Abstract
Carcinomas rarely occur in the heart, and cardiac surgeons are generally not familiar with cardiac tumors. Some characteristics of cardiac and intrapericardial tumors are reviewed to understand the features of cardiac tumors. Cardiac tumors are discussed separately from intrapericardial tumors. Primary cardiac tumors are predominantly benign whereas primary intrapericardial tumors are usually malignant. The prevalence of each tumor types is presented in this review. In both cardiac and intrapericardial tumors, the incidences of metastatic tumors from cancers outside of the heart are high with carcinomas occupying more than half of the cases. Generally, the prognosis of primary malignant cardiac tumors is very poor. Cardiac tumors metastasize to other organs as hematogenous metastases. Surgery must be performed based on all the above-mentioned features of both cardiac and intrapericardial tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, it is believed especially by non-medical populations that cancers rarely occur in the heart. In addition, unlike general surgeons, many cardiac surgeons are not familiar with tumors in the heart [1]. Only 602 cardiac tumor surgeries were performed out of 66,453 cardiovascular surgeries performed in Japan during 2014.
The reality is that carcinomas do not occur in the heart, but sarcomas are more common in the heart. Carcinomas begin in the epithelial tissues, and this is the reason why they do not occur in the heart. The heart consists mostly of mesenchymal tissues, and the only epithelial tissues are the endocardium and the pericardium. This lack of carcinomas is a feature that can differentiate the heart from other organs. It can be speculated that this lack of carcinomas leads to the general belief that cancers are rare in the heart.
This report details some features of cardiac tumors that may well lead cardiac surgeons to better management of such tumors. Not only diagnosing the existence of such tumors and surgically removing them, but also treating them with a better understanding is a key to patient survival and quality of life.
Primary cardiac tumors
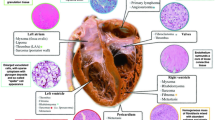
Cardiac tumors can be classified into cardiac tumors and intrapericardial tumors [2]. Cardiac tumors include intracavity and mural types based on their location [3]. To avoid confusion, we narrowly define here cardiac tumors as intracavity and mural tumors. The tumors that are located mostly in the intrapericardial space or originating from the pericardium are defined here as intrapericardial tumors.
According to the frequency of malignancies, primary cardiac tumors predominate as benign tumors and primary intrapericardial tumors are generally malignant.
According to the Atlas of Tumor Pathology, published by the Armed Forces Institute of Pathology (AFIP) [4], and based on a single institution series other than the AFIP, the most prevalent primary cardiac tumor of 124 surgical and autopsy cases was myxoma (42%), followed by sarcoma (14%), rhabdomyoma (11%), papilloma (9%), and fibroma (7%). The sarcomas were rhabdomyosarcoma (5%), angiosarcoma (3%), and other sarcomas (6%). In an exclusive surgical series, myxomas accounted for over 75% of 986 cases. The tumors were classified as myxoma (77%), sarcoma (10%), fibroma (4%), and lipoma (3%).
In the Atlas of Tumor Pathology [4], the most prevalent intrapericardial tumor in the AFIP was mesothelial cyst (58%) followed by malignant mesothelioma (13%), and sarcoma (13%) out of 64 primary cysts and tumors of the pericardium. If one excludes cysts, then malignant tumors (27%) were more common than benign tumors (13%).
According to Japanese surgical statistics, the types of cardiac tumor resections are as follows [1]: benign tumor (88%) and malignant tumor (12%). The benign tumors were classified as myxoma (70%), papillary fibroelastoma (8%), rhabdomyoma (1%), and others (10%). The malignant tumors were classified as primary (7%) and metastatic (4%).
Primary or metastatic
In both cardiac and intrapericardial tumors, the incidences of metastatic tumors originating from primary tumors outside of the heart are much higher than that of primary cardiac tumors. In fact, metastasis to the heart from other primary cancers is 30 times more common than primary cardiac tumors [5]. Secondary neoplasms of the pericardium account for greater than 95% of pericardial neoplastic diseases [6]. Primary pericardial tumors are rare. They are 40 times less common than the metastatic ones from tumors outside of the heart [7].
