Echmann SM, Pfannenberg C, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol. 2010;28:942–8.
Steger V, Spengler W, Hetzel J, Veit S, Walker T, Mustafi M, et al. Pneumonectomy: calculable or non-tolerable risk factor in trimodal therapy for stage III non-small-cell lung cancer? Eur J Cardiothorac Surg. 2012;41:880–5.
Allen AM, Mentzer SJ, Yeap BY, Soto R, Baldini EH, Rabin MS, et al. Pneumonectomy after chemoradiation; the Dana-Farber Cancer Institute/Brigham and Women’s Hospital experience. Cancer. 2008;112:1106–13.
Chen F, Okubo K, Sonobe M, Shibuya K, Matsuo Y, Kim YH, et al. Hyperfractionated irradiation with 3 cycles of induction chemotherapy in stage IIIA-N2 lung cancer. World J Surg. 2012;36:2858–64.
Toyooka S, Kiura K, Shien K, Katsui K, Hotta K, Kanazawa S, et al. Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg. 2012;15:954–60.
Fujita S, Katakami N, Takahashi Y, Hirokawa K, Ikeda A, Tabata C, et al. Postoperative complications after induction chemoradiotherapy in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg. 2006;29:896–901.
Acknowledgements
The authors thank Reiko Moriwake for laborious efforts in data management.
Funding
No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose with regard to commercial support. No potential conflicts exist.
Rights and permissions
About this article
Cite this article
Kayawake, H., Okumura, N., Yamanashi, K. et al. Surgical outcomes and complications of pneumonectomy after induction therapy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 66, 658–663 (2018). https://doi.org/10.1007/s11748-018-0980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-018-0980-4