Although the incidence of metastases in the heart is high, these tumors are seldom treated surgically. As the Japanese surgical statistics show, only 4% of all cardiac tumor surgeries are performed for resection of metastatic cardiac tumors [1]. The discrepancy between the incidence and surgically treated metastatic cases is easily understandable. Most cardiac or intrapericardially metastasized cases are systemically metastasized, and are not curable by surgical resection of only these lesions.
Common cancers metastasized
Mukai et al. found an increased incidence of secondary cardiac tumors compared with that of primary cardiac tumors in a series of autopsies [8]. They found only one instance of a primary tumor among the 2,649 autopsies of malignant tumors. In contrast, there were 407 cases (15%) of metastasis to the heart from a malignant tumor originating from other organs, including 78 cases (3%) of pericardial metastasis and 329 cases (12%) of cardiac metastasis with or without pericardial involvement. Based on the literature, Amano et al. cites the incidence of primary cardiac tumors diagnosed at autopsy as 0.0017–0.33% which is much lower than the frequency of metastasis (13.6%) to both the heart and the pericardium at autopsy [9]. According to Burke and Virmani, among their data of 6,240 autopsies, 654 cases (10%) were identified as cardiac metastases and 299 cases (5%) as pericardial metastases [10]. A high frequency of metastasis was seen in malignant melanoma (49%), malignant germ cell tumors (42%), and leukemia (34%). A lower frequency of metastasis was seen in lung cancer (28%), lymphoma (21%), breast cancer (20%), and esophageal cancer (17%) while the lowest frequency of metastasis was seen in gastric cancer (7%), hepato-biliary cancer (2%), ovarian cancer (4%), and colorectal cancer (6%). Since the absolute number of cases of each original malignancy was different, lung cancer, breast cancer, lymphoma, leukemia, and esophageal cancer had a high number of metastatic cases. Carcinomas as a whole occupied more than half of both the cardiac and pericardial metastatic cases.
Survival
According to the Case Western Reserve University group, primary malignant cardiac tumors continue to be associated with a poor prognosis [11]. Their data were obtained by accumulating 516 sarcoma, lymphoma, or mesothelioma patients from 18 cancer registries in the United States. This series included patients receiving surgery in 44% of the cases, and receiving radiation in 19% of the cases, but the use of chemotherapy was not documented. The 1-, 3-, 5-year survival rates of 32, 17, and 14% for 1973 to 1989 improved to 50, 24, and 19% for 2000–2011 (P = 0.009). Their survival, although still poor, appears to have increased over the past 5 decades. Some cases have good prognosis even if diagnosed as malignant. For example, the author has a case of a malignant solitary fibrous tumor (SFT) with a good prognosis after resection [12, 13].
Survival is highly dependent on the completeness of the surgical excision. In a series of 34 primary sarcomas from the Mayo Clinic, the median survival for those who underwent a complete surgical excision was 17 months compared with 6 months for those where a surgical complete excision could not be achieved (P = 0.01) [14]. Yu et al. had a similar conclusion in a series of 29 patients. Patients who had positive margins had a median survival duration of only 16 months, whereas patients with negative surgical margins had a median overall survival duration of 27 months (P = 0.002) [15]. There were no significant differences in survival whether the patients received post-surgical adjuvant chemotherapy or not. However, Habertheuer et al. insisted on the importance of adjuvant chemotherapy and radiation [16]. In a series of 10 sarcoma patients who underwent open-heart surgery, patients who underwent adjuvant combination chemotherapy or doxorubicin (mono-chemotherapy) and radiation had a mean survival of 45.7 months compared to 4.2 months for patients who were treated with either radiation alone (median survival 4 months), mono-chemotherapy (median survival 5 months), or who did not receive any adjuvant therapy (P < 0.05).
Metastasis to other organs
Cardiac tumors are also known to metastasize to other organs. Approximately 80% of primary cardiac angiosarcomas have metastatic disease at the time of diagnosis [17]. The lung is the most common site of metastasis, and other common sites include the liver, lymph nodes, bone, and adrenals. Out of 47 cases of cardiac malignant fibrous histiocytoma, 9 cases involved metastasis to the brain, 5 cases to the lung, 5 cases to the bone, and 4 cases to the adrenal glands [18]. The overall frequency of metastasis was 20 cases (43%), and the results suggested hematogenous metastasis. Reported organs that had metastases from a cardiac sarcoma include lungs, which are the most common site (53%), soft tissue including the mediastinum (19%), bone (11%), brain (8%), and liver (6%) [19].
Diagnostic procedures
Echocardiography is the best diagnostic tool for intracavity and mural cardiac tumors. Recent advances in echocardiography have led to a determination of both the precise localization and size of the tumors prior to surgery. Computed tomography (CT), magnetic resonance imaging (MRI), and/or angiography further add to the diagnostic information.
For intrapericardial tumors, CT and MRI are helpful for clarifying the anatomy of the tumor and invasiveness into the cardiac structures [2]. These methods even reveal the vascularity of the tumors and are superior to echocardiography.
Shown in Fig. 1 is an example of an intrapericardial tumor attached to the left ventricular (LV) wall. It was taken from the above-mentioned intrapericardial SFT. The CT scan reveals a heterogeneous mass on the left side of the heart which is slightly enhanced (Fig. 1a). The MRI reveals the mass with low signal intensity regions on the T2-weighted images (Fig. 1b).
Tumors, such as neurogenic tumors, SFT, sarcoma, and mesothelioma, can be included among the differential diagnosis [12]. The problematic aspect is that the malignancy of the tumors cannot be assessed with these diagnostic measures.
Hemodynamically, the effect of these cardiac and intrapericardial tumors is diverse. Even if malignancy is suspected, there are cases of cardiac tumors that require emergency surgery due to hemodynamic deterioration. For example, Yamamoto et al. reported a case of right ventricular sarcoma with pulmonary perfusion defect [20]. We reported a case of an intrapericardial lipoma with non-sustained ventricular tachycardia [21].
Surgical indications
Benign intracavity tumors should be removed surgically due to the possibility of embolism. Other cases that require surgery include benign tumors causing hemodynamic dysfunction, pericarditis, cardiac tamponade, and arrhythmia [21].
For malignant tumors, surgery is indicated to debulk the tumor as either a palliative procedure [22] or for tissue diagnosis [23]. Malignant primary cardiac neoplasms often require palliative surgery, because 78% of the patients present with a mechanical blood flow obstruction [24]. In such cases, emergency surgery is often indicated.
Whether malignant or benign, the indications for emergency surgery are based on the clinical symptoms. Cardiac tumors can cause a large variety of symptoms, including the following involving four indications [23]: (1) obstruction of intracardiac blood flow or interference with valve function, (2) arrhythmias or pericardial effusions with tamponade due to local invasion, (3) embolization, causing systemic or pulmonary deficits, and (4) systemic or constitutional symptoms caused by the tumors.
The most common clinical presentation is heart failure, followed by symptoms caused by peripheral emboli to the cerebral, systemic and coronary arterial circulatory systems [23]. Congestive heart failure symptoms (28%) and thromboembolism (17%) accounted for close to half of the presenting signs and symptoms in one series of patients [25]. In another series, congestive heart failure was indicated in 68% of the cases and peripheral embolization in 20% of the cases [26]. Although dependent on the patients’ condition, these symptoms generally required emergency surgery.
Surgical techniques
Surgery should be performed after a precise and careful pre-surgical assessment that has determined all of the above-mentioned features of cardiac or intrapericardial tumors. Whether to use cardiopulmonary bypass [21, 27], whether to arrest the heart for resection [12, 21, 27], whether to pursue complete resection [12], and so on are basic approaches that will be determined with full knowledge of the tumor location, size, metastatic status, and condition of the patient. Broader aspects of surgery, such as whether to pursue longer survival by a palliative procedure or rather a better quality of life by conservative therapy also must be considered.
The surgery for the malignant SFT of the LV wall described above was technically difficult. Shown are the related CT scan and MRI (Fig. 1) [12]. The tumor was attached to the LV wall with a broad stalk (Fig. 2a). Since malignancy was not diagnosed before the surgery, as much as possible of the tumor had to be removed. For that purpose, the tumorectomy was performed under cardiac arrest using a harmonic scalpel with secure hemostasis. The tumor apparently invaded the LV wall and complete resection was not accomplished (Fig. 2b). Histopathological examination of the tumor showed spindle cell proliferation with myxoid degeneration, and nuclear atypia was present (Fig. 3). The tumor was diagnosed to be SFT of low-grade malignancy. To minimize the risk of recurrence, partial resection of the LV wall with a patch reconstruction might have been indicated. In such a case, an assist device might have been needed.
Macroscopic view of a cardiac tumor. The patient’s head is located at the bottom of the pictures. a The tumor (left side of the picture) was attached to the ventricular wall (right side of the picture). It was yellowish in color with scattered white solid parts. b Tumorectomy being performed with a harmonic scalpel. The left ventricular wall was deeply excised during the tumor removal
Another technique that can be considered in a case of malignancy is an ex situ resection of the tumor [28], or so-called auto-transplant. This technique refers to removing the heart out of the thorax to facilitate complete resection of the tumor, and the heart is then re-implanted in its original position. This technique is indicated in tumors involving either the left atrium or the dorsal great vessels. Depending on domestic laws, a heart transplant can also be considered as a final treatment option, provided distant metastases can be ruled out [28].
Other surgical techniques include robotic technology. Some institutions aggressively use robotics, especially for atrial myxomas [29]. Also, either cryoablation of the atrial myxoma stalk [30] or tumor implantation site [31] is becoming more common as a surgical therapy. These methods can be applied in cases with good prognoses.
Other methods of treatment
In addition to surgery, other treatment approaches include chemotherapy and radiotherapy [20, 28, 32], as mentioned above. These methods are important when the cardiac tumors are malignant with dissemination to other organs, or when the tumors begin cardiac metastases originating from tumors in other organs. These adjuvant therapies require individual regimens and specifically designed protocols [20]. It is important for oncologists, radiotherapists, and surgeons to cooperate closely in such cases [28]. Since the numbers of cases are small, treating cardiac tumors as a team with full use of each specialist’ knowledge and experience is crucial for optimal treatment and outcome.
Conclusions
In summary, several important points can be made with regard to cardiac tumors. Carcinomas do not generally occur in the heart or in the intrapericardial space. As primary tumors, the frequency of benign tumors is high for cardiac tumors, and frequency of malignant tumors is high for intrapericardial tumors. For both cardiac and intrapericardial tumors, metastatic tumors originating from other organs are more common than primary cardiac tumors.
References
Masuda M, Okumura M, Doki Y, Endo S, Hirata Y, Kobayashi J, et al. Thoracic and cardiovascular surgery in Japan during 2014. Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665–97.
Taguchi S, Yozu R. Surgery for primary intrapericardial tumors in adults. J Card Surg. 2013;28:529–32.
Goldberg HP, Steinberg I. Primary tumors of the heart. Circulation. 1955;11:963–70.
Burke A, Virmani R. Classification and incidence of cardiac tumors. Tumors of the heart and great vessels. In: Burke A, Virmani R, editors. Atlas of tumor pathology, series 3, fascicle 16. Washington, D.C.: Armed Forces Institute of Pathology; 1996. pp. 1–11.
Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38:261–2.
Craig JM, Walker JD. Pericardial disease. In: Cohn LH, editor. Cardiac surgery in the adult. 4th ed. New York: McGraw-Hill; 2012. pp. 1229–44.
Maisch B, Seferovic PM, Ristic AD, Erbel R, Rienmuller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases. Executive summary. The task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610.
Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol. 1988;18:195–201.
Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. 2013;61:435 – 47.
Burke A, Virmani R. Classification and incidence of cardiac tumors. Tumors of the heart and great vessels. In: Burke A, Virmani R, editors. Atlas of tumor pathology, series 3, fascicle 16. Washington, D.C.: Armed Forces Institute of Pathology; 1996. pp. 195–209.
Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors: a 40-year analysis of over 500 patients. Circulation. 2015;132:2395 – 402.
Taguchi S, Mori A, Yamabe K, Suzuki R, Nishizawa K, Hasegawa I, et al. Malignant solitary fibrous tumor of the left ventricular epicardium. Ann Thorac Surg. 2013;95:1447–50.
Taguchi S. Primary cardiac solitary fibrous tumors. Ann Thorac Cardiovasc Surg. 2015;21:329 – 31.
Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, et al. Malignant primary cardiac tumors. Cancer. 2008;112:2440–6.
Yu L, Gu T, Shi E, Xiu Z, Fang Q, Wang C, et al. Primary malignant cardiac tumors. J Cancer Res Clin Oncol. 2014;140:1047–55.
Habertheuer A, Laufer G, Wiedemann D, Andreas M, Ehrlich M, Rath C, et al. Primary cardiac tumors on the verge of oblivion: a European experience over 15 years. J Cardiothorac Surg. 2015;10:56.
Riles E, Gupta S, Wang DD, Tobin K. Primary cardiac angiosarcoma: a diagnostic challenge in a young man with recurrent pericardial effusions. Exp Clin Cardiol. 2012;17:39–42.
Okamoto K, Kato S, Katsuki S, Wada Y, Toyozumi Y, Morimatsu M, et al. Malignant fibrous histiocytoma of the heart: case report and review of 46 cases in the literature. Int Med. 2001;40:1222–6.
Fujino S, Miyoshi N, Ohue M, Noura S, Hamamoto S, Oshima K, et al. Primary osteosarcoma of the heart with long-term survival: a case report of laparoscopic resection of a metastatic sarcoma in the intestine. Oncol Lett. 2014;8:1599 – 602.
Yamamoto H, Yamamoto F, Ishibashi K, Matsukawa M, Liu KX, Hasegawa H. Primary sarcoma of the right ventricle: surgical and adjuvant therapy. Gen Thorac Cardiovasc Surg. 2009;57:421–5.
Taguchi S, Mori A, Suzuki R, Ishida O, Hasegawa I, Irie R. Resection of an intrapericardial lipoma with nonsustained ventricular tachycardia. J Card Surg. 2013;28:268–70.
Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics 2000;20:1073–103.
Ekmektzoglou KA, Sameli GF, Xanthos T. Heart and tumors: location, metastasis, clinical manifestations, diagnostic approaches and therapeutic considerations. J Cardiovasc Med. 2008;9:769–77.
Miralles A, Bracamonte L, Soncul H, Diaz del Castillo R, Akhtar R, Bors V, et al. Cardiac tumors: clinical experience and surgical results in 74 patients. Ann Thorac Surg. 1991;52:886–95.
Odim J, Reehal V, Laks H, Mehta U, Fishbein MC. Surgical pathology of cardiac tumors. Two decades at an urban institution. Cardiovasc Pathol. 2003;12:267–70.
Larrieu AJ, Jamieson WR, Tyers GF, Burr LH, Munro AI, Miyagishima RT, et al. Primary cardiac tumors: experience with 25 cases. J Thorac Cardiovasc Surg. 1982;83:339–48.
Taguchi S, Mori A, Suzuki R, Hasegawa I, Sato H, Sugiura H, et al. Mediastinal schwannoma diagnosed preoperatively as a cyst. Tex Heart Inst J. 2014;41:76–9.
Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111:205–11.
Gao C, Yang M, Wang G, Wang J, Xiao C, Wu Y, et al. Excision of atrial myxoma using robotic technology. J Thorac Cardiovasc Surg. 2010;139:1282–5.
Marinakis S, Mircev D, Wauthy P. Cryoablation for a right atrial myxoma arising from the Koch’s triangle: a case report. J Cardiothorac Surg. 2013;8:222.
Sarraj A, Gonzalez AR, Calle Valda CM, Munoz DE, Antonio Anton ND, Monguio E, et al. Cryotherapy in benign heart tumors. Am J Cardiovasc Thorac Surg. 2016;1(1):2.
Ghiam AF, Dawson LA, Abuzeid W, Rauth S, Jang RW, Horlick E, et al. Role of palliative radiotherapy in the management of mural cardiac metastases: who, when and how to treat? A case series of 10 patients. Cancer Med. 2016;5:989–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Taguchi, S. Comprehensive review of the epidemiology and treatments for malignant adult cardiac tumors. Gen Thorac Cardiovasc Surg 66, 257–262 (2018). https://doi.org/10.1007/s11748-018-0912-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-018-0912-3